Abstract
Detailed knowledge on species–habitat relationships is of crucial importance for the understanding of processes in marine ecosystems. Being top-predators, birds are important bio-indicators for marine systems. The aim of this study was to elucidate precise information on foraging habitat use and foraging times of oystercatchers (Haematopus ostralegus) on wide tidal flats using global positioning system (GPS) data loggers. The study was conducted to collect hints for the negative population trends in oystercatchers in the Wadden Sea. It is the first time that GPS technique has been used in a shorebird species. Although oystercatchers are known to exhibit foraging site fidelity, a number of individuals visited multiple sites. Foraging trips at night were longer, and the targeted sites were further away than those used during the day. These patterns were likely to be caused by higher risks of clutch predation by avian predators during the day that led adults to reduce their absence to defend their clutches. Our methodological approach enabled the subtle spatio-temporal patterns of habitat use to be determined on a very fine spatio-temporal scale. We suggest further potential studies using GPS data loggers that may help to reveal the reasons for the current declines in oystercatcher populations in the German Wadden Sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the breeding period, birds need to balance the risks of clutch predation and loss of breeding territory against the risk of starvation for themselves and their offspring (e.g., Low et al. 2008). Adults therefore have to weigh the time spent foraging (to maintain body condition) against the time spent in the breeding habitat (to defend their clutch against predators and their territories against conspecifics). This balance is particularly important in shorebirds such as Eurasian oystercatchers (Haematopus ostralegus) that feed in tidally structured environments, because the time frame available for foraging is restricted by the tidal cycle. Although it has been shown that oystercatchers are able to conserve energy during the breeding season, and may even spend some time apparently inactive (Kersten 1996; Kersten and Visser 1996), some of this “spare time” needs to be devoted to digestion (Zwarts et al. 1996a). Furthermore, although they are inactive at the breeding colony for a certain proportion of time, individuals benefit from staying at the breeding site for as long as possible, to be available to defend their territory and clutch. In accordance with this idea, breeding oystercatchers were found to spend much less time foraging on tidal flats and had higher food intake rates compared to non-breeders, enabling them to return to the nest site sooner (Hulscher 1982). Taking these constraints into account, oystercatchers would be expected to time their foraging flights and choose their foraging areas carefully to allow them to return to their breeding territories as quickly as possible, while also enabling them to gain sufficient energy for their own metabolism.
Oystercatchers in the Wadden Sea are known to establish foraging territories on the mudflats, which they defend against conspecifics (e.g., Ens et al. 1992), enabling effective foraging within a known environment and thus allowing them to return quickly to their clutches. In accordance with that, we assumed site fidelity by foraging oystercatchers (i.e., constantly returning to a known habitat patch). While oystercatchers mainly forage using visual cues during daylight, they are thought to use tactile cues at night, which might make feeding slower (Hulscher 1976; Sutherland 1982b; Sitters 2000). In addition, the availability of benthic prey may differ between day and night (Esser et al. 2008), as well as during the tidal cycle (e.g., Hulscher 1996; de Vlas et al. 1996). Furthermore, the predation risks to both foraging adults and to clutches or chicks may differ between day and night. We therefore investigated differences in the temporal and spatial foraging flight patterns of oystercatchers between day and night, and at different tidal stages.
Like many other wader species, Eurasian oystercatchers have declined significantly in the German Wadden Sea during recent years (e.g., Blew et al. 2007; CWSS 2010). Although food availability has been reduced as a result of the extensive mussel fisheries in parts of the Dutch Wadden Sea (e.g., Piersma et al. 2001; Verhulst et al. 2004; Scheiffarth and Frank 2005), these effects alone are not sufficient to explain the negative trends in other parts of the Wadden Sea. It is therefore essential to investigate the foraging behaviour of oystercatchers in more detail, as information on the temporal and spatial patterns of foraging site selection will provide further insights into the possible relationships between oystercatchers and their prey. However, such studies have been hampered by logistic problems, especially on large tidal flats where precise information on habitat use during day and night cannot be obtained using visual methods.
We therefore equipped several individual oystercatchers with global positioning system (GPS) data loggers to gain detailed information on their temporal and spatial patterns of habitat use. These devices have already been used on larger seabirds (e.g., Weimerskirch et al. 2002; Grémillet et al. 2004; Garthe et al. 2007a, b), but to the best of our knowledge, this study is the first to use GPS data loggers to provide results for a shorebird species. GPS loggers allow the birds to be tracked during both day and night, at any distance from the observer. The German Wadden Sea includes vast tidal flats up to several kilometres in width, making information on foraging site utilization by oystercatchers impossible to gain by visual observations.
Remote techniques provide an upcoming methodology for studying habitat utilization by different marine animals, including birds (e.g., Hedd et al. 2009; Harris et al. 2010), sharks (e.g., Meyer et al. 2010; Stevens et al. 2010) and turtles (e.g., Girard et al. 2009). Three types of devices other than GPS data loggers have been used to reveal the spatial distribution patterns of shorebirds: (1) Radio transmitters have previously been used on oystercatchers (e.g., Exo 1992; Exo et al. 1996; Sitters 2000), but the temporal and spatial resolutions of the data obtained from GPS data loggers are more accurate and not range-restricted. (2) Satellite telemetry is suitable for tracking long-distance migrants on their flyways, when relatively coarse temporal and spatial resolutions are sufficient (e.g., Hamer et al. 2000; Kenow et al. 2002). The same is true of (3) geolocators, which provide an indirect measure of position by measuring the time of sunset and sunrise (for further details see e.g., Harris et al. 2010). The major aim of the current study was to gain information on the fine-scale distribution patterns within foraging habitats, and GPS data loggers were therefore used because of their superior temporal and spatial resolutions.
Because the use of GPS technology in shorebirds is novel, in addition to answering the questions raised, we also briefly discuss the applicability of these devices to solving questions related to habitat in this species group. Finally, we provide some suggestions for follow-up studies using GPS technology that may help to finally shed light on the reasons for the negative population trends in oystercatchers in the German Wadden Sea.
Materials and methods
Study area, GPS data loggers and equipment
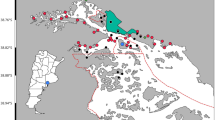
The study was conducted on the German Wadden Sea island of Oland (see small map in Fig. 1a) during the incubation period from 18 to 27 May 2008. About 340 pairs of oystercatchers were breeding on this island in 2008 (K. Lutz pers. comm.), mainly using grazed pastures as their breeding sites. Oland is located about 3 km off the mainland coast in the German Wadden Sea (Fig. 1a) and is surrounded by vast tidal flats that are frequented by oystercatchers and other breeding birds. There are large tidal creek systems at the north and south of the island (Fig. 1). During low tide, these tidal creeks are located at a distance of 1.2–2.5 km from the oystercatcher nest sites. Oland is only connected to the mainland by a railway track, which is not accessible to mammalian predators (Lutz 2008).
Examples of foraging trips of oystercatchers revealed by GPS data loggers: a four trips and home range of bird #18, including map of the location of Hallig Oland in the German Wadden Sea (top right corner); b nine trips and home range of bird #13; c five trips and home range of bird #5. Points on lines indicate fixes of GPS loggers, lines indicate shortest distance between fixes, different colours of lines represent different foraging trips, triangles indicate nest sites of sampled individuals. d All 61 foraging trips of the eight individuals (same colours indicate same individuals)
Nine individual oystercatchers were trapped using walk-in-traps while incubating, between 18 and 27 May 2008. The first chicks hatched in the second week of June. GPS data loggers (Earth & Ocean Technologies, Kiel, Germany) were attached to the tail feathers using adhesive TESA® tape (Wilson et al. 1997). Therefore, the device was positioned at the proximal end of the five middle tail feathers. The devices weighed 18 g. Three of the nine trapped oystercatchers were weighted and showed a mean body weight of 521 g (range: 487–574 g). The devices therefore accounted for 3.4% (range: 3.7–3.1%) of the body weight. As a comparison, Exo et al. (1996) used radiotelemetry devices equivalent to 3.5–4% of the body weight. Oystercatchers were released immediately after attaching the device, and after dyeing some tail and breast feathers to facilitate identification of the equipped individual in the field.
After a delayed start of 1–12 h, the devices recorded time, geographical position (80% of the data deviated by less than 10 m) and speed (km h−1) at programmed intervals of 20–60 s (for individual logging intervals, see Table 1). The additional standard period for the satellite uplink to gain a GPS-fix was 6–22 s. Dependent on the logging intervals, the batteries in the devices lasted long enough to provide data for 15–38 h for each individual bird (for the total number of individual GPS fixes recorded, see Table 1). Devices with a longer operating duration were available, but they would have been too heavy (exceeding 5% body mass) to put on oystercatchers and would have been likely caused device effects (Phillips et al. 2003). The devices were left on the birds for 2–3 days before re-capture using the same catching method. The device was then removed and the data were downloaded. We found no indications of behavioural differences between equipped and unequipped birds on the basis of visual observations. Biometric sexing of oystercatchers (Zwarts et al. 1996b) revealed a strong bias; all the individuals caught were males, except for individual #18 which was a female, and two further individuals that could not be sexed. However, Van de Pol et al. (2009) stated that biometric sexing of oystercatchers may be unreliable because of variances in biometric parameters between years. We therefore did not analyse any effects of sex on temporal and spatial patterns of habitat use. Eight of the nine individuals equipped with data loggers were recaptured.
Statistical analyses
For all correlations of foraging trip duration and foraging trip length with time of day, the data were separated as follows: “day” was classified as all daylight hours, including the time of civil twilight; “night” encompassed the hours of darkness. Four tidal stages were classified: (1) low tide (1.5 h prior to low tide to 1.5 h after low tide); (2) flood tide (1.5 h after low tide to 1.5 h prior to high tide); (3) high tide (1.5 h prior to high tide to 1.5 h after high tide); (4) ebb tide (1.5 h after high tide to 1.5 h prior to low tide). Only four trips were performed by the birds during high tide, while similar numbers of trips were performed during the other tidal stages (Table 1). A generalized linear mixed effect model (GLMM; details are given below) was applied to account for tidal effects (and for disproportionate coverage of different tidal stages) when testing for the influence of time of day on trip length/distance, and vice versa (Pinheiro and Bates 2009).
Geographical information system ArcView 3.2 was used to visualize the spatial patterns of the foraging trips of the eight individuals. A foraging trip was identified by the absence of the bird from the breeding territory at the same time as the recorded speed over several measurements showed values of 0–2 km h−1, which represented the characteristic pattern of individuals searching for food, determined by comparing the results of several hours of direct field observations (when the birds were near enough) with logger data. Foraging trips that led further away from the breeding territory could also be identified by the different location and high flight speeds at the beginning and end of the foraging trips. Foraging site fidelity of the sampled individuals was estimated by performing kernel home range analyses (e.g., Worton 1989) using the Animal Movement extension in ArcView 3.2 (http://www.absc.usgs.gov/glba/gistools/animal_mvmt.htm). The total foraging areas in which 25, 50, 75 and 95% of the positions recorded by GPS loggers were located were recorded (e.g., Wood et al. 2000).
We tested for differences in trip length (min) (i.e., time between departure from and arrival at the nest) and trip distance (m) (i.e., maximum distance from the nest site reached during a foraging trip), respectively, between night and day (referred to as “time class”), as well as between different tidal stages (referred to as “tide”). Each individual oystercatcher performed more than one trip, and these were treated as replicates. This individual effect was accounted for using a GLMM based on restricted maximum likelihood, using the individual as a random effect (Venables and Ripley 2002; Faraway 2006). To test for differences in trip distance and duration between time classes (fixed effect), we additionally used tide as random effect. Accordingly, to test for differences in trip distance and duration between different tidal stages (fixed effect), we used time class as a random effect. A second model keeping only the random effects was computed for comparison to test for the significance of the fixed effects. As the model output of GLMMs does not provide AIC, we applied an analysis of variance (e.g., Zar 1999) comparing both models using the explained variance as response variable. This statistical approach for comparing random versus fixed effects was suggested by Faraway (2006). The data were left-skewed, and we therefore considered a quasi-poisson distribution to be appropriate. All tests were conducted using the R 2.8.1 open-source software package (R Development Core Team 2007) using the library lme4 (Bates and Maechler 2009).
Results
Spatial patterns and foraging site fidelity
A total of 61 foraging trips were recorded from the eight oystercatchers. Individual #8 recorded the most foraging trips (n = 12), while individual #18 showed the fewest (n = 4), which was largely because of the short log interval, and consequently short operating period of its device (Table 1). There was high variability in both trip duration and trip distance both between and within individuals (Table 2). Several individuals regularly performed trips of between 70 and 150 m (e.g., #7 and #8), whereas most other birds commuted to foraging areas several kilometres from the nest. The longest trip was performed by individual #13, which flew to a freshwater pond on the mainland at a distance of 5.7 km from the nest, during daytime flood tide (Fig. 1b; Table 2). This was the only example of a trip to a foraging area other than in the intertidal zone. The minimum distance travelled from the nest was 70 m, by individual #8 (Table 2). The mean overall distance from the nest to the foraging site was 1.1 km (SD: ±1.1 km).
Individuals exhibited differences in foraging site fidelity (i.e., their return rate to known patches differed; Table 3). Home range analyses revealed relatively high site fidelities in five of the eight sampled individuals (#1, #5, #7, #8, #10), while three individuals (#13, #15, #18) used wider foraging areas (Table 3; Fig. 1 for examples). The five foraging trips performed by individual #5 were to almost the same foraging spot in the north of the island (Fig. 1c). In contrast, individual #18 used multiple sites in the north of the island and also commuted to the south of the island on one of its four recorded trips (Fig. 1a). Five of the nine trips recorded by individual #13 led to the same foraging area in the south, while the other four trips went to the east and west of the island (Fig. 1b). With the exception of individual #8, which constantly performed very short trips, all other individuals visited multiple sites at least once or twice (Fig. 1d). Many foraging trips were to areas located close to the two tidal creek systems in the north and south of Oland.
Influence of time of day and tide
Oystercatchers spent most of their time in their breeding territories (mean for the eight individuals: 81.7 ± 7.4%) and only a small time on foraging trips (mean: 18.3 ± 7.4%). Foraging trips lasted between 143 and 8 min (Table 2). The overall mean foraging trip duration was 37 min (±32 min). There was a significant positive correlation between distance travelled from the nest to the foraging site and the trip length (min) (Fig. 2; GLM: t = 3.0; P < 0.01; R 2 = 0.14). There were also high variabilities in both trip length and distance travelled for most oystercatchers (different symbols in Fig. 2), indicating that the same individuals used different places and stayed at these for different periods of time.
Some of this variability could be explained by the predictors time class and tide (Fig. 3): For trip length, the model was significantly improved by adding time class as a fixed effect versus a model using only the random effects (individual and tide) (ANOVA: χ2 = 38.8; P < 0.001). Individuals performed significantly longer trips during the night than during the day (Fig. 3a). Accordingly, the model for trip distance was significantly improved by adding time class (ANOVA: χ2 = 392.3; P < 0.001). Trip distances were significantly higher during the night than during the day, although this relationship is unclear in Fig. 3b, most likely because of the strong random effects of the individuals.
The model for trip length was not significantly improved by adding tidal stage (ANOVA: χ2 = 0.3; P = 0.53; Fig. 3a), while that for trip distance was significantly improved (ANOVA: χ2 = 311.1; P < 0.001). Individuals performed their longest trips during low and flood tide (Fig. 3b).
Discussion
Device effects
It is not possible to completely rule out an influence of the devices on the temporal and spatial patterns observed in the equipped birds. There was no opportunity to compare the distances and durations of trips between equipped and unequipped birds, but we did carry out detailed visual observations of the behaviour of five of the sampled birds within their breeding territory and (if close enough) during their foraging on the mudflats. We found no indication that relative time spent incubating (t = −0.99), preening (t = −0.17), sleeping (t = 0.08), or absent from the nest (t = 0.4) differed between five equipped and five unequipped individuals (P > 0.5 and df = 9 in all cases). After deployment of the devices each individual returned to their breeding territory within a few minutes following release. To avoid cumulative effects, the deployment period was restricted to a maximum of 2–3 days. Furthermore, the data loggers were programmed with a delayed start of 1–12 h to reduce the possible effects of catching and handling on the recorded data. It is not known if this time period was sufficient to allow for habituation to the device. Using radio tags on oystercatchers in the German Wadden Sea, Exo et al. (1996) suggested a period of 1–3 days for habituation. No losses of eggs of equipped birds were noted during the study period (except for one oystercatcher that could not be re-captured because it lost its clutch). The fledging success of all the other equipped individuals appeared comparable to that of unequipped breeding pairs, although this was not measured quantitatively. There was no indication of any long-term effects, and five out of the eight individuals that had been equipped with GPS data loggers during this study were re-sighted or re-captured the following year while incubating on Oland in the same breeding territories (we were only able to check about 15% of the total breeding population on Oland for re-sightings). The fledging success in the following year was similar to that of unequipped individuals.
Spatial patterns
The mean foraging trip distance for oystercatchers nesting on Oland was 1.1 km, which was more than twice the distance recorded for individuals on Schiermonikoog (Ens et al. 1992). The tidal flats around Oland (as in many parts of the German Wadden Sea) are vast, and oystercatchers seem to utilize the whole range of the flats up to the tidal creek systems that border them. Moreover, our data showed that many individuals preferred foraging at the edges of tidal creeks, i.e., in low-lying areas with a low exposure time. The long submerged period means that the quality of benthic prey such as cockles (Cardium edule) is highest in these areas (e.g., Sutherland 1982a; Goss-Custard et al. 1991).
Several individuals in the current study showed a moderate degree of foraging site fidelity (i.e., between 0.01 and 0.54 km² used at 95% encounter probability; Table 3), though at least three of the eight individuals showed low fidelity (i.e., between 5.80 and 12.78 km² at 95% encounter probability; Table 3). Thus, specific foraging territories on the tidal flats could not be identified for all individuals, contradicting our assumption. There are several possible explanations for the moderate site fidelity. (1) Foraging territories might not have been finally established prior to fledging. Foraging areas may change in relation to breeding stage, as documented for other shorebird species (e.g., American avocets, Recurvirostra americana; Demers et al. 2008). (2) Only birds breeding at the edge of the island with direct access to tidal habitats (“residents”; see Ens et al. 1992) developed foraging territories, whereas the others varied their habitat use. This was demonstrated by individuals #7 and #8, which were the only ones with direct access to tidal flats and also showed the highest site fidelity. (3) Although previous studies suggested that site choice did not usually differ between day and night (Sitters 2000), our data showed that oystercatchers often used sites further away from the nest at night, a fact that would decrease the apparent site fidelity. (4) Variation in habitat use might have been caused by insufficient availability and/or quality of prey, thus forcing the oystercatchers to visit various sites to locate a rich site. The last hypotheses may provide further indications for the population declines, but this issue cannot be addressed on the basis of the data collected within the scope of the current study. Further studies using GPS techniques are needed to collect additional data on prey availability and site quality for sites used by oystercatchers, to reveal any implications at the population level.
However, more data are needed to confirm these interpretations, as the sample sizes and recording periods in the current study were too low to allow final conclusions to be drawn. Despite the fact that a total of 61 foraging trips were studied and GLMM was used to correct for individual effects, the relatively small sample size might still have contributed to the high variances in trip distances and durations within and among individuals. Moreover, the data may not be sufficiently temporally independent, and further data collected over longer time periods are needed to confirm the patterns found in this study. However, relatively small sample sizes may still hamper logger or transmitter studies, as (1) devices are often expensive, and (2) often malfunction (though this was not the case in the present study), and (3) it is often not possible to retrieve the data either because the device is lost or because the bird cannot be recaptured (which was the case with only one individual in this study). Consequently, sample sizes of around 10 or fewer are still common in studies employing electronic devices to study habitat use (compare e.g., Garthe et al. 2007b; Hedd et al. 2009; Harris et al. 2010; Meyer et al. 2010).
Temporal patterns
Nocturnal foraging is common in shorebirds (e.g., McNeil et al. 1993; Thomas et al. 2006; Kuwae 2007). This is true for oystercatchers in tidally structured sites (e.g., Hulscher 1974, 1976, 1982; Sutherland 1982b; Exo et al. 1996; Kersten and Visser 1996; Zwarts et al. 1996c; Sitters 2000) and contrasts with the situation in individuals that breed inland, which do not forage at night (Wolf 1998).
In accordance with the studies mentioned earlier, the results of the current study indicated extensive foraging during the night; moreover, oystercatchers made trips to more distant sites during the night than during the day, and stayed longer in the foraging areas (Figs. 2, 3). This could be because oystercatchers had difficulty in finding sufficient energy at night. However, food intake by oystercatchers during the night is not necessarily lower compared to daytime (see review in Hulscher 1996).
Predation risk obviously is a crucial factor for the temporal patterns of habitat use found in our study: No clutch losses caused by nocturnal mammals have been found on the island of Oland (Lutz 2008), whereas numbers of gulls, i.e., herring (Larus argentatus), black-headed (L. ridibundus) and common gull (L. canus) have increased dramatically both on the island and throughout the whole Wadden Sea area (Garthe et al. 2000; Koffijberg et al. 2006). All the recorded clutch predation events on Oland have so far occurred during the daytime (Lutz 2008), suggesting that gulls or birds of prey are the most likely predators. Shorter foraging trips by oystercatchers during the day may therefore be a response to the risks of egg and chick predation posed by diurnal gulls. This possibility is emphasized by several observations of gulls and less frequently birds of prey, such as marsh harrier (Circus aeruginosus) and short-eared owl (Asio flammeus) attacking oystercatchers during the fieldwork period (own observations). Oystercatchers might counter this risk by being present in the breeding territory for longer during the day. This could be the crucial reason why the maximum distance travelled from the nest was significantly lower during the day than at night (Figs. 2, 3). Predation risk has already been revealed as an important factor contributing to differences between diurnal and nocturnal foraging behaviours in various bird species (e.g., Kalejta 1992; Wolf 1998; Beauchamp 2008; Merkel and Mosbech 2008). As mentioned earlier, larger data sets, ideally at additional sites, are need to confirm these findings and interpretations.
Tidal effects
The tidal cycle significantly affected both distance travelled from the nest and trip duration: both parameters were highest during low tide and showed moderate values during ebb and flood tides. This supports the hypothesis that oystercatchers forage at the line of the retreating/incoming water. As for time of day, the tidal cycle is also likely to have a major impact on prey availability. De Vlas et al. (1996) found that oystercatchers took more ragworms (Nereis diversicolor) at low tide, but more Macoma balthica during the early and late stages of the tidal cycle, shortly after exposure of the flats. This suggests that oystercatchers should preferentially search for certain prey species in the vicinity of the water line, supporting our findings.
Only four foraging trips were recorded during high tide, indicating that oystercatchers were able to fulfil their energetic demands on exposed tidal flats (for activity budgets in relation to tidal cycle, also see Exo 1995). However, one individual performed one long foraging trip to the mainland coast, where it foraged at the edge of a freshwater pond. This indicates that foraging might also be necessary at high tide in some individuals. Again, more data are needed to address this question properly.
Oystercatchers spent most of their time (mean: 81.7%) within their breeding territory. Visual observations of individuals equipped with GPS data loggers indicated that they did not search for terrestrial prey in their territories, but spent their time exclusively preening, resting, and incubating, or defending their clutch against potential predators and conspecifics. Data on the body weights of different individuals on Schiermonnikoog (Kersten and Visser 1996) suggest that energy is conserved during the incubation period, rather than expended. However, no data are available to confirm this in oystercatchers breeding on Oland. Thus, it remains unknown if oystercatchers spend most of their time within their breeding territory because they only need a small amount of time to fulfil their energetic demands, or if they accept a net weight loss during incubation for the sake of defending their clutches against potential predators.
Conclusions and outlook
The results of the current study suggest that oystercatchers on Oland exhibited moderately strong site fidelity and performed longer foraging trips at night than during the day. Oystercatchers apparently timed their foraging flights to allow them to spend more time within their breeding territory and less time foraging, particularly during the crucial daytime hours when clutches were susceptible to predation by diurnal avian predators. The patterns identified in this study need to be validated by further analyses of data from studies with larger sample sizes and from different regions. Moreover, it is important to consider that oystercatchers are large-bodied, long-lived birds. Their complex foraging ecology therefore needs to take account of life-long considerations, such as the gathering of information throughout the season and over consecutive years, as well as predation pressures at foraging sites. Risks of predation at the foraging site did not appear to be an issue on Oland, where no predators of adult individuals were observed.
GPS data logger technology advances quickly (Ens et al. 2008). In the near future, more powerful batteries will allow loggers to collect data continuously for longer periods, and device masses will be significantly reduced (e.g., Wikelski et al. 2007). This will enable a higher density of data points to be recorded, so improving the findings of the present study. Moreover, resource selection analyses by sampling the abundance and quality of potential prey items at foraging sites identified by GPS will allow further insights into the reasons for habitat choice and may thus provide explanations for the negative population trend in oystercatchers in the eastern Wadden Sea. Predation of benthic species by oystercatchers has already been studied elsewhere by visual observations (e.g., Sutherland 1982b). However, visual observations alone will not be sufficient to shed light on the habitat choice of oystercatchers and their possible relationships to food supply on the vast tidal flats of the German Wadden Sea, and so direct observations in the breeding habitats, in addition to the use of GPS data loggers, will be useful for evaluating the role and intensity of predation pressure on nests and chicks at the breeding grounds.
References
Bates D, Maechler M (2009) lme4: linear mixed-effects models using S4 classes. http://cran.r-project.org/web/packages/lme4/index.html
Beauchamp G (2008) Risk factors for predation attempts by peregrine falcons (Falco peregrinus) on staging semipalmated sandpipers (Calidris pusilla). Waterbirds 31:651–655
Blew J, Günther K, Laursen K, van Roomen M, Südbeck P, Eskildsen K, Potel P (2007) Trends of waterbird populations in the International Wadden Sea 1987-2004: an update. In: Reineking P, Südbeck P (eds) Seriously declining trends in migratory waterbirds: Causes concerns consequences. Proceedings of the international workshop on 31 August 2006 in Wilhelmshaven, Germany. Wadden Sea Ecosystem No. 23, pp 9–31
CWSS (2010) http://www.waddensea-secretariat.org/TMAP/Migratory_birds.html. Common Wadden Sea Secretariat, last accessed 20 March 2010
de Vlas SJ, Bunskoeke AEJ, Ens B, Hulscher JB (1996) Tidal changes in the choice of Nereis diversicolor or Macoma balthica as main prey species in the diet of the oystercatcher Haematopus ostralegus. Ardea 84A:105–116
Demers SA, Colwell MA, Takekawa JY, Ackerman JT (2008) Breeding stage influences space use of female American Avocets in San Francisco Bay, California. Waterbirds 31:365–371
Ens BJ, Kersten M, Brenninkmeijer A, Hulscher JB (1992) Territory quality, parental effort and reproductive success of oystercatchers (Haematopus ostralegus). J Appl Ecol 61:703–715
Ens BJ, Baaij EW, Bairlein F, Bouten W, de Boer P, Exo K-M, Klaasen RHG, Oosterbeek H, Shamoun-Baranes J, van der Jeugd H, van Gasteren H (2008) State of the art in the study of bird movement using GPS-transmitters. Wader Study Group Bull 115:197
Esser W, Vöge S, Exo K-M (2008) Day-night activity of intertidal invertebrates and methods to estimate prey accessibility for shorebirds. Senckenb Marit 38:115–122
Exo K-M (1992) Methoden zur Aufnahme von Raum-Zeit-Budgets bei Vögeln, dargestellt am Beispiel des Austernfischers (Haematopus ostralegus). Vogelwarte 36:311–325
Exo K-M (1995) Aktivitäts- und Verhaltensmuster von Wattenmeer- und Binnenlandsbrütern des Austernfischers (Haematopus ostralegus). Jber Institut Vogelforschung 2:10–11
Exo K-M, Scheiffarth G, Haesihus U (1996) The application of motion-sensitive transmitters to record activity and foraging patterns of oystercatchers Haematopus ostralegus. Ardea 84A:29–38
Faraway JJ (2006) Extending the linear model with R. Generalized linear, mixed effects and nonparametric regression models. Chapman & Hall, Boca Raton
Garthe S, Flore BO, Hälterlein B, Hüppop O, Kubetzki U, Südbeck P (2000) Brutbestandsentwicklung der Möwen (Laridae) an der deutschen Nordseeküste in der zweiten Hälfte des 20. Jahrhunderts. Vogelwelt 121:1–13
Garthe S, Montevecchi WA, Chapdelaine G, Rail J-F, Hedd A (2007a) Contrasting foraging tactics by northern gannets (Sula bassana) breeding in different oceanographic domains with different prey fields. Mar Biol 151:687–694
Garthe S, Montevecchi WA, Davoren GK (2007b) Flight destinations and foraging behaviour of northern gannets (Sula bassana) preying on a small forage fish in a low-Arctic ecosystem. Deep-Sea Res II 54:311–320
Girard C, Tucker AD, Calmettes B (2009) Post-nesting migrations of loggerhead sea turtles in the Gulf of Mexico: dispersal in highly dynamic conditions. Mar Biol 156:1827–1839
Goss-Custard JD, Warwick RM, Kirby R, McGrorty S, Clarke RT, Pearson B, Rispin WE, Durell dit le V, Rose RJ (1991) Towards predicting wading bird densities from predicted prey densities in a post-barrage Severn Estuary. J Appl Ecol 28:1004–1026
Grémillet D, Dell’Omo G, Ryan PG, Peters G, Ropert-Coudert Y, Weeks SJ (2004) Offshore diplomacy, or how seabirds mitigate intra-specific competition: a case study based on GPS tracking of cape gannets from neighbouring colonies. Mar Ecol Prog Ser 268:265–279
Hamer KC, Phillips RA, Wanless S, Harris MP, Wood AG (2000) Foraging ranges, diets and feeding locations of gannets Morus bassanus in the North Sea: evidence from satellite telemetry. Mar Ecol Prog Ser 200:257–264
Harris MP, Daunt F, Newell M, Phillips RA, Wanless S (2010) Wintering areas of adult Atlantic puffins Fratercula arctica from a North Sea colony as revealed by geolocation technology. Mar Biol 157:827–836
Hedd A, Regular AE, Montevecchi WA, Buren AD, Burke CM, Fifield DA (2009) Going deep: common murres dive into frigid water for aggregated, persistent and slow-moving capelin. Mar Biol 156:741–751
Hulscher JB (1974) An experimental study of the food intake of the oystercatcher Haematopus ostralegus L. in captivity during the summer. Ardea 62:156–171
Hulscher JB (1976) Localization of cockles (Cardium edule L.) by the oystercatcher (Haematopus ostralegus L.) in darkness and daylight. Ardea 64:292–310
Hulscher JB (1982) The oystercatcher Haematopus ostralegus as a predator of the bivalve Macoma balthica in the Dutch Wadden Sea. Ardea 70:89–152
Hulscher JB (1996) Food and feeding behaviour. In: Goss-Custard JD (ed) The oystercatcher. From individuals to populations. University Press, Oxford
Kalejta B (1992) Time budgets and predatory impact of waders at the Berg River estuary, South Africa. Ardea 80:327–342
Kenow KP, Meyer MW, Evers DC, Douglas DC, Hines J (2002) Use of satellite telemetry to identify Common Loon migration routes, staging areas and wintering range. Waterbirds 25:449–458
Kersten M (1996) Time and energy budgets of oystercatchers Haematopus ostralegus occupying territories of different quality. Ardea 84A:291–310
Kersten M, Visser W (1996) Food intake of oystercatchers Haematopus ostralegus by day and by night measured with an electronic nest balance. Ardea 84A:57–72
Koffijberg K, Dijksen L, Hälterlein B, Laursen K, Potel P, Südbeck P (2006) Breeding birds in the Wadden Sea in 2001. Results of the total survey in 2001 and trends in numbers between 1991–2001. Wadden Sea Ecosystem Rep. No. 22, Wilhelmshaven, Germany
Kuwae T (2007) Diurnal and nocturnal feeding rate in kentish plovers (Charadrius alexandrinus) on an intertidal flat as recorded by telescopic video systems. Mar Biol 151:663–673
Low M, Eggers S, Arlt D, Pärt T (2008) Daily patterns of nest visits are correlated with ambient temperature in the northern wheatear. J Ornithol 149:515–519
Lutz K (2008) Brutvogeluntersuchungen auf der Hallig Oland 2008. Untersuchungen im Auftrage des Landesbetriebes für Küstenschutz, Nationalpark und Meeresschutz, Husum. Unpublished Report
McNeil R, Drapeau P, Pierotti R (1993) Nocturnality in colonial waterbirds: occurrence, special adaptations, and suspected benefits. In: Power DM (ed) Current ornithology. Volume 10. Plenum Press, New York, pp 187–246
Merkel FR, Mosbech A (2008) Diurnal and nocturnal feeding strategies in Common Eiders. Waterbirds 31:580–586
Meyer DG, Papastamatiou YP, Holland KN (2010) A multiple instrument approach to quantifying the movement patterns and habitat use of tiger (Galeocerdo cuvier) and Galapagos sharks (Carcharhinus galapagensis) at French Frigate Shoals, Hawaii. Mar Biol 157:1857–1868
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Piersma T, Koolhaas A, Dekinga A, Beukema JJ, Dekker R, Essink K (2001) Long-term indirect effects of mechanical cockle-dredging on intertidal bivalve stocks in the Wadden Sea. J Appl Ecol 38:976–990
Pinheiro JC, Bates DM (2009) Mixed-effects models in S and S-PLUS. Springer, Berlin
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available at http://www.R-project.org (last accessed 22 March 2010)
Scheiffarth G, Frank D (2005) Shellfish-eating birds in the Wadden Sea—what can we learn from current monitoring programs? In: Blew J, Südbeck P (eds). Migratory Waterbirds in the Wadden Sea 1980–2000. Wadden Sea Ecosystem No. 20, pp 187–200
Sitters H (2000) The role of night-feeding in shorebirds in an estuarine environment with specific reference to mussel-feeding oystercatchers. Dissertation, University of Oxford
Stevens JD, Bradford RW, West GJ (2010) Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: depth behaviour, temperature experience and movements. Mar Biol 157:575–591
Sutherland WJ (1982a) Spatial variation in the predation of cockles by oystercatchers at Traeth Melynog, Anglesey. II. The pattern of mortality. J Anim Ecol 51:491–500
Sutherland WJ (1982b) Do oystercatchers select the most profitable cockles? Anim Behav 30:857–861
Thomas RJ, Székely T, Powell RF, Cluthill IC (2006) Eye size, foraging methods and the timing of foraging in shorebirds. Funct Ecol 20:157–165
van de Pol M, Oosterbeek K, Rutten AL, Ens BJ, Tinbergen JM, Verhulst S (2009) Biometric sex discrimination is unreliable when sexual dimorphism varies within and between years: an example in Eurasian Oystercatchers Haematopus ostralegus. Ibis 151:171–180
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
Verhulst S, Oosterbeek K, Rutten AL, Ens B (2004) Shellfish fishery severely reduces condition and survival of oystercatchers despite creation of large marine protected areas. Ecol Soc 9:17
Weimerskirch H, Bonadonna F, Bailleul F, Mabille G, Dell’Omo G, Lipp HP (2002) GPS tracking of foraging albatrosses. Science 295:1259
Wikelski M, Kays RW, Kasdin NJ, Thorup K, Smith JA, Swenson GW Jr (2007) Going wild: what a global small-animal tracking system could do for experimental biologists. J Exp Biol 210:181–186
Wilson RP, Pütz K, Peters G, Culik B, Scolaro JA, Charrassin J-B, Ropert-Coudert Y (1997) Long-term attachment of transmitting and recording devices to penguins and other seabirds. Wildl Soc Bull 25:101–106
Wolf C (1998) Ethoökologische Untersuchungen am Austernfischer (Haematopus ostralegus). Chancen und Risiken für einen Küstenvogel im Binnenland. Dissertation, University of Cologne
Wood AG, Naef-Daenzer B, Prince PA, Croxall JP (2000) Quantifying habitat use in satellite-tracked pelagic seabirds: application of kernel estimation to albatross locations. J Avian Biol 31:278–286
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Zwarts L, Ens BJ, Goss-Custard JD, Hulscher JB, Kersten M (1996a) Why oystercatchers Haematopus ostralegus cannot meet their daily energy requirements in a single low water period. Ardea 84A:269–290
Zwarts L, Hulscher JB, Koopman K, Zegers PM (1996b) Discriminating the sex of oystercatchers Haematopus ostralegus. Ardea 84A:1–12
Zwarts L, Hulscher JB, Koopman K, Zegers PM (1996c) Short-term variation in the body weight of oystercatchers Haematopus ostralegus: effect of available feeding time by day and night, temperature and wind force. Ardea 84A:357–372
Acknowledgments
We greatly thank Angelika and Frank Kühn for access to the oystercatcher breeding sites and for extensive help with field work. H. Seibel and H. Dries helped with bird handling and recapture. G. Peters from Earth & Ocean Technologies helped to solve problems with the GPS data loggers. S. Adler provided statistical advice. We are thankful to M. Exo for commenting on an earlier version of the manuscript. All parts of the study complied with current German laws. All necessary permissions regarding animal welfare were issued prior to the study. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. E. Hauber.
Rights and permissions
About this article
Cite this article
Schwemmer, P., Garthe, S. Spatial and temporal patterns of habitat use by Eurasian oystercatchers (Haematopus ostralegus) in the eastern Wadden Sea revealed using GPS data loggers. Mar Biol 158, 541–550 (2011). https://doi.org/10.1007/s00227-010-1579-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1579-1