Abstract
Worldwide, seabird populations are facing significant declines due to various threats, making them valuable bioindicators of marine ecosystem changes. Understanding their foraging behavior and identifying crucial foraging areas (FAs) is essential for their conservation. In this study, the focus was on Olrog’s gull (Larus atlanticus), a near-threatened species endemic to the Atlantic coast of Argentina, Uruguay, and southern Brazil. The objectives were to assess the distribution of FAs and home ranges of Olrog’s gull and to evaluate the overlap between FAs and protected areas (PAs) in the Bahía Blanca Estuary (BBE), Argentina. Global positioning system (GPS) tracking and dynamic Brownian bridge movement models (dBBMM) were used to analyze Olrog’s gull behavior during their breeding season. The results revealed well-defined FAs, with 72% of them located outside PAs, raising concerns about potential threats to the species. Sex-related differences were observed, with females covering longer distances during foraging flights. In addition, several FAs were located near industrial sites, posing health risks due to pollution. This study contributes novel insights into Olrog’s gull foraging ecology, particularly in the most critical breeding area for the species. A dynamic web application was developed to provide rapid access to comprehensive information applicable to conservation actions. These findings underscore the need for enhanced protection of critical FAs, particularly those lying outside currently PAs.
This research emphasizes the importance of well-informed policy decisions to enhance ongoing conservation initiatives for Olrog’s gull populations and their habitats, particularly in the face of increasing threats posed by human activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, populations of seabirds are declining (Paleczny et al. 2015). The threats faced by seabirds are numerous, and include predation, habitat loss, bycatch, pollution, and overfishing (Dias et al. 2019). Therefore, seabirds can provide great insight into marine ecosystem changes (Piatt et al. 2007). Furthermore, seabird foraging ranges can be used to identify candidate locations for protection (Thaxter et al. 2012), and safeguarding these areas can benefit whole ecosystems (Hooker and Gerber 2004).
The Olrog’s gull (Larus atlanticus) is a near-threatened species (BirdLife International 2018) endemic to the Atlantic coast of Argentina, Uruguay, and southern Brazil (Yorio et al. 2013). Few Olrog’s gull breeding sites have been identified, and all of them are located in Argentina (Yorio et al. 2013). Most of the breeding population concentrates in the Bahía Blanca Estuary (BBE), Buenos Aires province (Petracci and Sotelo 2013), with the largest colony known to this date located on Isla del Puerto (Delhey et al. 2001a).
During their breeding season in the BBE, Olrog’s gull adults prey mainly on the grapsid crabs Neohelice granulata (Delhey et al. 2001b) and Cyrtograpsus angulatus, which they feed to their young. The specialized feeding ecology of Olrog’s gull makes it highly dependent on intertidal habitats during this critical period. These aspects not only make this species highly dependent on coastal habitats but also expose it to a myriad of hazards linked to human-related activities (Berón and Favero 2009; Berón and Seco Pon 2021). With this background, the identification of foraging areas (FAs) for Olrog’s gull becomes crucial for the conservation of this species through the strategic protection of these key areas.
The main objectives of this research were (1) to assess the distribution of FAs and home range used by Olrog’s gull in the BBE and (2) to evaluate the overlap between FAs and protected areas (PAs).
Materials and methods
Study area and species
The study was conducted in the BBE in southwest Buenos Aires province, Argentina. This wetland is characterized by extensive mudflats and marshes of Spartina spp. and Salicornia ambigua. It is also home to large populations of N. granulata and C. angulatus crabs (Zalba et al. 2008). This wetland includes three protected areas: Reserva Natural de Usos Múltiples Bahía Blanca, Bahía Falsa, Bahía Verde (2600 km2); Reserva Natural Municipal Costera Bahía Blanca (3.1 km2); and Reserva Natural Islote de la Gaviota Cangrejera/Isla del Puerto (16.1 km2). Annually, the latter hosts the largest known breeding colony of Olrog’s gull (38° 49′ S, 62° 16′ W). Comprising approximately 3500 pairs, this colony is distributed among subcolonies alongside the intertidal area (Yorio et al. 2013).
Within the study area, Olrog’s gulls commence egg laying in early September, with hatching taking place between late September and early October. The full fledging of chicks is completed by late December (La Sala et al. 2011a). Although there are additional Olrog’s gull colonies in the region, they are notably smaller (Petracci and Sotelo 2013).
Sampling
The fieldwork was conducted at the Isla del Puerto breeding colony during the late incubation period of the 2015 breeding season (November 1 through November 11). Given the difficult accessibility of the studied colony and the need to reduce human disturbance during the breeding period, a convenience sampling approach was employed. Coulson traps (Weaver and Kadlec 1970) were placed over active nests with at least one egg, ensuring easy access while minimizing disturbance to other breeding adults. Hooding was used during animal manipulation to minimize stress on the birds. Following each capture, the trap was relocated to a different location, and only one adult Olrog’s gull per nest was captured. Each bird was fitted with metal and plastic leg bands with unique alpha-numeric identification codes to avoid recapture.
To determine the sex of each bird, the individuals were weighed with a handheld spring scale (nearest 10 g), and four measurements were recorded using a caliper: bill depth (at the proximal edge of the nostrils; nearest 0.01 mm), tarsus length (from joint between the tarsus and toes to the intertarsal joint; nearest 0.5 mm), head-bill length (from hindmost point of the head to the tip of the bill; nearest 0.01 mm), and wing length (from carpal joint to the tip of the wing in naturally folded wing; nearest mm) (Eck et al. 2011). Subsequently, the sex of each individual was determined through morphometric measurements, following the methodology recommended by Petracci et al. (2018).
GPS tracking
Seabirds exhibit a pattern of moving between profitable prey patches and decrease their movement upon reaching regions abundant in prey, as highlighted by Weimerskirch (2007). Consequently, monitoring seabirds can serve as an effective method to identify locations with high abundance of prey, as suggested by Piatt et al. (2007).
To assess the habitat utilization of Olrog’s gull, each individual was equipped with a global positioning system (GPS) store-on-board logger (i-gotU GT-120, Mobile Action Technology). The devices were streamlined by repackaging them with rubber shrink tubes, and were affixed to the bird’s dorsal mantle cover feathers using adhesive tape (3M™ Vinyl Duct Tape 3903). Recording intervals for time and position were set at every 11 s.
It is acknowledged that externally attached devices can influence the behavior, time allocation, and energy budget of birds (Elliott et al. 2007; Vandenabeele et al. 2012). However, studies using GPS technology on other gull species suggest minimal impact on weight, breeding success, and/or survival (Masello et al. 2013; Camphuysen et al. 2015; Thaxter et al. 2016).
In this study, potential adverse effects of GPS on the studied birds were mitigated by using small tags (20 g, including attachment), representing only 2.6% and 2.2% of the average body mass of the captured females and males, respectively. Loggers were deployed on one member of each pair from 17 nests. Following release, the behavior of each gull was closely observed for any indications of disturbance, such as nest abandonment, aggression, or interruptions in incubation. Each GPS device operated on the bird for approximately 24 h, after which the birds were recaptured, and the GPS units were removed for data transfer. Only one GPS device could not be recovered, but both pair members were observed nesting normally, indicating that the GPS had no negative effects on this individual.
Movement analysis and habitat utilization
Contemporary techniques for estimating home ranges go beyond merely measuring the size of an area. They also assess the intensity with which animals use different areas within their home range. This utilization distribution (UD), as introduced by Worton (1989), provides a more detailed understanding of how animals distribute their activities across different parts of their habitat.
In this context, dynamic Brownian bridge movement models (dBBMM) were selected over the traditional kernel method (Worton 1989), based on their superior ability to accommodate the autocorrelated nature of telemetry-derived data. Unlike the kernel method, the dBBMM ground their kernel density estimations on the entire movement path rather than relying solely on individual location estimates (Horne et al. 2007; Kranstauber et al. 2012). In addition, the dBBMM employ Brownian motion variance as a measure for behavioral state. This one-dimensional measure offers the advantage of simultaneously capturing changes in both turning angles and speed, enhancing the method’s sensitivity to nuanced behavioral patterns (Kranstauber et al. 2012). This method has been acknowledged for its versatile potential, as demonstrated in ecological studies (e.g., Farmer et al. 2010; Jones et al. 2017; Cerecedo-Iglesias et al. 2023), assessments of the efficacy of marine reserves for marine birds (Mason et al. 2018), and disease outbreak investigations (Takekawa et al. 2010).
In the present study, the analysis was conducted using the “move” package (Kranstauber et al. 2012) of R (R Core Team 2022). The parameters used included an error tolerance of 10 m, a time step of 5 min, a margin encompassing 21 locations, and window sizes set at 43, in accordance with the methodology outlined by Kranstauber et al. (2012). The GPS locations falling within the boundaries of the breeding colony were excluded from subsequent analyses. Graphics were created using the “ggplot2” package (Wickham 2016) of R, and mapping was conducted using Quantum GIS (QGIS Development Team 2023).
Due to the limited availability of information on the foraging temporal pattern for the studied species, and despite the brief tracking period (mean: 24.2 h) used in the present research, the results of a descriptive analysis are presented.
Identification of foraging areas
To identify the optimal percentage of UD that effectively enclosed the FAs, a receiver operating characteristic curve (ROC) analysis was conducted to avoid relying on arbitrary thresholds (Delong et al. 1988; Elith et al. 2006). A subset of randomly selected gulls, comprising two males and two females to mitigate potential biases linked to the birds’ sex, was used for this analysis. The GPS locations of this subset were classified as FAs (n = 201 areas) or non-foraging areas (NFAs; n = 103 areas) by way of expert opinion (Petracci and La Sala, pers. comm.). Subsequently, 101 values ranging from 0 to 100% were used as threshold levels in each of the corresponding UD, and the threshold that exhibited optimal discrimination between FAs and NFAs was chosen. Evaluation of classification performance involved metrics such as accuracy, sensitivity, and specificity. To ensure consistency, the threshold values selected for the four individual gulls were averaged, and this value was then uniformly applied across the UD models of all 14 studied gulls.
To visually represent FAs, minimum convex polygons (MCP) were constructed, encompassing the pixels associated with each identified FA. In addition, the centroid of each MCP is displayed to enhance the examination of smaller FAs.
Foraging areas’ distribution and use
The total and percentage area corresponding to FAs located inside or outside any of the PAs were calculated. In addition, the difference in the proportion of protected FA used by males and females was assessed using a logistic regression model. The distance between each pixel classified as FA and the centroid of the breeding colony was calculated for each gull. Subsequently, the difference in traveled distance between sexes was estimated using a mixed-effects model that included each gull as a random intercept, using the “nlme” R package (Pinheiro and Bates 2000).
For a more interactive exploration of the results, a dynamic application was developed utilizing Google Earth Engine (Gorelick et al. 2017) through its JavaScript API (https://lucianolasala.users.earthengine.app/view/olroggull). This application facilitates a comprehensive and user-friendly visualization of the spatial relationships and variations in foraging patterns among the gulls.
Results
Tracking and foraging patterns
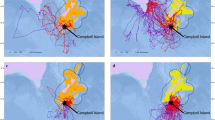
In this study, GPS tags were operational for an average of 24.2 h (SD: 6.5; range: 14.8–40.2, n = 14 gulls). When considering only locations outside the boundaries of the colony, a mean of 2743 (range: 961–6623) locations per gull was recorded. The accumulated tracking time for all the birds was 338.72 h (mean: 24.19; range: 15.83–40.87). The distribution of flight trajectories showed varying distribution among individuals and between sexes (Fig. 1; see web application for greater detail).
Foraging areas for male (black circles) and female (red circles) Olrog’s gull (Larus atlanticus) in the Bahía Blanca Estuary, southwest Buenos Aires province, Argentina (map inset). The Olrog’s gull breeding colony on Isla del Puerto (blue circle), sewage effluents (green diamonds), industrial area (green polygon), and protected areas (dashed red lines) are shown (color figure online)
Our model showed that Olrog’s gulls use well-defined areas during their foraging trips. Each gull performed between one and four foraging trips and used between two and eight FAs (Table 1). We identified a total of 57 FAs distributed across the study area, 41 (72%) of which were located outside the limits of PAs, three (5%) overlapped partially with the latter, and 13 (23%) lay completely inside their boundaries. Also, eight (14%) of the FAs that were located outside PAs lay inside areas recently incorporated in the Western Hemisphere Shorebird Reserve Network (WHSRN). Finally, 21 (37%) FAs were located along the northern shore of the BBE, in proximity to industrial and sewage effluents.
The mean home range (100% MCP) was larger (p = 0.002) for females (mean: 3082 ha; range: 1821–5030.4) compared to males (mean: 1211.8 ha; range: 440.5–1932) (Fig. 2). The mean size of FAs was 13.7% larger for females (mean: 1.23 ha; SD: 0.875; range: 0.21–2.81) compared to males (mean: 1.07 ha; SD: 1.11; range: 0.04–3.07), but this difference was not significant (p = 0.758) (Fig. 3). On average, however, the odds of using FAs under protection were 35 times larger for males than those for females (OR: 36.1; p = 0.037; males: 37.7%; females: 1.2%) (Table 2). Three FAs were located in artificial water bodies within the premises of two industrial plants (gulls OG0760 and OG0757), eight were under the direct influence of industrial wastewater discharge points (OG0763, OG0770, OG0756, OG0766, OG0767, and OG0771), and at least other four were adjacent to the main municipal sewage outfall (gulls OG0763 and OG0771) (see web application).
Home range (ha) estimated for females (n = 7) and males (n = 7) of Olrog’s gull (L. atlanticus) in the Bahía Blanca Estuary. Boxes represent the first quartile (lower end), median (middle line), and third quartile (upper end). Whiskers show minimum and maximum values. Double asterisks represent statistically significant (p ≤ 0.01) differences between sexes
Foraging area (ha) estimated for females (n = 7) and males (n = 7) of Olrog’s gull (L. atlanticus) in the Bahía Blanca Estuary (BBE). Boxes represent the first quartile (lower end), median (middle line), and third quartile (upper end) quartile. Whiskers show minimum and maximum values. Non-significant difference between sexes is shown (NS)
The mean distance traveled by each gull between the nest and each foraging pixel was 8.32 km (range: 2.6–15.1), and this distance was larger (p = 0.032) for females (mean: 10.9 km; SD: 4.52) compared to males (mean: 8 km; SD: 2.75) (Fig. 4). Foraging occurred during night and day hours, and it was highly variable among individuals (Fig. 5).
Distance (km) between the breeding colony and foraging areas (FAs) for females (n = 7) and males (n = 7) of Olrog’s gull (L. atlanticus) in the Bahía Blanca Estuary (BBE). Boxes represent the first quartile (lower end), median (middle line), and third quartile (upper end). Whiskers show minimum and maximum values. Single asterisk represents statistically significant (p ≤ 0.05) differences between sexes
The model classification process yielded an optimum threshold of 0.83 (range: 0.75–0.88), for which mean accuracy was 0.82 (0.74–0.95), mean sensibility was 0.89 (range: 0.79–1), and mean specificity was 0.7 (range: 0.39–0.94).
Discussion
The findings reveal a notable underrepresentation of Olrog’s gull FAs within protected areas in the BBE, Argentina. Specifically, more than 60% and nearly 100% of the FAs for males and females, respectively, were located outside PAs, and this strongly suggests a spatial mismatch between Olrog’s gull foraging activities and the existing PAs in the region.
A handful of studies have addressed the foraging patterns and areas of Olrog’s gull in other regions during the breeding season (Yorio et al. 2005; Suárez et al. 2012). However, to date, there were no studies addressing this topic in the BBE, which represents the most relevant breeding area for the species.
This study stands out as a unique contribution by filling a crucial knowledge gap and providing essential insights into the foraging ecology of Olrog’s gull in general and in the most important breeding area for the species in particular. Furthermore, this research successfully introduced the use of dBBMM as a novel approach for studying the foraging ecology of this species. In addition, a dynamic web application was developed, providing scientists, managers, and decision makers with accessible and relevant information. This application serves as a valuable conservation tool, offering insights that can be used independently or synergistically with other resources for effective conservation and management strategies.
Female Olrog’s gulls were observed to cover larger distances during their foraging flights compared to males. These variations in flight distance and habitat utilization might be attributed to sex-specific differences in habitat use and selection, as has been also found in other gull species (e.g., Camphuysen et al. 2015; Kazama et al. 2018; Navarro et al. 2010).
Previous research has suggested that mercury (Hg) concentrations in the feathers of adult Olrog’s gulls from this colony could have potential for long-term effects, with reported values largely falling within the toxic range for this metal in seabirds (La Sala et al. 2011b). Furthermore, gulls from the same colony have been found to carry pathogens of both human and animal origin, including Salmonella enterica var. typhimurium, Salmonella enterica serovar Gallinarum, and Escherichia coli (La Sala et al. 2014). In addition, prey items for Olrog’s gull, specifically crabs N. granulata and C. angulatus from some of the FAs identified in this study, were found to carry Clostridium perfringens type A in their gastrointestinal tracts (La Sala et al. 2015). This pathogen has been found to cause necrotic enteritis in both poultry and wild birds (Asaoka et al. 2004; Shojadoost et al. 2012). Given this background, our results raise additional concerns about the health risks for Olrog’s gull in the study area, particularly in FAs located near industrial effluents and sewage outfalls.
Olrog’s gulls used FAs located both inside and outside PAs, but unprotected FAs were considerably more common than protected ones. Moreover, the number of FAs exposed to the impacts of industrial activities or wastewater pollution raises additional concerns about their potential negative effects on this population.
Recently, new areas totaling 4760 ha were incorporated into the WHSRN. In our study, 14% of the FAs were located within these areas. However, despite their international importance for the conservation of shorebirds, these sites are not protected by law and remain vulnerable to future human-related activities.
In conclusion, our research underscores the necessity of intensifying policy efforts and implementing targeted conservation strategies to effectively protect FAs that are crucial for Olrog’s gull, especially those lying outside the boundaries of currently PAs.
Data availability
The datasets used in this research are not publicly available due to confidentiality reasons; however, they are available from the corresponding author on reasonable request.
References
Asaoka Y, Yanai T, Hirayama H, Une Y, Saito E, Sakai H, Goryo M, Fukushi H, Masegi T (2004) Fatal necrotic enteritis associated with Clostridium perfringens in wild crows (Corvus macrorhynchos). Avian Pathol 33(1):19–24
Berón MP, Favero M (2009) Mortality and injuries of Olrog’s gulls Larus atlanticus associated with sport fishing activities in Mar Chiquita coastal lagoon. El Hornero 24:99–102
Berón MP, Seco Pon JP (2021) Fishing gear-related injuries and mortality of seabirds in coastal northern Argentina. Mar Ornithol 49:321–327
BirdLife International (2018) Larus atlanticus. The IUCN red list of threatened species 2018: e.T22694286A132538305. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22694286A132538305.en. Accessed 18 Jul 2021
Camphuysen KCJ, Shamoun-Baranes J, van Loon EE, Bouten W (2015) Sexually distinct foraging strategies in an omnivorous seabird. Mar Biol 162:1417–1428
Cerecedo-Iglesias C, Bartumeus F, Cortés-Avizanda A, Pretus JL, Hernández-Matías A, Real J (2023) Resource predictability modulates spatial-use networks in an endangered scavenger species. Mov Ecol 11:22
Delhey JKV, Petracci PF, Grassini CM (2001a) Hallazgo de una nueva colonia de gaviota de Olrog (Larus atlanticus) en la ría de Bahía Blanca, Argentina. Hornero 16:39–42
Delhey JKV, Carrete M, Martínez MM (2001b) Diet and feeding behaviour of Olrog’s Gull Larus atlanticus in Bahía Blanca, Argentina. Ardea 8:319–329
Delong ER, Delong DM, Clarke-Pearson DL (1998) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Garcia Borboroglu P, Croxall JP (2019) Threats to seabirds: a global assessment. Biol Conserv 237:525–537
Eck S, Fiebig J, Fiedler W, Heynen I, Nicolai B, Töpfer T, Van den Elzen R, Winkler R, Woog F (2011) Measuring birds. The German Ornithologists’ Society, Wilhelmshaven
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ et al (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Elliott KH, Davoren GK, Gaston AJ (2007) Increasing energy expenditure for a deep-diving bird alters time allocation during the dive cycle. Anim Behav 75:1311–1317
Farmer C, Safi K, Barber DR, Martel M, Bildstein KL (2010) Efficacy of migration counts for monitoring continental populations of raptors: an example using the osprey (Pandion haliaetus). Auk 127:863–870
Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R (2017) Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sen Environ 202:18–27
Hooker SK, Gerber LR (2004) Marine reserves as a tool for ecosystem-based management: the potential importance of megafauna. Bioscience 54:27–39
Horne JS, Garton EO, Krone SM, Lewis JS (2007) Analyzing animal movements using Brownian bridges. Ecology 88:2354–2363
Jones PL, Hämsch F, Page RA, Kalko EKV, O’Mara MT (2017) Foraging and roosting behaviour of the fringe-lipped bat, Trachops cirrhosus, on Barro Colorado Island, Panamá. Acta Chiropterol 19(2):337–346
Kazama K, Nishizawa B, Tsukamoto S, Gonzalez JE, Kazama MT, Watanuki Y (2018) Male and female Black-tailed Gulls Larus crassirostris feed on the same prey species but use different feeding habitats. J Ornithol 159:923–934
Kranstauber B, Kays R, LaPoint SD, Wikelski M, Safi K (2012) A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. J Anim Ecol 81:738–746
La Sala LF, Pérez AM, Martorelli S, Smits J (2011a) Breeding biology of Olrog’s Gull in Bahía Blanca Estuary, Argentina. Wilson J Ornithol 123(2):243–250
La Sala LF, Petracci PF, Smits JE, Botté S, Furness RW (2011b) Mercury levels and health parameters in the threatened Olrog’s Gull (Larus atlanticus) from Argentina. Environ Monit Assess 181:1–11
La Sala LF, Petracci PF, Randazzo V, Fernández-Miyakawa ME (2014) Enteric bacteria in Olrog’s Gull (Larus atlanticus) and Kelp Gull (Larus dominicanus) from the Estuary of Bahía Blanca, Argentina. Hornero 28:59–64
La Sala LF, Redondo LM, Díaz Carrasco JM, Pereyra AM, Farber M, Jost H, Fernández-Miyakawa ME (2015) Carriage of Clostridium perfringens by benthic crabs in a sewage-polluted estuary. Mar Pollut Bull 97:365–372
Masello JF, Wikelski M, Voigt CC, Quillfeldt P (2013) Distribution patterns predict individual specialization in the diet of dolphin gulls. PLoS ONE 8:e67714
Mason C, Alderman R, McGowan J, Possingham HP, Hobday AJ, Sumner M, Shaw J (2018) Telemetry reveals existing marine protected areas are worse than random for protecting the foraging habitat of threatened shy albatross (Thalassarche cauta). Divers Distrib 24:1744–1755
Navarro J, Oro D, Bertolero A, Genovart M, Delgado A, Forero MG (2010) Age and sexual differences in the exploitation of two anthropogenic food resources for an opportunistic seabird. Mar Biol 157:2453–2459
Paleczny M, Hammill E, Karpouzi V, Pauly D (2015) Population trend of the world’s monitored seabirds, 1950–2010. PLoS ONE 10:e0129342
Petracci P, Sotelo M (2013) Aves del estuario de Bahía Blanca: una herramienta para su conocimiento y conservación. Editorial Muelle Sur, Bahía Blanca, p 196
Petracci PF, Burgos JM, López GC, Delhey K, Carrizo M, Sarria R, Sotelo M, Somoza GM, La Sala LF (2018) Field sexing Olrog’s Gull (Larus atlanticus) using morphometry. Waterbirds 41(4):411–416
Piatt JF, Sydeman WJ, Wiese F (2007) Introduction: a modern role for seabirds as indicators. Mar Ecol Prog Ser 352:199–204
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
QGIS Development Team (2023) QGIS geographic information system. Open Source Geospatial Foundation Project. https://qgis.org/en/site/
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Shojadoost B, Vince AR, Prescott JF (2012) The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet Res 43(1):74
Suárez N, Retana MV, Yorio P (2012) Spatial patterns in the use of foraging areas and its relationship with prey resources in the threatened Olrog’s Gull (Larus atlanticus). J Ornithol 153:861–871
Takekawa JY, Newman SH, Xiao X, Prosser DJ, Spragens KA, Palm EC, Yan B, Li T, Lei F, Zhao D, Douglas DC, Muzaffar SB, Ji W (2010) Migration of waterfowl in the east Asian flyway and spatial relationship to HPAI H5N1 outbreaks. Avian Dis 54(s1):466–476
Thaxter CB, Lascelles B, Sugar K, Cook AS, Roos S, Bolton M, Langston RHW, Burton NHK (2012) Seabird foraging ranges as a preliminary tool for identifying candidate marine protected areas. Biol Conserv 156:53–61
Thaxter CN, Ross-Smith VH, Clark JA, Clark NA, Conway GJ, Masden EA, Wade HM, Leat EHK, Gear SC, Marsh M, Booth C, Furness RW, Votier SC, Burton NHK (2016) Contrasting effects of GPS device and harness attachment on adult survival of lesser black-backed Gulls Larus fuscus and great skuas Stercorarius skua. Ibis 158:279–290
Vandenabeele SP, Shepard EL, Grogan A, Wilson RP (2012) When three per cent may not be three percent; device-equipped seabirds experience variable flight constraints. Mar Biol 159:1–14
Weaver DK, Kadlec JA (1970) A method for trapping breeding adult gulls. Bird-Banding 41(1):28–31
Weimerskirch H (2007) Are seabirds foraging for unpredictable resources? Deep Sea Res II: Top Stud Oceanogr 54:211–223
Western Hemisphere Shorebird Network (WHSRN) https://whsrn.org/whsrn-sites
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70(1):164–168
Yorio P, Suárez N, Quintana F, Gatto A (2005) Nocturnal activity in breeding Olrog’s gulls (Larus atlanticus). Ornitol Neotrop 16:123–126
Yorio P, Petracci P, Garcia Borboroglu P (2013) Current status of the threatened Olrog’s Gull Larus atlanticus: global population, breeding distribution and threats. Bird Conserv Int 23(4):477–486
Zalba SM, Nebbia AJ, Fiori SM (2008) Propuesta de plan de manejo de la Reserva Natural de Uso Múltiple Bahía San Blas. Universidad Nacional del Sur, Bahía Blanca
Acknowledgements
We thank Carlos Re and Oscar Pedro Salvatori for transportation to the study site.
All activities related to this work were conducted under scientific research and collecting permits issued by Organismo Provincial para el Desarrollo Sostenible de la provincia de Buenos Aires.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, field work, and data collection were performed by Pablo Petracci, Leandro Marbán, Martín Sotelo, Martín Carrizo, and Rodolfo Sarria. All the analyses and first draft of the manuscript were performed by Luciano La Sala and Nicolás Caruso, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed under the permits by Ministerio de Ambiente de la Provincia de Buenos Aires.
Compliance with ethical standards
The authors declare that there is no conflict of interest. High animal welfare standards were followed, and stages of our field work were closely overseen by government authorities (park rangers). No informed consent was necessary as humans were not involved in our work.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petracci, P., Caruso, N., La Sala, L.F. et al. Foraging areas of Orlog’s gulls in an estuarine ecosystem of Argentina: are they underprotected?. Mar Biol 171, 96 (2024). https://doi.org/10.1007/s00227-024-04404-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04404-7