Abstract
Owing to the necessity of delivering food to offspring at colonies, breeding seabirds are highly constrained in their foraging options. To minimize constraints imposed by central-place foraging and to optimize foraging behavior, many species exhibit flexible foraging tactics. Here we document the behavioral flexibility of pursuit-diving common murres Uria aalge when foraging on female capelin Mallotus villosus in the northwest Atlantic. Quite unexpectedly, being visual foragers, we found that common murres dived throughout the day and night. Twenty-one percent of recorded dives (n = 272 of 1,308 dives) were deep (≥50 m; maximum depth = 152 m, maximum duration = 212 s), bringing murres into sub-0°C water in the Cold Intermediate Layer (CIL; 40–180 m) of the Labrador Current. Deep dives occurred almost exclusively during the day when murres would have encountered spatially predictable aggregations of capelin between 100 and 150 m in the water column. Temperatures within the CIL shaped trophic interactions and involved trade-offs for both predators and prey. Sub-0°C temperatures limit a fish’s ability to escape from endothermic predators by reducing burst/escape speeds and also lengthening the time needed to recover from burst-type activity. Thus, while deep diving may be energetically costly, it likely increases certainty of prey capture. Decreased murre foraging efficiency at night (indicated by an increase in the number of dives per bout) reflects both lower light conditions and changing prey behavior, as capelin migrate to warmer surface waters at night where their potential to escape from avian predators could increase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biophysical variability in dynamic marine environments affects the performance, behavioral ecology and distribution of both predators and prey (Domenici et al. 2007). During breeding, the demands of central-place foraging greatly restrict the foraging options and provisioning opportunities of seabirds (Orians and Pearson 1979), often challenging their behavioral and physiological capabilities (Weimerskirch et al. 2003; Jodice et al. 2006; Elliott et al. 2008a). Seabirds may consequently employ flexible foraging tactics in an effort to minimize constraints and effectively cope with breeding demands.
Seabird species that fly and dive are compromised in both forms of locomotion (Burger 1991). Pursuit-diving alcids have taken these adaptations to extremes and exhibit considerable performance capability and agility—“flying” underwater to capture prey (Tremblay et al. 2003). Common murres Uria aalge, the deepest diving species among birds that fly (Piatt and Nettleship 1985; Burger 1991), have been shown to make major adjustments in foraging behavior and effort in response to fluctuating prey conditions during chick-rearing (Burger and Piatt 1990; Davoren et al. 2003a; Harding et al. 2007).
Off Newfoundland, in the northwest Atlantic, breeding common murres specialize on capelin Mallotus villosus, preferentially feeding themselves and their chicks on mature females (Piatt 1990; Davoren and Montevecchi 2003a; Burke and Montevecchi 2008). During spring and early summer, capelin migrate from over-wintering areas near the edge of the continental shelf to the coastal waters of Newfoundland to spawn (Nakashima 1992). Suitable habitat for demersal spawning and staging along the northeast coast of Newfoundland creates persistent aggregations of capelin (Davoren et al. 2006) which, in turn, provide predictable foraging areas for the massive concentration of murres breeding at the nearby Funk Island Seabird Ecological Reserve (Davoren and Montevecchi 2003a, Davoren and Montevecchi 2003b). Spatially persistent demersal spawning sites (<38 m) and staging areas (100–150 m) for capelin have been documented between Funk Island and the northeast coast of Newfoundland (see Davoren et al. 2006 for locations).
Prior to spawning, mixed-sex shoals of maturing capelin stage deep in the water column, remaining within or below the Cold Intermediate Layer (CIL; <0°C) of the Labrador Current during the day and migrating to warmer (~9–13°C) surface waters at night (Davoren et al. 2006). The CIL is a band of sub-0°C water (approximately 50–240 m) that is a prominent oceanographic feature of the Newfoundland Shelf area through much of the year (Petrie et al. 1988, Davoren et al. 2006). When capelin mature, they move inshore to spawn on beaches and at demersal sites located near the coast (Davoren et al. 2006). Capelin that survive spawning may return to deep water staging areas to forage and replenish fat reserves before the onset of winter (Winters 1970).
In this paper, we examine diurnal patterning and physiological efficiency of diving by parental common murres, and focus on deep (≥50 m) dives for capelin located within the CIL. Because the probability of an endothermic avian predator capturing ectothermic prey is at least partially mediated by temperature effects on the fish’s ability to perform and recover from burst-type exercise (Kieffer 2000; Cairns et al. 2008), we hypothesize that capelin located within the CIL will be slow-moving and relatively easy for murres to catch. On this premise, if murres are successful at capturing prey during a diving bout (Camphuysen 2005), we predict that they will perform fewer dives per bout as the average depth of dives within the bout increases. Also, if the time spent foraging within a patch (i.e., dive bottom time) relates to pursuit time for prey, we predict that dive bottom times will be shorter in colder waters. Lastly, as the foraging efficiency of penguins and cormorants has been shown to vary with light conditions (Wilson et al. 1993, Wanless et al. 1999), we predict that murres will perform more dives per bout as light availability declines. We consider how the physical environment has shaped interactions between murres and capelin within the Newfoundland Shelf ecosystem.
Materials and methods
Study sites
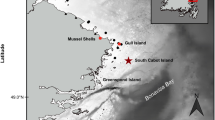
Research was conducted between 14 July and 6 August 2007 at Gull Island (47°16′N, 52°46′W; ~1,632 breeding pairs of common murres; Robertson et al. 2004), Witless Bay, and between 25 July and 4 August 2007 at Funk Island (49°45′N, 53°11′W, ~400,000 pairs, Chardine et al. 2003), Newfoundland, Canada, at a time when most murres were rearing chicks (Fig. 1).
Oceanographic setting
Temperature data collected at hydrographic Station 27 (Fig. 1; 47°31′50″N, 52°35′10″W; Fisheries and Oceans Canada Oceanographic database, https://doi.org/www.mar.dfo-mpo.gc.ca/science/ocean/database/data_query.html) on 4 days between 13 and 31 July 2007 were used to describe the thermal profile of the water column (5–165 m), and to delineate the position of the CIL. Temperatures were available at 10 m intervals between the surface and 50 m, then subsequently at 75, 100, 150, and 165 m. Station 27 is located within the Avalon Channel branch of the Labrador Current and provides a robust index of oceanographic conditions over the Newfoundland and Labrador Shelf (Petrie et al. 1988; Drinkwater 1996).
Field protocol: devices and procedures
Adults attending chicks (n = 6 at Gull Island from 14 to 24 July, and n = 15 at Funk Island from 25–30 July) were caught with a 6 m telescoping noose pole and equipped with a Lotek LTD 1110 logger (5 g, 32 × 11 mm; 128 Kb memory) on the left leg and a Canadian Wildlife Service metal band on the right leg. Loggers were secured to plastic leg bands (Pro-Touch Engraving, Saskatoon, Saskatchewan) using cable ties. These loggers recorded pressure (depth resolution ± 0.49 m when maximum depth < 125 m, and ±0.98 m when maximum depth 125–250 m) and temperature (±0.3°C) every 2 s for 36 consecutive hour until their memory filled. Devices remained on the birds for the full 36 h, capturing information across successive foraging trips for each bird. Upon recapture, loggers were removed and murres were weighed with a Pesola® 1 or 1.5 kg spring balance, and 0.5 ml of blood was collected from the brachial vein to determine sex using W-chromosome analysis (Fridolfsson and Ellegren 1999). Birds were held with eyes covered for ~4 and ~6 min during logger deployment and recapture, respectively. Nine of the 15 loggers were recovered from murres on Funk Island as were four of the six deployed on Gull Island (60% overall recovery rate). Of the loggers recovered, data were lost from one (Funk Island) and data from two others (Gull Island) were distorted and unrecoverable.
Throughout logger deployments, dawn to dusk observations (05:00–21:00 h Newfoundland Daylight Savings time) were conducted at Gull Island, and equipped birds were observed at Funk Island when work time allowed. Arrival and departure times and prey items delivered to chicks were noted for equipped birds. When observational data were not available, colony arrival and departure times were estimated using continuous temperature and depth records obtained from the data loggers (Tremblay et al. 2003; Elliott et al. 2008a).
At Funk Island, food samples were collected throughout the period of logger deployment using dip-nets on long poles to catch prey-carrying murres as they returned to the colony. Fish were weighed (using an 100 g Pesola® scale), measured (total length ± 1 mm using a ruler), identified, sexed and classed as gravid or spent (which may have included a few immature fish).
Data analysis
As drift in the LTD 0-level exceeded ±1 m in some cases, we defined dives as submersions ≥2 m. Start and end times for each dive were determined, along with the following descriptive parameters: dive duration, maximum depth, minimum temperature, dive bottom time, surface interval duration, and dive and bottom efficiencies. Dive bottom time (generally assumed to represent the time available for foraging) was defined as the time elapsing from the first and last instant when vertical velocity (calculated between successive records) fell below 0.5 ms−1 (e.g., Halsey et al. 2007). Dive efficiency was defined as the ratio of the duration of the dive to the duration of the dive + the subsequent surface time, and bottom efficiency was the ratio of bottom time to the dive + the subsequent surface time (Camphuysen 2005). V-shaped dives (n = 165, 12.6%) had no bottom time, and hence had a bottom efficiency of zero. Bouts of diving were identified using a surface interval criterion (e.g., Huin and Prince 1997). A frequency distribution of post-dive surface intervals showed a rapid decline to 220 s after which they remained relatively constant. A bout of diving was therefore defined as a single dive or as a series of dives in which each dive started ≤ 220 s after the previous dive had ended.
Dives were classified as either “shallow” (<50 m) or “deep” (≥50 m). We chose 50 m as the criterion to define a deep dive, as this depth ensured that birds had reached, and were presumably foraging within the CIL (see “Results”). We plotted frequency distributions of the time of diving (by hour), examining both overall patterns and those for deep dives separately. We used Chi-square tests for independence to determine whether frequency distributions of diving depth and duration were dependent on time of day (day vs. night). Daytime commenced with nautical twilight in the morning (the instant when the rising sun is 12° below the horizon) and continued until nautical twilight in the evening (the instant when the setting sun is 12° below the horizon); nighttime was the intervening period (RASC 2007).
We also assessed whether dive depth varied as a function of time of day by fitting a generalized linear model with a gamma error distribution and inverse links using SAS 9.1 PROC GENMOD (SAS Institute 2005). Data are presented as mean ± SE, and an alpha level (α) of 0.05 was used to assess statistical significance.
Results
Oceanographic setting and parental prey deliveries
During July 2007, the CIL or band of sub-0°C water across the Newfoundland and Labrador Shelf extended from 40 m to below 165 m (Fig. 2). Hence when below 40 m in the water column, murres and capelin were in <0°C water. Temperature profiles collected by the dive loggers confirmed that murres reached the CIL on all deep dives.
Coincident with the diving records, parental prey deliveries (n = 86) by unequipped murres at Funk Island consisted of 100% female capelin; 23% were gravid, 65% were spent, 2% were immature and for the remaining 10% the maturity status was unknown. Concurrent hydro-acoustic surveys detected no capelin at known demersal spawning sites (18–38 m water) within the murre’s foraging range from Funk Island when murre diets were collected at the colony (G. K. Davoren and P. Penton, unpublished data).
Diving performance
Diving profiles were obtained for nine individuals (n = 7 from Funk Island, n = 2 from Gull Island) during 20 (plus 5 partial) foraging trips, involving a total of 1,308 dives (Table 1). Birds dived throughout the day and night, with a peak in activity near dusk (~21:00–22:00 h; Fig. 3a). Overall, mean ± SE (and maximum) dive depth and duration were 30 ± 0.8 m (152 m) and 64 ± 1.3 s (212 s), respectively. The distribution of both dive depth (χ 27 = 82.3, P < 0.01) and duration (χ 210 = 287.6, P < 0.01) was dependent on time of day (Fig. 4), with long and deep dives occurring almost exclusively during daylight.
By our definition, 21% (n = 272) of all dives were deep (≥50 m) and brought murres into the CIL. All murres performed deep dives, and these comprised 9–68% of the dives performed by each individual (Table 1). Most (97%) deep diving occurred during the day, largely from mid-afternoon to dusk, with a smaller peak in early morning (Fig. 3b). Mean dive depth varied with time of day (χ 223 = 122.04, P < 0.0001), being significantly deeper in the 2 h after sunrise (05:00–06:59 h) than through the remainder of the day (contrast test: χ 21 = 29.15, P < 0.0001, Fig. 5). Murres dived frequently at night, but almost exclusively to depths less than 50 m (Figs. 3a, 5; Table 1). Although the number of individuals studied was small, the data suggested a colony difference in the time of diving, with only Funk Island birds diving at night (Table 1).
Dive efficiency was low for the shallowest dives (2–9 m), and highest for dives between 10 and 29 m (Fig. 6a). Efficiency declined for dives below 30 m and remained relatively constant for deeper depths. Bottom efficiency was also highest for dives between 10 and 29 m, declined progressively between 30 and 79 m, and was extremely low for dives deeper than 80 m (Fig. 6b).
Individual murres performed, on average, 5.0 ± 1.3 dives per bout (range of individual means = 2.5–12.2; Table 1), and as anticipated, they undertook fewer dives per bout as the average depth of dives within the bout increased (Fig. 7). Bouts that consisted of just 1 or 2 dives had average maximum depths ≥50 m (Fig. 7). The mean number of dives per bout was also significantly greater during dark conditions at night than during the day (independent t-test = −2.54, df = 231, P < 0.05). There was no clear relationship between dive depth and time spent at the bottom of the dive, though the longest bottom times corresponded with the deepest dives. Contrary to our expectations, dive bottom time increased with decreasing temperature (Fig. 8), but the proportion of variance accounted for was small (7%).
Discussion
This study documents the remarkable behavioral flexibility of common murres. Quite unexpectedly, as murres are considered to be largely visual foragers, birds dived throughout the day and night, and reached depths in excess of 150 m during the day. Our findings exceed depths recorded previously for this species using data loggers (Table 2), but they confirm the impressive diving depths recorded using maximum depth gauges (Burger and Simpson 1986) and inferred through fisheries bycatch data (Piatt and Nettleship 1985) for murres in the region.
A most intriguing feature of this dataset is the incidence of long, deep dives; more than 20% of dives exceeded 50 m, and 8% exceeded 75 m, which is the maximum depth recorded for this species in the eastern Atlantic (Table 2). Deep diving, then, is clearly an important aspect of parental foraging in the western Atlantic where birds specialize on capelin (Piatt 1990; Davoren and Montevecchi 2003a; Davoren 2007; Burke and Montevecchi 2008). Presumably these deep dives would incur high energetic costs as the birds are hunting in frigid (sub-0°C) waters. So why do they perform these deep dives and, when they do, how successful are they at capturing prey?
In all likelihood, murres in the Newfoundland region dive deeply simply because they have to, but we hypothesize that when they do, their prey capture rates are high. The study area near Funk Island has gradually sloping bathymetry from the coast line, with water depth increasing with increasing distance from the coast (see Fig. 1, Davoren et al. 2006). As flapping flight in air is energetically costly for murres, birds may choose to forage close to the colony in deeper water. Murres from Funk Island forage on shoals of capelin located between the colony and the coast (Davoren et al. 2003a, b) that are known to be persistent both in space (at the scale of hundreds of meters) and time (across years; Davoren et al. 2006). These capelin shoals are found deep in the water column, within the CIL, during the day. Temperature can limit fishes’ swimming performance (swimming speed halves with every 10°C decrease in water temperature; Q10°C = 2; Videler and Wardle 1991), and perhaps more importantly in the context of predator-prey interactions, the cold temperatures would increase the duration of recovery from burst-type exercise (Kieffer 2000). Capelin located in the CIL would therefore have relatively slow escape speeds and would require extended periods of time to recover, which would presumably place them at a disadvantage when facing endothermic predators. So, while deep foraging in the CIL is perhaps energetically demanding, it is also likely an effective tactic, as capelin shoals are easy to find (Davoren et al. 2006) and we hypothesize, the fish are easy to catch.
Murres from Funk Island dived throughout the day and night, and as anticipated from studies of other diving avian predators (Wilson et al. 1993, Wanless et al. 1999), both diving depth and foraging efficiency decreased during dark conditions at night. At night, murres dived frequently to shallow depths (<50 m) and showed decreased foraging efficiency as reflected by an increase in the number of dives per bout. Temporal patterns in murre diving behavior and efficiency reflect diel variation in both light conditions and prey behavior. Capelin shoals occupying deep waters around Funk Island engage in diel vertical migrations (Davoren et al. 2006). During the day, when murres perform deep dives, capelin occur in discrete shoals within and below the CIL. At dusk, capelin migrate vertically through the CIL to reach the warm surface layer, where they disperse and spend the night (Davoren et al. 2006). At dawn, the pattern is reversed; shoals re-group and migrate down into the CIL and spend the day at depth (Bailey et al. 1977). The murres’ deepest dives occurred just after sunrise, when capelin are migrating toward the seabed, perhaps indicating birds were chasing fish to the bottom. Yet, in our study, most diving occurred near sunset, when the capelin are migrating toward the surface. The shoaling behavior and upward movement of capelin at dusk, when light levels are still relatively high, could provide murres with the most favorable foraging conditions.
During the 1990s, capelin shifted deeper in the water column and likely experienced considerable predator–prey tradeoffs (Mowbray 2002). Capelin produce very little anti-freeze protein but can super-cool and have been documented in water temperatures below the freezing point of their bodily fluids (−0.4 to –1.5°C; Raymond and Hassell 2000, Nakshima and Wheeler 2002). It has been hypothesized that capelin shoal in deep, sub-0°C water during the day to reduce predation risk from their primary predator––northern cod Gadus morhua that generally occupy waters between −0.5 and 8.5°C (Rose and Leggett 1990; Mowbray 2002). Capelin might also encounter improved feeding conditions at deeper depths during the day when zooplankton biomass is considerably elevated in the lower (>50 m) compared with the upper (<50 m) water column (Mowbray 2002; Davoren et al. 2006). Migrating into warmer surface waters at night likely increases metabolic rates, thereby increasing escape response capabilities (Videler and Wardle 1991) and also accelerates digestion and gonadal development (Winters 1970; Davoren et al. 2006). To summarize, by aggregating at depth in sub-0°C water during the day, capelin are inaccessible to cod and most species of seabirds (Regehr and Montevecchi 1997; Regehr and Rodway 1999) but they remain vulnerable to deep-diving murres. This “cost” for capelin is likely a minor trade-off, as the consumption of capelin by murres pales in comparison to that of cod (Montevecchi 2001).
While we did not find the anticipated relationship between time spent foraging within a patch (i.e., dive bottom time) and water temperature, murres did perform fewer dives per bout as the average depth of dives within the bout increased, supporting our hypothesis that foraging success is high during deep dives. Contrary to our initial rationale, it is possible that bottom time increases in cold (deep) water owing to lower light levels at depth and associated increases in prey handling time. Using stomach temperature loggers, Camphuysen (2005) reported that though foraging success was variable at the level of an individual dive, murres successfully captured prey on most diving bouts. If the murres in our study had similar success, fewer deep than shallow dives were needed to catch prey. Standard (physiological) assessment of diving and bout efficiency suggested that the murres deep dives were inefficient, however, these physiological costs need to be integrated with information on catch per unit effort in order to assess overall foraging efficiency. Resolving this will require an understanding of how murres trade-off energetic costs of flying (horizontal movement) and diving (vertical movement) with rates of prey intake in different areas. We are addressing these issues with tracking studies and will attempt to quantify rates of prey capture with stomach thermal (Weimerskirch et al. 2005) and/or beak sensors (Wilson et al. 2002).
References
Bailey RFJ, Able KW, Leggett WC (1977) Seasonal and vertical distribution and growth of juvenile and adult capelin (Mallotus villosus) in the St Lawrence Estuary and western Gulf of St Lawrence. J Fish Res Board Can 34:2030–2040
Benvenuti S, Dall’Antonia L, Falk K (2002) Diving behaviour differs between incubating and brooding Brünnich’s guillemots, Uria lomvia. Polar Biol 25:474–478
Burger AE (1991) Maximum diving depths and underwater foraging in alcids and penguins. In: Montevecchi WA, Gaston AJ (eds) Studies of high-latitude seabirds. 1. Behavioural, energetic, and oceanographic aspects of seabird feeding ecology, vol 68. Can Wild Serv Occas Pap, pp 9–15
Burger AE, Piatt JF (1990) Flexible time budgets in breeding common murres: buffers against variable prey abundance. Stud Avian Biol 14:71–83
Burger AE, Simpson M (1986) Diving depths of Atlantic puffins and common murres. Auk 103:828–830
Burke CM, Montevecchi WA (2008) Fish and chicks: forage fish and chick success in two co-existing auks. Waterbirds 31:372–384
Cairns DK, Gaston AJ, Huettmann F (2008) Endothermy, ectothermy and the global structure of marine vertebrate communities. Mar Ecol Prog Ser 356:239–250. doi:https://doi.org/10.3354/meps07286
Camphuysen CJ (ed) (2005) Understanding marine foodweb processes: an ecosystem approach to sustainable sandeel fisheries in the North Sea. IMPRESS Final Report. Royal Netherlands Institute for Sea Research, Texel
Chardine JW, Robertson GJ, Ryan PC, Turner B (2003) Abundance and distribution of common murres breeding at Funk Island, Newfoundland in 1972 and 2000, vol 404. Can Wild Ser Tech Rep Ser, Atlantic Region
Croll DA, Gaston AJ, Burger AE, Konnoff D (1992) Foraging behavior and physiological adaptation for diving in thick-billed murres. Ecology 73:344–356. doi:https://doi.org/10.2307/1938746
Davoren GK (2007) Effects of gill-net fishing on marine birds in a biological hotspot in the Northwest Atlantic. Conserv Biol 21:1032–1045. doi:https://doi.org/10.1111/j.1523-1739.2007.00694.x
Davoren GK, Montevecchi WA (2003a) Signals from seabirds indicate changing biology of capelin stocks. Mar Ecol Prog Ser 258:253–261. doi:https://doi.org/10.3354/meps258253
Davoren GK, Montevecchi WA (2003b) Consequences of foraging trip duration on provisioning behaviour and fledging condition of common murres Uria aalgae. J Avian Biol 34:44–53. doi:https://doi.org/10.1034/j.1600-048X.2003.03008.x
Davoren GK, Montevecchi WA, Anderson JT (2003a) Distributional patterns of a marine bird and its prey: habitat selection based on prey and conspecific behaviour. Mar Ecol Prog Ser 256:229–242. doi:https://doi.org/10.3354/meps256229
Davoren GK, Montevecchi WA, Anderson JT (2003b) Search strategies of a pursuit-diving marine bird and the persistence of prey patches. Ecol Monogr 73:463–481. doi:https://doi.org/10.1890/02-0208
Davoren GK, Anderson JT, Montevecchi WA (2006) Shoal behaviour and maturity relations of spawning capelin (Mallotus villosus) off Newfoundland: demersal spawning and diel vertical movement patterns. Can J Fish Aquat Sci 63:268–284. doi:https://doi.org/10.1139/f05-204
Domenici P, Claireaux G, McKenzie D (2007) Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms: an introduction. Philos Trans R Soc B Biol Sci 362:1929–1936. doi:https://doi.org/10.1098/rstb.2007.2078
Drinkwater KF (1996) Atmospheric and oceanic variability in the Northwest Atlantic during the 1980s and early 1990s. J Northwest Atl Fish Sci 18:77–97. doi:https://doi.org/10.2960/J.v18.a6
Elliott KH, Davoren GK, Gaston AJ (2007) The influence of buoyancy and drag on the dive behaviour of an Arctic seabird, the thick-billed murre. Can J Zool 85:353–361. doi:https://doi.org/10.1139/Z07-012
Elliott KH, Woo K, Gaston AJ, Benvenuti S, Dall’Antonia L, Davoren GK (2008a) Seabird foraging behaviour indicates prey type. Mar Ecol Prog Ser 354:289–303. doi:https://doi.org/10.3354/meps07221
Elliott KH, Davoren GK, Gaston AJ (2008b) Time allocation by a deep-diving bird reflects prey type energy gain. Anim Behav 75:1301–1310. doi:https://doi.org/10.1016/j.anbehav.2007.09.024
Falk K, Benvenuti S, Dall’Antonia L, Kampp K, Ribolini A (2000) Time allocation and foraging behaviour of chick-rearing Brünnich’s guillemots Uria lomvia in high-arctic Greenland. Ibis 142:82–92. doi:https://doi.org/10.1111/j.1474-919X.2000.tb07687.x
Falk K, Benvenutti S, Dall’Antonia L, Gilchrist G, Kampp K (2002) Foraging behaviour of thick-billed murres breeding in different sectors of the North Water polynya: an inter-colony comparison. Mar Ecol Prog Ser 231:293–302. doi:https://doi.org/10.3354/meps231293
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121. doi:https://doi.org/10.2307/3677252
Halsey LG, Bost CA, Handrich Y (2007) A thorough and quantified method for classifying seabird diving behaviour. Polar Biol 30:991–1004. doi:https://doi.org/10.1007/s00300-007-0257-3
Harding AMA, Piatt JF, Schmutz JA, Shultz MT, Van Pelt TI, Kettel AB, Speckman SG (2007) Prey density and the behavioural flexibility of a marine predator: the common murre (Uria aalge). Ecology 88:2024–2033. doi:https://doi.org/10.1890/06-1695.1
Huin N, Prince PA (1997) Diving behaviour of the grey-headed albatross. Antarct Sci 9:243–249. doi:https://doi.org/10.1017/S0954102097000321
Jodice PGR, Roby DD, Suryan RM, Irons DB, Turco KR, Brown ED, Thedinga JF, Visser GH (2006) Increased energy expenditure by a seabird in response to higher food abundance. Mar Ecol Prog Ser 306:283–293. doi:https://doi.org/10.3354/meps306283
Jones IL, Rowe S, Carr SM, Fraser G, Taylor P (2002) Different patterns of parental effort during chick-rearing by female and male thick-billed murres (Uria lomvia) at a low-arctic colony. Auk 119:1064–1074. doi:https://doi.org/10.1642/0004-8038(2002)119[1064:DPOPED]2.0.CO;2
Kieffer JD (2000) Limits to exhaustive exercise in fish. Comp Biochem Physiol A 126:161–179
Mehlum F, Watanuki Y, Takahashi A (2001) Diving behaviour and foraging habits of Brünnich’s guillemots (Uria lomvia) in the High Arctic. J Zool (Lond) 255:413–423. doi:https://doi.org/10.1017/S0952836901001509
Montevecchi WA (2001) Interactions between fisheries and seabirds. In: Schrieber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, pp 527–557
Mowbray F (2002) Changes in the vertical distribution of capelin (Mallotus villosus) off Newfoundland. ICES J Mar Sci 59:942–949. doi:https://doi.org/10.1006/jmsc.2002.1259
Nakashima BS (1992) Patterns in coastal migration and stock structure of capelin (Mallotus villosus). Can J Fish Aquat Sci 49:2423–2429
Nakshima BS, Wheeler JP (2002) Capelin (Mallotus villosus) spawning behaviour in Newfoundland waters. ICES J Mar Sci 59:909–916. doi:https://doi.org/10.1006/jmsc.2002.1261
Nevins HM (2004) Diet, demography, and diving behaviour of the common murre (Uria aalge) in central California. MSc thesis, San Francisco State University
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RJ, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 154–177
Petrie B, Akenhead S, Lazier J, Loder J (1988) The cold intermediate layer on the Labrador and Northeast Newfoundland Shelves, 1978–86. NAFO Sci Counc Stud 12:57–69
Piatt JF (1990) The aggregative response of common murres and Atlantic puffins to their prey. Stud Avian Biol 14:36–51
Piatt JF, Nettleship DN (1985) Diving depths of four alcids. Auk 102:293–297
RASC (2007) Observers handbook 2008. In: Kelly P (ed) Royal Astronomical Society of Canada, Toronto
Raymond JA, Hassell A (2000) Some characteristics of freezing avoidance in two osmerids, rainbow smelt and capelin. J Fish Biol 57:1–7
Regehr HM, Montevecchi WA (1997) Interactive effects of food shortage and predation on breeding failures of black-legged kittiwakes: indirect effects of fisheries activities and implications for indicator species. Mar Ecol Prog Ser 155:249–260. doi:https://doi.org/10.3354/meps155249
Regehr HM, Rodway MS (1999) Seabird breeding performance during two years of delayed capelin arrival in the Northwest Atlantic: a multi-species comparison. Waterbirds 22:60–67. doi:https://doi.org/10.2307/1521994
Robertson GJ, Wilhelm SI, Taylor PA (2004) Population size and trends of seabirds breeding on Gull and Great Islands, Witless Bay Islands Ecological Reserve, Newfoundland, up to 2003. Can Wild Serv Tech Rep Ser 418, Atlantic Region
Rose GA, Leggett WC (1990) The importance of scale to predator–prey spatial correlations: an example of Atlantic fishes. Ecology 71:33–43. doi:https://doi.org/10.2307/1940245
SAS INSTITUTE (2005) SAS/STAT User’s guide, version 9.1.2. SAS Institute, Inc., Cary
Tremblay Y, Cherel Y, Oremus M, Tveraa T, Chastel O (2003) Unconventional ventral attachment of time-depth recorders as a new method for investigating time budget and diving behaviour of seabirds. J Exp Biol 206:1929–1940. doi:https://doi.org/10.1242/jeb.00363
United Nations Foundation (UN) (2007) UN Atlas of the Oceans. https://doi.org/www.oceansatlas.org/unatlas/about/physicalandchemicalproperties/background/seemore2.html (November 6, 2007)
Videler JJ, Wardle CS (1991) Fish swimming stride by stride: speed limits and endurance. Rev Fish Biol Fish 1:23–40. doi:https://doi.org/10.1007/BF00042660
Wanless S, Morris A, Harris MP (1988) Diving behaviour of guillemot Uria aalge, puffin ratercula arctica and razorbill Alca torda as shown by radio-telemetry. J Zool (Lond) 216:73–81
Wanless S, Finney SK, Harris MP, McCafferty DJ (1999) Effect of diel light cycle on the diving behaviour of two bottom feeding marine birds: the blue-eyed shag Phalacrocorax atriceps and the European shag P. aristotelis. Mar Ecol Prog Ser 188:219–224. doi:https://doi.org/10.3354/meps188219
Weimerskirch H, Ancel A, Caloin M, Zahariev A, Spagiari J, Kersten M, Chastel O (2003) Foraging efficiency and adjustment of energy expenditure in a pelagic seabird provisioning its chick. J Anim Ecol 72:500–508. doi:https://doi.org/10.1046/j.1365-2656.2002.00720.x
Weimerskirch H, Gault A, Cherel Y (2005) Prey distribution and patchiness: Factors in foraging success and efficiency of wandering albatrosses. Ecology 86:2611–2622. doi:https://doi.org/10.1890/04-1866
Wilson RP, Puetz K, Bost CA, Culik BM, Bannasch R, Reins R, Adelung D (1993) Diel dive depth in penguins in relation to diel vertical migration of prey: whose dinner by candlelight? Mar Ecol Prog Ser 94:101–104. doi:https://doi.org/10.3354/meps094101
Wilson RP, Steinfurth A, Ropert-Coudert Y, Kato A, Kurita M (2002) Lip-reading in remote subjects: an attempt to quantify and separate ingestion, breathing and vocalization in free-living animals using penguins as a model. Mar Biol (Berl) 140:17–27. doi:https://doi.org/10.1007/s002270100659
Winters GH (1970) Biological changes in coastal capelin from the over-wintering to the spawning condition. J Fish Res Board Can 27:2215–2224
Acknowledgments
We thank Gail Davoren, Don Deibel, Garth Fletcher, Jack Lawson, Laura McFarlane-Tranquilla, Steven Peake, Paulette Penton, and Becky Sjare for discussions and helpful comments. We thank Dawn Marshall and Steven Carr for molecular sexing of the murres. Nick White and Peter Mallum helped with the field work at Gull Island. Research was supported by grants to W. A. M. from NSERC, the Murre Fund of the Newfoundland and Labrador Legacy Nature Trust and the Government of Canada’s Program for International Polar Year.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Lewison.
Rights and permissions
About this article
Cite this article
Hedd, A., Regular, P.M., Montevecchi, W.A. et al. Going deep: common murres dive into frigid water for aggregated, persistent and slow-moving capelin. Mar Biol 156, 741–751 (2009). https://doi.org/10.1007/s00227-008-1125-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1125-6