Abstract
Despite the important roles played by parasites in local population dynamics and community structure of marine ecosystems, there is a lack of information on the geographical variation in infection levels displayed by particular host–parasite species combinations. This study examines geographical variation in infection levels by the metacercarial stages of trematode parasites in crustacean and bivalve second intermediate hosts. Analyses were based on a dataset compiled from the literature, consisting of 164 local samples representing 49 host–parasite species pairs for crustaceans, and 338 entries representing 36 host–parasite species pairs for bivalves. The analyses indicate that for all measures of infection levels [prevalence (percentage of individuals infected), intensity (mean no. of metacercariae per infected individual), abundance (mean no. of metacercariae across all individuals in a sample)], there was statistically significant repeatability of infection values within host–parasite species pairs. However, it is only for values of intensity and abundance of infection in crustacean hosts that the repeatability was strong; this suggests that infection levels are specific properties of crustacean–trematode species pairs, showing significant consistency across localities despite spatial variation in abiotic and biotic conditions. Although the magnitude of variation in infection levels within parasite species pairs (measured as coefficients of variation) was independent of scale in crustacean hosts, infection levels in bivalves increased in variability at large (>100 km) spatial scales. These results suggest that there is a considerable geographical consistency in parasite load, especially in crustacean hosts, which should lead to consistent ecological and ecosystem effects of marine trematodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites play fundamental roles in natural ecosystems. They can regulate host population abundance, influence the diversity and composition of communities, and stabilize food webs (Minchella and Scott 1991; Thomas et al. 2005; Lafferty et al. 2008). In particular, trematode parasites are important, although often ignored, elements of marine systems, especially intertidal ones (Sousa 1991; Mouritsen and Poulin 2002). They are known to modulate the population dynamics of invertebrate hosts (Lafferty 1993; Fredensborg et al. 2005; Thieltges 2006a) and to indirectly determine the diversity and structure of intertidal communities (Mouritsen and Poulin 2005; Wood et al. 2007). What remains unclear; however, is whether the impact of a particular trematode species is confined to a few localities, or whether this impact is detectable over larger spatial scales.
The potential impact of a parasite is obviously dependent on the infection levels it achieves in a given ecosystem. On the one hand, local factors may be the key determinants of how abundant a particular parasite will be in a locality; on the other hand, different parasite species may have different intrinsic properties that pre-determine how abundant they will be, at least within a species-specific range of abundance values. There is evidence from a wide range of parasite taxa that the levels of infection attained by a parasite species vary much less than expected by chance across localities, and therefore represent parasite species characters (Arneberg et al. 1997; Poulin 2006a; Krasnov et al. 2006). Understanding the spatial variation in infection levels displayed by a particular parasite species is important as it underpins the geographical dimension of host–parasite interactions, a spatial scale often ignored because of its complexity. Given that the pathological effects of marine trematode parasites are generally dependent on the number of parasites per host (e.g., Fredensborg et al. 2004; Thieltges 2006b), it is important to investigate what regulates infection levels by these parasites to gain insights into their role in the population dynamics and community structure of marine hosts.
Spatial variation in infection levels has received very little attention in trematodes parasitic of marine animals. Intertidal trematodes have complex life cycles. They generally use either shorebirds or fish as definitive hosts, in which the parasites reproduce sexually. Eggs released in the definitive host’s feces will then infect snails, which serve as first intermediate hosts. A few studies have shown that some of the variation among localities in trematode infection levels of snails acting as first intermediate hosts is explained by the frequency and number of visits to each site by bird definitive hosts (Smith 2001; Skirnisson et al. 2004; Fredensborg et al. 2006; Byers et al. 2008). Trematodes castrate their snail host and exploit its resources for the production of infective stages (cercariae) that will then leave the snails to infect a second intermediate host, which can be crustaceans, bivalves, or a range of other animals depending on the parasite species, or encyst outside their hosts in some cases. Within the second intermediate hosts, the cercariae develop into metacercariae, and over time a host can accumulate large numbers of metacercariae before these are transmitted to the definitive host via predation on the second intermediate host.
Little is known about the spatial variation in infection levels of second intermediate hosts by particular trematode species. Similar to avian definitive hosts in first intermediate snail hosts, the distribution and abundance of the first intermediate hosts seem to be important in determining metacercarial infection levels in second intermediate hosts on the scale of several kilometres (Thieltges and Reise 2007; Thieltges 2007). Other factors that contribute to variation in infection levels in second intermediate hosts among localities are habitat properties like residual water in pools at low tide and the density of the second intermediate hosts themselves, which can result in spatial heterogeneity of infection levels on the scale of a few meters (Thieltges and Reise 2007). The abundance and distribution of alternative host species as well as non-hosts (e.g., predators of infective cercarial stages) also affect infection levels in second intermediate hosts (Thieltges et al. 2008a, b). Differences in temperature among localities might add further variation because the rate at which cercariae are produced by (and released from) snails is known to be sensitive to temperature (Fredensborg et al. 2005; Thieltges and Rick 2006; Poulin 2006b). Therefore, local factors may be the dominant determinants of infection levels achieved by trematodes in their second intermediate hosts, ranging from very small (within localities) to larger spatial scales (among localities and regions). Given that differences in ambient environmental conditions increase with the distance between localities, we can expect the magnitude of variation to increase with the spatial scale investigated. However, given that parasites of all kinds show highly repeatable infection levels across localities (Arneberg et al. 1997; Poulin and Mouritsen 2003; Poulin 2006a; Krasnov et al. 2006), there may be a certain consistency in trematode infections of second intermediate hosts on a geographic scale.
The objective of this study is to assess the consistency (or repeatability) of infection levels by trematodes in crustacean and bivalve second intermediate hosts. We determine whether the infection levels [prevalence (percentage of individuals infected), intensity (mean no. of metacercariae per infected individual), abundance (mean no. of metacercariae across all individuals in a sample)] observed for particular trematode and host species are more similar within than among species, as a test of whether or not local factors can offset any intrinsic tendency for a trematode species to reach low or high abundance. As data on trematode infection levels in second intermediate hosts are limited we cannot cover the entire range of a parasite species but can only compare sites within this range, spanning from small (<10 km) to large spatial scales (>1,000 km), depending on the data available. In addition to assessing the consistency of infection levels, we test if variation in infection levels is greater on large compared with small spatial scales.
Materials and methods
We searched online databases and our own reprint collections for studies reporting on metacercarial infections in marine crustaceans or bivalves. As we were interested in the variance of metacercarial infection levels within the parasite–host species pairs, we included all parasite–host records from each locality. Hence, a single data entry consisted of a parasite–host species pair, i.e., a single parasite species and a single host species. As a result of this procedure some parasite species occur in combination with different host species in the dataset, both within and among localities. For each entry corresponding to a particular parasite–host species pair in one locality, we recorded data on three measures of infection levels: prevalence (proportion of infected hosts in the sample), intensity (mean number of parasites per host among infected hosts only) and abundance (mean number of parasites per host across the whole sample). Where possible we calculated missing data if the other two measures were available (abundance = prevalence × intensity). Data from graphs were read using the free software Engauge (http://digitizer.sourceforge.net/). Only studies investigating at least 15 individual hosts of the same species and from the same locality were included, in order to achieve a compromise between the level of accuracy of the recorded infection levels and the number of records for which data would be available. We retained only parasite–host species pairs for which we had at least two entries, i.e., samples from two different localities. To identify the spatial scale covered by the samples included in each parasite–host species pairs, we determined the approximate maximum distance among sampling sites using the following scale index: 1:<10 km, 2: 10–100 km, 3: 100–1,000 km, 4:>1,000 km. We also noted maximum host size, i.e., maximum shell or body length, from the original publications, identification guides or databases.
To test whether metacercarial infection levels in marine invertebrate hosts are parasite species characters (i.e., varying less among populations of the same parasite species in a particular host than among species), we used two approaches. First, for each parasite–host species pair, we plotted the lowest infection level values against all other values and performed a linear regression analysis. This was done separately for crustacean and bivalve hosts. Although this procedure is likely to be skewed towards finding a correlation as data cannot fall below the 1:1 line, we did so for the following reason: the data do not necessarily have to fall on the 1:1 line a priori. If variation among samples is large, many points in the plot will accumulate in the upper-left corner of the graph and there would not be a strong positive correlation between the lowest and all other infection levels in a pair. In contrast, a tight band of points along the 1:1 line would suggest that infection levels among sites for given pairs are consistent. The graph; thus, visually helps to interpret the data in this respect. A statistical test for the significance of the regression line serves as support to the conclusion that species have consistent infection levels and helps to estimate how large the scatter in the data is. However, one has to keep in mind that the nature of the data might confound the tests. For these analyses, we log-transformed intensity and abundance values to meet the assumptions of parametric tests.
Second, we conducted a repeatability analysis following Arneberg et al. (1997) and Poulin (2006a). Using separate ANOVAs for each infection level measure and separately for crustaceans and bivalves, we analyzed the variation in prevalence, intensity and abundance within and among parasite–host species pairs. Parasite–host species pairs served as levels in single factor analyses. A significant ANOVA result would indicate that the mean infection levels generally differ among pairs, although not all pairs necessarily have to differ from one another. This can only result if variances in infection levels within pairs are relatively small compared with variances among pairs, and would suggest repeatability of infection levels within pairs. Another indicator of repeatability we used is the proportion of the total variance that occurs among parasite–host species pairs, as opposed to within parasite–hosts species pairs. It was estimated using the coefficient of intraclass correlation as explained in Sokal and Rohlf (1995, p. 214). If a high proportion of the variance occurs among rather than within parasite–host species pairs, this suggests that infection levels in a parasite–host species pair at different localities are more similar to each other than to infection levels from other parasite–host species pairs. Hence, the proportion of variance associated with among and within parasite–host pair variation of infection levels can be used as an indicator for repeatability of infection levels. For all analyses, intensity and abundance data were log-transformed and prevalence data arcsine-transformed.
In addition to these analyses, we investigated whether local populations with high prevalence also exhibit higher infection intensities. To do so, we plotted log-transformed intensities from all samples in the dataset against prevalence and performed a linear regression analysis, separately for crustacean and bivalve hosts.
To analyse the effect of scale on variability in metacercarial loads, we calculated coefficients of variation (CV) for the three infection measures (prevalence, intensity, abundance) for each parasite–host species pair, grouped into the four scale index categories (see above). We tested for differences in CV among the four scale categories using one-way ANCOVAs with CV as the dependent variable and scale as the predictor variable. Maximum host size was added as a covariate to account for potential effects of host size on infection levels, as host size might influence parasite accumulation (e.g., Thieltges 2008). In a strict sense, the data used for this analysis are technically pseudo-replicated. Some data in the dataset come from studies investigating multiple host species at the same locality and are thus not strictly independent from each other. There might also be taxonomic effects on infection levels. However, we decided to use the analysis in its present form as this is likely the best approach for the data at hand.
Results
The data base included 49 and 36 host–parasite species pairs for crustaceans (164 total entries) and bivalves (338 total entries), respectively; data on all three infection measures are not available for all entries, however. The dataset is included as supplementary material in an electronic appendix (see Supplementary material S1–S4).
Over all samples, intensity was positively correlated with prevalence in crustacean hosts (R2 = 0.33, P = 0.015). However, there is a considerable scatter in the plotted data (Fig. 1). The pattern was stronger in bivalves as indicated by a higher regression coefficient (R2 = 0.53, P < 0.001) but there is still a lot of scatter in the plotted data, especially at prevalence levels of 100% (Fig. 1). Indeed, in bivalve samples with a 100% prevalence of infection, intensity values range over three orders of magnitude.
Relationship between prevalence and intensity of metacercarial infections in marine crustacean (above) and bivalve (below) hosts. The lines represent the best fit of a regression. Crustaceans: y = 0.0123x + 0.2604; bivalves: y = 0.0164x − 0.0416. The graphs use a subset of the total dataset as prevalence and intensity values were not available for all samples
When the lowest prevalence value for a particular host–parasite species pair was plotted against all other values for that pair, across all pairs involving crustaceans, a strong positive correlation emerged (R2 = 0.62, P < 0.001) (Fig. 2). However, the triangular shape of the scatterplot indicates that many crustacean host–parasite species pairs show a wide range of prevalences between localities, from very low to very high values. The pattern was clearer for intensity and abundance values, where the lowest and all other values per parasite–host species pair were positively and significantly correlated (intensity R2 = 0.76, P < 0.001; abundance R2 = 0.50, P < 0.001). In both cases, there was less scatter in the data and the roughly linear arrangement of points suggests a relatively narrow range of intensity and abundance values within parasite–hosts species pairs at different localities (Fig. 2). The ANOVA analyses indicated a similar pattern. Prevalence values were repeatable within the parasite–host species pairs (F47,97 = 4.43, P < 0.001) but only 20% of the variation in prevalence among all samples was associated with differences among parasite–host species pairs as opposed to differences within parasite–host species pairs. Repeatability patterns were stronger for intensity (F21,44 = 13.67, P < 0.001) and abundance (F22,62 = 13.43, P < 0.001), with 81 and 78% of the variation being associated with among parasite–host pair variation, respectively. Hence, most of the variation in intensity and abundance in crustacean parasite–host systems can be explained by the identity of the parasite–host species pair.
Relationship of the lowest infection level value (x axis) within a parasite–host species pair and all other values of the respective pair for either prevalence (top), intensity (middle) or abundance (bottom) of metacercarial infections in marine crustaceans. The graphs use subsets of the total datasets as infection level values were not available for all samples, depending on the measure used. For the number of samples within each parasite–host species pair see Supplementary material S3
In bivalves, infection levels among parasite–host species pairs showed a similar but much weaker pattern (Fig. 3). Although there was a significant correlation between the lowest prevalence values of each parasite–host species pair and all other prevalence values, the associated correlation coefficient was very low (R2 = 0.043, P < 0.001). The pattern was stronger for intensity (R2 = 0.126, P < 0.001) and abundance (R2 = 0.146, P < 0.001), but there was still considerable scatter in the data plots (Fig. 3), also indicated by the relatively low correlation coefficients. Similar to crustacean parasite–host systems, prevalence patterns were repeatable among populations of the same parasite–host species pairs (F32,294) = 4.24, P < 0.001). However, only 25.8% of the variation was associated with among parasite–host pair variation. Intensity (F30,234 = 5.91, P < 0.001) and abundance (F31,239 = 6.47, P < 0.001) patterns also showed repeatability among populations of parasite–host species pairs in bivalves, but the proportion of the variance associated with variation among parasite–host pairs was much lower than in crustacean systems, with 37.4% for intensity and 40.2% for abundance.
Relationship of the lowest infection level value (x axis) within a parasite–host species pair and all other values of the respective pair for either prevalence (top), intensity (middle) or abundance (bottom) of metacercarial infections in marine bivalves. The graphs use subsets of the total datasets as infection level values were not available for all samples, depending on the measure used. For the number of samples within each parasite–host species pair see Supplementary material S4
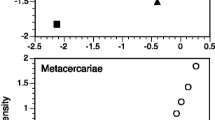
The spatial scale covered by the samples within each parasite–host species pair had no effect on the variability in metacercarial infection levels in crustacean hosts as indicated by similar coefficients of variation (CV) and non-significant effects in ANCOVAs (Fig. 4; Table 1). Maximum host size also had no effect on variability of infection levels (Table 1). In contrast, variation in all three infection measures (prevalence, intensity, abundance) increased with scale above 100 km in bivalves (Fig. 4; Table 1). However, again, maximum host size had no effect on the variability of infection levels (Table 1).
Mean coefficients of variation (CV) (+SE) within parasite–host species pairs at different spatial scales (<10 km, 10–100 km, 100–1,000 km, >1,000 km) for the three measures (prevalence, intensity, abundance) of metacercarial infections of marine crustacean and bivalve hosts. For the number of samples within each parasite–host species pair see Supplementary material S3 and S4
Discussion
Intensity and abundance showed some level of repeatability among parasite–host species pairs, i.e., there was a tendency for parasite species to occur within a relatively narrow range of values in a certain host species among localities. This suggests intensity and abundance to be specific for a given parasite–host species pair. In contrast, prevalences varied widely and the variation among localities within parasite–host species pairs was extensive in some cases. Hence, prevalence does not appear to be predictable for a specific parasite–host species pair but strongly determined by local factors.
This pattern is consistent with the previous studies on parasites in final hosts. Arneberg et al. (1997) found that intensity and abundance, but not prevalence, were repeatable among nematode species parasitic in mammals, and a similar pattern was reported from helminth parasites in freshwater fish (Poulin 2006a). Prevalence is a measure of the proportion of hosts infected with a specific parasite and is thus an indicator of the encounter rate of the hosts with infective stages of the parasite. Encounter rates are largely governed by processes outside the host such as the distribution and abundance of free-living infective stages, or the availability of infected prey items in parasites transmitted via predation. In the case of the crustacean and bivalve second intermediate hosts, it will be the local supply with cercarial stages that determines encounter rates of the hosts. Shedding rates of cercariae from snail first intermediate hosts and other external processes are likely to be determined by local factors that differ among localities. These include the distribution and abundance of first intermediate hosts, the density of the target hosts themselves, and varying temperatures (Fredensborg et al. 2005; Thieltges and Rick 2006; Poulin 2006b; Thieltges 2007; Thieltges and Reise 2007). In addition, ambient biota can further modulate infection levels by interfering with cercarial transmission (Mouritsen and Poulin 2003; Kaplan et al. 2008; Thieltges et al. 2008a, b). As a consequence of varying local factors, prevalences vary substantially among localities. Such a high variation in prevalences makes it difficult to predict the prevalence of a parasite in a certain host species among localities, and hence prevalence cannot be considered to be a characteristic of a given parasite–host species pair.
In contrast, intensities of infection with endoparasites are strongly affected by processes acting within the host (Poulin 2006a). Host size may limit the number of parasites that can fit within the body or particular organs of a given host (Poulin 2006a). However, our analyses did not indicate any effect of maximum host size on infection levels, although a density-dependence of infection levels has been documented in small second intermediate hosts like amphipods (5–20 mm body length): both the establishment success and the growth of metacercariae decrease with increasing infection doses in amphipods (Brown et al. 2003; Fredensborg et al. 2004; Fredensborg and Poulin 2005). More important in our case might be a density-dependent mortality of hosts. An increasing mortality of hosts with increasing density of metacercarial cysts has been well documented, particularly in crustacean hosts (e.g., Meissner and Bick 1997; Fredensborg et al. 2004). Assuming similar density-dependent mortality effects among localities, parasites infecting a certain host species will face a similar density-dependent limitation wherever they occur. As a result, intensities will vary within a narrow range of values since there is a ceiling on the maximum possible value; thus intensity values will be more similar within than among parasite–host species pairs. Hence, intensity (and abundance, i.e., the product of intensity and prevalence) is predictable among localities and can be considered to be a characteristic of a given parasite–host species pair.
Although intensity and abundance seem to be relatively predictable for a parasite species in a given host species, local factors still modulate infection levels in crustaceans and bivalves, as indicated by the strong scatter in the data. This is particularly evident for bivalves. Hence, local factors can override any predetermined ranges in infection levels in many parasite-bivalve systems. However, in crustaceans our analyses suggest that local factors play a minor role. What could be responsible for the different patterns observed in the two host groups? Most crustacean records in our dataset are from amphipods, isopods and barnacles which are all relatively small organisms (see supplementary material S1, S3). As discussed above, the small body size of crustacean hosts may impose a physical limit on the numbers of metacercariae that can accumulate inside a host. In addition, density-dependent mortality can also set an upper limit of infections. This may explain the relatively small variation of intensity and abundance within parasite–host species pairs. Local factors might still alter infection levels but not beyond certain size-imposed limits. In contrast, bivalves such as mussels, clams and cockles are much larger and offer ample space for the accumulation of metacercariae (which are usually below 200 μm in diameter). In addition, effects of metacercarial infections on bivalve hosts are less dramatic and rarely involve mortalities, although they may affect growth and other parameters (e.g., Thieltges 2006b). Hence, space limitations and density-dependent mortalities should be of minor importance for metacercariae in bivalves, and variation in local factors among sites can result in a relatively wide range of infection levels in bivalve hosts.
The observed difference in the strength of the repeatability patterns between crustacean and bivalve hosts may also have methodological reasons. For parasite–host species pairs in bivalve hosts, data were available from many sampling localities, whereas the number of localities available for crustaceans was more restricted; this might affect the scatter of values for a parasite–host species pair. More important might be spatial issues. For bivalves, many data were available from sites ranging over large geographical distances. In contrast, many entries in the crustacean dataset are from localities relatively close to each other (e.g., in the vicinity of islands, or bays) (see Supplementary material S3, S4). Hence, it could be that the spatial proximity results in a high similarity of local factors among localities for crustacean hosts, thus reducing the measurable effect of local factors on the repeatability of infection levels compared with bivalves. However, in crustaceans, the spatial scale had no effect on variation in infection levels. This suggests that intrinsic host factors like host size and density-dependent mortality are more important than environmental factors, even on large spatial scales. In contrast, variation in infection levels in bivalves was stronger at larger scales (>100 km), suggesting that environmental factors are more important in this host group at larger scales.
Intensity and abundance may not only be characters of a parasite species, but may also be characters of the host species. For example, inventories of trematode parasites in bivalve communities suggest that some host species generally acquire higher infection levels than others (de Montaudouin et al. 2000; Thieltges 2006). Unfortunately, with our dataset it is not possible to distinguish between the role of parasites and hosts. Not all parasites occur in all hosts and this results in incomplete designs with many missing cells for a direct comparison, e.g., a 2-way ANOVA with both parasite species and host species as factors. We were only interested in the potential repeatability of infection levels of specific parasite species in specific host species. Further inventories comparing infection levels among various crustacean and bivalve hosts and/or experimental infections are needed to disentangle the relative roles of parasite and host species.
What are the implications of these findings? First, the data show that metacercarial infections in crustaceans and bivalves occur with often high infection levels not just at single localities but over a larger spatial scale. Hence, any effects of trematode infections on their hosts can extend to many populations. This underlines the large-scale importance of parasites for host population and community dynamics (Sousa 1991; Mouritsen and Poulin 2002). Second, although there is some repeatability in intensity and abundance, particularly for metacercarial infections in crustacean hosts, local factors have their share in the observed patterns. This may become important in the course of climate change which is predicted to result in increases in infection levels in marine systems (Harvell et al. 2002; Mouritsen et al. 2005; Poulin and Mouritsen 2006). As local cercarial supply depends strongly on temperature (Poulin 2006b), encounter rates of hosts with infective stages are likely to increase with global warming, resulting in greater infection. And third, the high variation caused by local factors, particularly in metacercarial infections of bivalves, points toward the need to investigate transmission patterns in marine systems. Currently, our understanding of cercarial transmission processes in marine environments is limited. Although some factors are known to affect transmission (see above), we have no idea of the relative importance of these factors and of the interactions between them, or of the variation in their relative importance over large geographical scales. Further studies, preferably using experiments testing various factors in multivariate designs, are needed to disentangle the role of local factors on cercarial transmission processes in marine environments.
References
Arneberg P, Skorping A, Read AF (1997) Is population density a species character? Comparative analyses of the nematode parasites of mammals. Oikos 80:289–300. doi:https://doi.org/10.2307/3546597
Brown SP, De Lorgeril J, Joly C, Thomas F (2003) Field evidence for density-dependent effects in the trematode Microphallus papillorobustus in its manipulated host, Gammarus insensibilis. J Parasitol 89:668–672. doi:https://doi.org/10.1645/GE-3122
Byers JE, Blakeslee AMH, Linder E, Cooper AB, Maguire TJ (2008) Controls of spatial variation in the prevalence of trematode parasites infecting a marine snail. Ecology 89:439–451. doi:https://doi.org/10.1890/06-1036.1
Fredensborg BL, Poulin R (2005) Larval helminths in intermediate hosts: does competition early in life determine the fitness of adult parasites? Int J Parasitol 35:1061–1070. doi:https://doi.org/10.1016/j.ijpara.2005.05.005
Fredensborg BL, Mouritsen KN, Poulin R (2004) Intensity-dependent mortality of Paracalliope novizealandiae (Amphipoda: Crustacea) infected by a trematode: experimental infections and field observations. J Exp Mar Biol Ecol 311:253–265. doi:https://doi.org/10.1016/j.jembe.2004.05.011
Fredensborg BL, Mouritsen KN, Poulin R (2005) Impact of trematodes on host survival and population density in the intertidal gastropod Zeacumantus subcarinatus. Mar Ecol Prog Ser 290:109–117. doi:https://doi.org/10.3354/meps290109
Fredensborg BL, Mouritsen KN, Poulin R (2006) Relating bird host distribution and spatial heterogeneity in trematode infections in an intertidal snail: from small to large scale. Mar Biol (Berl) 149:275–283. doi:https://doi.org/10.1007/s00227-005-0184-1
Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samual MD (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162. doi:https://doi.org/10.1126/science.1063699
Kaplan AT, Halling S, Lafferty KD, Kuris AM (2008) Small estuarine fishes feed on large trematode cercariae: lab and field investigations. J Parasitol (in press)
Krasnov BR, Shenbrot GI, Khokhlova IS, Poulin R (2006) Is abundance a species attribute? An example with haematophagous ectoparasites. Oecologia 150:132–140. doi:https://doi.org/10.1007/s00442-006-0498-9
Lafferty KD (1993) Effects of parasitic castration on growth, reproduction and population dynamics of Cerithidea californica. Mar Ecol Prog Ser 96:229–237. doi:https://doi.org/10.3354/meps096229
Lafferty KD, Allesina S, Arim M, Briggs CJ, DeLeo G, Dobson AP, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546. doi:https://doi.org/10.1111/j.1461-0248.2008.01174.x
Meissner K, Bick A (1997) Population dynamics and ecoparasitological surveys of Coriophium colutator in coastal waters in the bay of Mecklenburg (southern Baltic Sea). Mar Ecol Prog Ser 29:169–179
Minchella DJ, Scott ME (1991) Parasitism: a cryptic determinant of animal community structure. Trends Ecol Evol 6:250–254. doi:https://doi.org/10.1016/0169-5347(91)90071-5
de Montaudouin X, Kisielewski I, Bachelet G, Desclaux C (2000) A census of macroparasites in an intertidal bivalve community, Arcachon Bay, France. Oceanol Acta 23:453–468
Mouritsen KN, Poulin R (2002) Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 124:S101–S117
Mouritsen KN, Poulin R (2003) The mud flat anemone–cockle association: mutualism in the intertidal zone? Oecologia 135:131–137
Mouritsen KN, Poulin R (2005) Parasites boost biodiversity and change animal community structure by trait-mediated indirect effects. Oikos 108:344–350. doi:https://doi.org/10.1111/j.0030-1299.2005.13507.x
Mouritsen KN, Tompkins DM, Poulin R (2005) Climate warming may cause a parasite-induced collapse in coastal amphipod populations. Oecologia 146:476–483. doi:https://doi.org/10.1007/s00442-005-0223-0
Poulin R (2006a) Variation in infection parameters among populations within parasite species: intrinsic properties versus local factors. Int J Parasitol 36:877–885. doi:https://doi.org/10.1016/j.ijpara.2006.02.021
Poulin R (2006b) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132:143–151. doi:https://doi.org/10.1017/S0031182005008693
Poulin R, Mouritsen KN (2003) Large-scale determinants of trematode infections in intertidal gastropods. Mar Ecol Prog Ser 254:187–198. doi:https://doi.org/10.3354/meps254187
Poulin R, Mouritsen KN (2006) Climate change, parasitism and the structure of intertidal ecosystems. J Helminthol 80:183–191. doi:https://doi.org/10.1079/JOH2006341
Skirnisson K, Galaktionov KV, Kozminsky EV (2004) Factors influencing the distribution of digenetic trematode infections in a mudsnail (Hydrobia ventrosa) population inhabiting salt marsh ponds in Iceland. J Parasitol 90:50–59. doi:https://doi.org/10.1645/GE-118R
Smith NF (2001) Spatial heterogeneity in recruitment of larval trematodes to snail intermediate hosts. Oecologia 127:115–122. doi:https://doi.org/10.1007/s004420000560
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman, New York
Sousa WP (1991) Can models of soft-sediment community structure be complete without parasites? Am Zool 31:821–830
Thieltges DW (2006a) Parasite induced summer mortality in common cockles Cerastoderma edule by the trematode Gymnophallus choledochus. Hydrobiologia 559:455–461. doi:https://doi.org/10.1007/s10750-005-1345-4
Thieltges DW (2006b) Effect of metacercarial trematode infections (Renicola roscovita) on growth in intertidal blue mussels (Mytilus edulis). Mar Ecol Prog Ser 319:129–134. doi:https://doi.org/10.3354/meps319129
Thieltges DW (2007) Habitat and transmission–effect of tidal level and upstream host density on metacercarial load in an intertidal bivalve. Parasitology 134:599–605. doi:https://doi.org/10.1017/S003118200600165X
Thieltges DW (2008) Effect of host size and exposure on metacercarial infection levels in the intertidal cockle Cerastoderma edule. J Mar Biol Assoc U K 88:613–616. doi:https://doi.org/10.1017/S0025315408001008
Thieltges DW, Reise K (2007) Spatial heterogeneity in parasite infections at different scales in an intertidal bivalve. Oecologia 150:569–581. doi:https://doi.org/10.1007/s00442-006-0557-2
Thieltges DW, Rick J (2006) Effect of temperature on emergence, survival and infectivity of cercariae of the marine trematode Renicola roscovita (Digenea: Renicolidae). Dis Aquat Organ 73:63–68. doi:https://doi.org/10.3354/dao073063
Thieltges DW, Krakau M, Andresen H, Fottner S, Reise K (2006) Macroparasite community in molluscs of a tidal basin in the Wadden Sea. Helgol Mar Res 60:307–316
Thieltges DW, Donas-Botto Bordalo M, Cabalero Hernández A, Prinz K, Jensen KT (2008a) Ambient fauna impairs parasite transmission in a marine parasite-host system. Parasitology 135:1111–1116
Thieltges DW, Jensen KT, Poulin R (2008b) The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135:407–426
Thomas F, Renaud F, Guegan J-F (eds) (2005) Parasitism and ecosystems. Oxford University Press, Oxford, New York
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AMH (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104:9335–9339. doi:https://doi.org/10.1073/pnas.0700062104
Acknowledgments
DWT acknowledges support by a fellowship from the German Research Foundation (DFG) (Th 1361/1-1). BLF acknowledges support by a University of Texas-Pan American Faculty Research Grant (135BIOL04). Sincere thanks to the anonymous reviewers who helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thieltges, D.W., Fredensborg, B.L. & Poulin, R. Geographical variation in metacercarial infection levels in marine invertebrate hosts: parasite species character versus local factors. Mar Biol 156, 983–990 (2009). https://doi.org/10.1007/s00227-009-1142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1142-0