Abstract
Population density is a fundamental property of a species and yet it varies among populations of the same species. The variation comes from the interplay between intrinsic features of a species that tend to produce repeatable density values across all populations of the same species and extrinsic environmental factors that differ among localities and thus tend to produce spatial variation in density. Is inter-population variation in density too large for density to be considered a true species character? We addressed this question using data on abundance (number of parasites per individual host, i.e. equivalent to density) of fleas ectoparasitic on small mammals. The data included samples of 548 flea populations, representing 145 flea species and obtained from 48 different geographical regions. Abundances of the same flea species on the same host species, but in different regions, were more similar to each other than expected by chance, and varied significantly among flea species, with 46% of the variation among samples accounted by differences between flea species. Thus, estimates of abundance are repeatable within the same flea species. The same repeatability was also observed, but to a lesser extent, across flea genera, tribes and subfamilies. Independently of the identity of the flea species, abundance values recorded on the same host species, or in the same geographical region, also showed significant statistical repeatability, though not nearly as strong as that associated with abundance values from the same flea species. There were also no strong indications that regional differences in abiotic variables were an important determinant of variation in abundance of a given flea species on a given host species. Abundance thus appears to be a true species trait in fleas, although it varies somewhat within bounds set by species-specific life history traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding of the processes that determine the population density of organisms is a central question in ecology. It is commonly accepted that the density (abundance per unit area) of a species in a location results from the interplay between the intrinsic properties of that species (Hughes et al. 2000; Blackburn and Gaston 2001; Lopez-Sepulcre and Kokko 2005) and the extrinsic, both biotic and abiotic, properties of the local habitat (Rosenzweig 1981; Morris 1987; Newton 1998). Consequently, because it results from interactions among a variety of factors, the predictability of the density level of any given species is often low, causing problems for conservationists and pest managers (e.g. Beissinger and Westphal 1998; Ludwig 1999; Fieberg and Ellner 2000). One of the likely reasons for this low predictability is the fact that the density of a species is determined simultaneously by extrinsic factors generating variation among populations of this species, and by intrinsic factors promoting among-population stability (= repeatability) in density.
Variability and repeatability of species density have been investigated mainly in free-living organisms (Begon et al. 2005). Parasites, in contrast, have received less attention. However, parasite populations represent convenient models to study intraspecific patterns of population parameters. In particular, this is because of the relative ease of obtaining replicated samples (e.g. host individuals, host populations or host species). Furthermore, the elucidation of spatial patterns of parasite population parameters is crucially important for epidemiology, veterinary medicine and pest management.
High intraspecific variation in the population parameters of parasites, such as their intensity of infestation, prevalence and abundance, is well documented. For example, the abundance of parasites is strongly dependent on the abundance of their host (see review in Anderson and May 1978; Arneberg et al. 1998; Tompkins et al. 2001), which, in turn, is spatially variable. This relationship has been documented in a variety of host–parasite systems (Grenfell and Dobson 1995). Moreover, the relationship between parasite and host abundance varies from being positive (e.g. Krasnov et al. 2002a) to being negative (e.g. Stanko et al. 2006) among different species of the same parasite taxa depending on species-specific reproductive rate and seasonality. In addition, the dependence of survival and, consequently, abundance of parasites on spatially variable abiotic factors has been reported for both endo- (Galaktionov 1996) and ectoparasites (Metzger and Rust 1997). However, in spite of the strong dependence of parasite population parameters on extrinsic factors and, therefore, the expected spatial variation of these parameters, species-specific features of parasites such as body size and egg production could constrain this variation (Poulin 1999). Indeed, Arneberg et al. (1997), studying nematodes parasitic in mammals, have demonstrated that intensity of infection as well as abundance were repeatable within nematode species, i.e. were less variable within than among species, suggesting that the levels of intensity of infection and abundance are “true” attributes of a nematode species. The only other study of intraspecific variability versus stability of parasite population parameters was carried out on different taxa of metazoan parasite species of Canadian freshwater fishes (Poulin 2006). Again, prevalence, intensity of infection and abundance values from different populations of the same parasite species were more similar to each other, and more different from those of other species, than expected by chance alone. These results suggest that intensity of infestation and abundance are real characters of parasite species, supporting the view that the biological features of parasite species can potentially override local environmental conditions in driving parasite population dynamics.
Periodic (e.g. fleas) and temporary (e.g. tabanids) ectoparasites of terrestrial hosts may be much more strongly influenced by their off-host environment than both the endo- or permanent ectoparasites studied by Arneberg et al. (1997) and Poulin (2006). For example, survival and abundance of fleas are strongly affected by the microclimate of the host’s nest/burrow (Krasnov et al. 2001, 2002b), host identity (Krasnov et al. 1998) and the total abundance and diversity of co-occurring flea species (Krasnov et al. 2005a). This suggests that the patterns reported by Arneberg et al. (1997) and Poulin (2006) may not be valid for terrestrial arthropod ectoparasites. However, another abundance-related parameter, the relationship between mean abundance and its variance, appears to be a true flea species character (Krasnov et al. 2006). This apparent contradiction can only be resolved by testing whether a key population parameter, such as abundance, of terrestrial periodic arthropod parasites, such as fleas, is a true species attribute or, alternatively, whether it is determined by extrinsic factors such as host species identity or the local environment.
The aim of this study was to test if the level of abundance of fleas parasitic on small mammals from 48 different geographic regions is species-specific or if it is determined mainly by host identity and/or parameters of the abiotic environment. We evaluated the repeatability of estimates of flea abundance across populations of the same flea species to determine if the abundance is repeatable within flea species; i.e. if the values of abundance are more similar among populations of the same flea species than among different flea species. We also tested the repeatability of flea abundance within host species and within regions. In addition, we searched for correlations between abiotic features of a region and flea population abundance, separately for several flea species. Our prediction was that if population abundance is a spatially repeatable feature, then the effect of local climatic factors on abundance should be weak.
Methods
Data were obtained from published surveys that reported flea distribution and abundance on small mammals (Insectivora, Rodentia and Lagomorpha) in 48 different regions (see Electronic Supplementary Material, Table S1). These sources provided data on the number of individuals of a particular flea species found on a number of individuals of a particular host species. Mammal sampling as reported in the original sources was done either by live- or by snap-trapping, or by hunting. Most sources reported that mammals were examined immediately upon capture/kill. However, there was no information about the time lag between capture/kill and parasitological examination in some sources. Nevertheless, we assumed that this time lag was too short to allow fleas to leave the host body because the aim of all studies was a survey of ectoparasite assemblages. In addition, the sources that provided information of the time of mammal sampling reported that sampling covered either the entire spectrum of seasons or most parts of the year. This suggests that the effect of seasonality on flea abundance, and thus on the results of this study, was negligible (if any).
We cross-checked the species lists with the catalogues of Lewis and Lewis (1990) and Medvedev et al. (2005) to resolve cases of synonymy. Initially, we listed all flea–mammal combinations for each region. Then, we excluded those mammal species for which fewer than 15 individuals have been examined per region, because estimates of flea abundance could be inaccurate for such small samples. We included in the analysis only those flea species that were recorded on the same host species in at least two regions. The same flea species was often recorded from several different host species within and/or among regions. Abundance values for the same flea species but from several different host species are not comparable. Consequently, we focused on the principal host species for each flea species. The parasite’s principal host is the host that supports the largest part of a parasite population. It should be noted, however, that in some cases a secondary host may have fewer parasites per host, but there might be greater numbers of the secondary hosts and, therefore, the secondary host may support the larger part of the parasite population. Nevertheless, the principal host is identified here either as the host species that supports a given parasite species across the largest part of its geographic range, or as the host species on which the parasite attains its highest abundance (Dogiel et al. 1961; Poulin 2005). Indeed, a comparative analysis across 1,334 host–flea species associations demonstrated that the abundance of a particular flea species on its principal host species was on average more than twice that on its auxiliary hosts (Krasnov et al. 2004a).
We used both these approaches. First, we identified the principal host as the host on which a flea occurred most frequently calculated across all regions from which this flea has been reported. Only records of the flea on the principal host were used in the analyses. Second, we identified the principal host as the mammal species on which the flea achieved its highest average abundance across all populations sampled. We then repeated all analyses using records of fleas on these principal hosts. This second run of the analyses provided the same results as the previous one. Consequently, in this paper we report only the results of analyses performed on the dataset where the principal host of a flea species was identified as the host on which a flea occurred most frequently across all regions inhabited by this flea. The principal host species for a flea genus, tribe, subfamily and family were identified in the same way. In total, we used data on 548 flea samples, representing 1,211,858 fleas of 145 species collected from 379,302 individual mammals of 48 species. In 108 of 145 flea species in the dataset, the principal host species identified by both methods was the same host species.
We used the mean number of fleas per individual host of a given species as a measure of flea abundance. Other measurements of infestation level, such as prevalence and intensity of flea infestation, were not available for most of the regions considered. To determine whether flea abundance is a true flea species attribute, i.e. a parameter that varies less among populations of the same flea species than among different flea species, we performed (1) correlation analyses between the values of flea abundance and (2) a repeatability analysis following that of Arneberg et al. (1997). In the former analysis, we correlated the lowest abundance recorded for a given flea species with all other values of abundance recorded in other regions for this species across all flea species. If abundances of the same flea species are consistent with each other across different regions, then a positive correlation is expected between the lowest value and other values of abundance.
In the repeatability analyses, we analysed the variation in flea abundance per host individual by a one-way ANOVA in which flea species was the independent factor, using only flea species for which at least two samples were available. A significant effect of flea species would indicate that the abundance is repeatable within flea species, i.e. that abundance values for the same flea species from different geographic regions are more similar to each other than to values from other flea species. We estimated the proportion of the total variance originating from differences among flea species, as opposed to within species, following Sokal and Rohlf (1995). We first carried out the repeatability analysis using all flea species in the dataset. Then, we carried out the repeatability analysis across flea genera (48 genera), tribes (20 tribes), subfamilies (13 subfamilies) and families (4 families) to see if flea abundance is also a trait characteristic of higher order taxonomic ranks. To assess whether flea abundance is also determined by host identity or by a complex of environmental conditions, we performed the repeatability analyses using host species or region (as a proxy for geographic differences in a set of environmental conditions) instead of flea species as the single factor. A significant effect of host species or region would indicate that the flea abundances are repeatable within host species or region, respectively.
Finally, to understand the possible causes of geographic variation in flea abundance within a flea–host association, we calculated the parameters that characterised the abiotic environment for each region and for each of flea species with at least six different regional records on the same host species. Environmental parameters were latitude, mean surface annual temperature, mean surface air temperature in January, mean surface air temperature in July, mean annual precipitation and mean altitude. These variables were calculated for each region using 30′ grid data (Kineman et al. 2000). Because most of the variables were strongly correlated with each other (r =−0.83–0.95, P<0.001 for all), we substituted them with the scores calculated from the principal component analysis of all these variables. The resulting two “environmental factors” explained 79.2% of the variance (56.4 and 22.8%, respectively) and their eigenvalues were 3.38 and 1.38, respectively. The first environmental factor (EF1) was strongly positively correlated with mean annual, mean July and mean January temperatures (factor loadings were 0.97, 0.78 and 0.90, respectively) and strongly negatively correlated with latitude (factor loading was −0.91), whereas the second environmental factor (EF2) was strongly positively correlated with mean altitude (factor loading was 0.76) and strongly negatively correlated with mean annual precipitation (factor loading was −0.80). For each flea species, we regressed measures of abundance on the principal host in a region against scores of these two principal components using multiple stepwise (forward procedure) regression.
Results
Each of the 145 flea species in the dataset was recorded from 2 to 18 regions. Twenty-four species were found on the same host species in at least six regions (see Electronic Supplementary Material, Table S2).
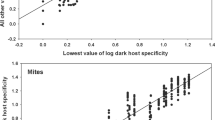
A strong positive correlation was found between the lowest abundances and all other abundance values across flea species (r =0.67, P<0.0001). In other words, different fleas demonstrated a relatively narrow range of abundances when exploiting the same host species in different regions (Fig. 1).
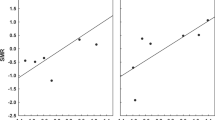
The repeatability analysis for the 145 flea species recorded in least two regions demonstrated that mean flea abundance per host individual can be considered as a flea species character (Fig. 2a). Abundances of the same flea species on the same host species are more similar to each other than expected by chance, and vary significantly among flea species (F 144,403=4.2, P<0.0001), with 45.9% of the variation among samples accounted by differences between flea species. Thus, estimates of abundance are repeatable within the same flea species. Furthermore, flea abundances on the same host species were also repeatable across flea genera, tribes and subfamilies, but were not repeatable across flea families (Table 1). In addition, the percentage of the variation among samples accounted by differences among flea taxa, as opposed to within-taxa, was lower for genera compared to species, for tribes compared to genera and for subfamilies compared to tribes (Table 1).
a Rank plot of flea abundance. The 145 flea species recorded in at least two regions are ranked according to their mean log-transformed abundance values, with rank 1 given to the species with the lowest mean abundance; all sample estimates are plotted for each species. If variation is small within compared to between flea species, we expect the points to fall in a region of the plot stretching from the lower left to the upper right corner, with few or no points in either the upper left or lower right corner. b Rank plot of flea abundance across 48 host species. c Rank plot of flea abundance across 48 regions. Values at tick marks of y-axis are back-transformed
The repeatability analyses using host species and regions instead of flea species as the main factor demonstrated significant repeatability of flea abundance independent of flea species both among host species and among regions (Table 2). However, the proportions of the variance accounted by differences among host species and among regions, as opposed to within-host and within-region, respectively, were lower than that accounted by differences among flea species, being 24.1 and 12.7%, respectively (Table 2; Fig. 2b, c).
Despite a significant, albeit weak, repeatability of flea abundances across regions, in 19 of 24 flea species that were recorded on the same host in at least six regions, no correlation was found between abundance and either of the two environmental composite factors (r 2=0.07–0.61, F=0.1–3.6, n=6–18; P>0.05 for all). However, in the other five species there was a correlation between abundance and one of the environmental composites (Table 3). The abundance of Amalaraeus penicilliger correlated with scores of EF2, increasing with an increase in the mean altitude and a decrease in the mean annual precipitation of a region. The abundance of the remaining four species correlated with scores of EF1 (increase in air temperature and decrease of latitude) (Table 3). However, the direction of this correlation varied among species, being either positive or negative (Table 3).
Discussion
The results of this study demonstrate that patterns found for mammalian endoparasites (Arneberg et al. 1997) and parasites of fish (Poulin 2006) are also valid for mammalian ectoparasites (at least for those included in this analysis) despite their greater sensitivity to external factors. Flea abundance appeared to be repeatable among populations of the same species exploiting the same host, albeit it varied to some degree and, thus, can be considered as a true flea species character. Furthermore, abundance can also be considered as an attribute characteristic of a flea genus, tribe or subfamily, but not family. In addition, flea abundance can be viewed to a lesser extent as a property of a host species and a property of a region. In other words, some host species and some locations are characterised by higher flea abundance than other hosts or locations.
Repeatability of abundance within flea species implies that some species-specific life history traits determine the limits of abundance. Lower limits of flea abundance can be affected by species-specific mating systems and/or the relationship between mating and blood feeding, whereas upper limits of abundance can be determined by species-specific reproductive outputs, generation times, preferences for blood-sucking on a specific body part of a host and/or the ability of both imago and larvae to withstand crowding. Indeed, some flea species can mate without a bloodmeal (e.g. Nosopsyllus fasciatus; Iqbal and Humphries 1974), whereas in other species an unfed male is unable to inseminate a female (e.g. Ctenocephalides felis; Dean and Meola 1997 and Leptopsylla segnis; Krampitz 1980). The necessity to feed prior to mating may possibly increase the number of fleas on a host body at any given time. For example, on Mus musculus, the average abundance of L. segnis is higher than that of N. fasciatus (0.75±0.31 vs 0.04±0.01 fleas per individual host). Flea species also differ in the number of mating events necessary to lay viable eggs (Hsu and Wu 2000), which can affect their mean abundance.
Another species-specific mating pattern related to the abundance of fleas on a host is the time that fleas spend either on or off the host body and mating on or off the host (“body” versus “nest” fleas; see Ioff 1941; Smit 1962; Marshall 1981; Vatschenok 1988). As a result, all else being equal, abundance of the “body” fleas should be higher than that of the “nest” fleas. Indeed, the mean abundance of the “body” flea, Citellophilus tesquorum, was higher than that of the “nest” flea, Neopsylla setosa, on the same host, Spermophilus undulatus (2.41±0.60 vs 0.43±0.08 fleas per individual host). Nevertheless, sampling of “nest” fleas from host bodies appeared to be just as reliable for the evaluation of flea abundance as sampling from host nests (Krasnov et al. 2004b).
Flea fecundity varies drastically among flea species (Marshall 1981; Vatschenok 1988; Krasnov et al. 2002c). For example, two fleas characteristic of M. musculus, Nosopsyllus mokrzeckyi and L. segnis, demonstrate sharp differences in fecundity (1–2 clutches per day with 2–3 eggs per clutch versus 2–3 clutches per day with 4 eggs per clutch, respectively; Kosminskyi 1965). This difference in fecundity is paralleled by a difference in mean abundance (0.14±0.08 vs 0.75±0.31, respectively). As flea species also vary in their generation time (Marshall 1981; Vatschenok 1988), variation in frequency and size of clutches undoubtedly causes variation in the lifetime fecundity (Marshall 1981) which, in turn, may be manifested by interspecific variation in mean abundance. Some fleas prefer to take a bloodmeal from a specific area on a host body (Linsdale and Davis 1956; Hsu et al. 2002), whereas other species are distributed evenly over it (Marshall 1981). Site-specific fleas might be more prone to crowding compared with less site-specific species and, thus, may achieve lower abundance. Finally, species-specific levels of intraspecific competition for food could also determine the mean abundance of a flea species, although there are no empirical data supporting this explanation.
Repeatability of flea abundance within flea genera, tribes and subfamilies suggests that limits of abundance as well as intrinsic properties of fleas that determine these limits are phylogenetically constrained. In other words, a relatively narrow, species-specific range of inter-population variation in abundance is the result, at least in part, of natural selection. Consequently, the observed range of abundance for a given flea species has not only evolved for some particular ecological reason, but it seems also to be nested within the flea phylogeny and, thus, it can be inherited by descent. This advocates the necessity of using phylogenetic information in comparative analyses of abundance (e.g. Stanko et al. 2002; Gaston and Blackburn 2003).
The repeatability of flea abundance within the same host species demonstrates that this parameter can be also seen as a property of the host. Indeed, some host species always harbour more fleas than other host species (Krasnov et al. 1998). Furthermore, Arneberg et al. (1997) reported the abundance of nematodes to be repeatable within mammalian species. In other words, independently of nematode species, some mammal species have many nematodes per individual, whereas other mammals have only a few nematodes per individual. However, the repeatability of nematode abundance among host species was weaker than that among nematode species (18% of the variation in abundance associated with differences among host species versus 36% associated with differences among nematode species; Arneberg et al. 1997). The results of our study provided a very similar ratio between percentages of the variation of flea abundance associated with differences among host species and among flea species (21.4% vs 45.9%, respectively).
The repeatability of flea abundance within host species suggests that some host properties constrain to some extent the number of fleas harboured by an individual. These constraints can be related to processes on the host body that affect adult fleas and/or to processes within a host burrow or nest that affect pre-imaginal fleas. The abundance of adult fleas can be limited by host body features, host immune defence and host antiparasitic behaviour. Host body size can determine the number of parasites per host, as more space is available for multiple parasite individuals on larger hosts (e.g. Morand and Guégan 2000, but see Krasnov et al. 2005a). Host species also differ in the level of their immune and behavioural defence against parasites (Klein and Nelson 1998; Mooring et al. 2000). A host species with lower immunocompetence and/or a more limited ability to self-groom can be exploited by a higher number of fleas than a host species with higher immunocompetence and/or higher self-grooming efficiency, all else being equal. In addition, the fecundity of fleas depends on which host species they exploit (e.g. Krasnov et al. 2002c, 2004c) which might, in turn, determine host-specific levels of flea abundance.
Species-specific differences in sheltering behaviour and burrow/nest structure (depth, length of tunnels, nest composition, defecation in or outside burrow, etc.) can determine the amount of organic matter in the burrow/nest substrate (food for flea larvae) as well as burrow microclimate (Kucheruk 1983). The amount of organic matter and microclimatic parameters affect flea abundance (Krasnov et al. 2001, 2005b) which is likely reflected in among-host differences in the abundance of adult fleas.
Burrow structure can differ not only among host species but also within host species although among different locations (Shenbrot et al. 2002). On the other hand, burrows of different host species in the same location can be characterised by similar microclimate conditions due to the same macroclimate (Kucheruk 1983). This can lead to among-location differences (as opposed to within-location similarities among host species) in flea abundance and, together with among-location climatic differences, can, at least partly, explain the repeatability of within-region flea abundances found in this study. However, the lack of a clear relationship between flea abundance on a given host species and regional climatic parameters found for most species in this study suggests that abiotic characteristics of a location play only a minor role in the observed pattern of within-region repeatability of flea abundance. From the point of view of each flea species, the biotic components of a location include not only the host species but also the co-occurring assemblage of other flea species. Recently, we demonstrated that the abundance of a given flea species (1) correlates positively with the total abundance of all other co-occurring flea species in the community and (2) correlates negatively with the diversity of the flea community (Krasnov et al. 2005a). The latter is also related to the diversity of host species in a location (Krasnov et al. 2004d). Consequently, the repeatability of flea abundance within regions as opposed to among regions can be explained by among-region differences in a set of biotic and abiotic factors. Nevertheless, only a small portion (12.7%) of population variation in abundance was associated with among-region differences.
In conclusion, our results suggest that the abundance of a flea species parasitic on a small mammalian host, although variable within some limits, is (1) a true species character, (2) to some extent is determined by host species identity and (3) to a small extent is affected by local biotic and abiotic conditions. This supports the ideas of Arneberg et al. (1997) and Poulin (2006) that the biological attributes of parasite species are primary determinants of parasite dynamics compared with characteristics of hosts and of the local environment.
References
Anderson RM, May RM (1978) Regulation and stability of host–parasite population interactions. I. Regulatory processes. J Anim Ecol 47:219–247
Arneberg P, Skorping A, Read AF (1997) Is population density a species character? Comparative analyses of the nematode parasites of mammals. Oikos 80:289–300
Arneberg P, Skorping A, Grenfell B, Read AF (1998) Host densities as determinants of abundance in parasite communities. Proc R Soc Lond B 265:1283–1289
Begon M, Townsend CR, Harper JL (2005) Ecology: from individuals to ecosystems, 4th edn. Blackwell Publishing, London
Beissinger SR, Westphal MI (1998) On the use of demographic models of population viability in endangered species management. J Wildl Manag 62:821–841
Blackburn TM, Gaston KJ (2001) Linking patterns in macroecology. J Anim Ecol 70:338–352
Dean SR, Meola RW (1997) Effect of juvenile hormone and juvenile hormone mimics on sperm transfer from the testes of the male cat flea (Siphonaptera: Pulicidae). J Med Entomol 34:485–488
Dogiel VA, Petrushevski GK, Polyanski YI (1961) Parasitology of fishes. Oliver & Boyd, Edinburgh
Fieberg J, Ellner SP (2000) When is it meaningful to estimate an extinction probability? Ecology 81:2040–2047
Galaktionov KV (1996) Life cycles and distribution of seabird helminths in Arctic and subarctic regions. Bull Scand Soc Parasitol 6:31–49
Gaston KJ, Blackburn TM (2003) Dispersal and the interspecific abundance–occupancy relationship in British birds. Glob Ecol Biogeogr 12:373–379
Grenfell BT, Dobson AP (eds) (1995) Ecology of infectious diseases in natural populations. Cambridge University Press, Cambridge
Hsu MH, Wu WJ (2000) Effects of multiple mating on females reproductive output in the cat flea (Siphonaptera: Pulicidae). J Med Entomol 37:828–834
Hsu MH, Hsu TC, Wu WJ (2002) Distribution of cat fleas (Siphonaptera: Pulicidae) on the cat. J Med Entomol 39:685–688
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81:2241–2249
Ioff IG (1941) Ecology of fleas in relevance to their medical importance. Ordzhonikidze Regional Publisher, Piatigorsk (in Russian)
Iqbal QJ, Humphries DA (1974) The mating behaviour of the rat flea Nosopsyllus fasciatus Bosc. Pak J Zool 8:39–41
Kineman JJ, Hastings DA, Ohrenschall MA, Colby J, Schoolcraft DC, Klaus J, Knight J, Krager L, Hayes P, Oloughlin K, Dunbar P, Ikleman J, Anderson C, Burland J, Dietz J, Fisher H, Hannaughan A, Kelly M, Boyle S, Callaghan M, Delamana S, Di L, Gomolski K, Green D, Hochberg S, Holquist W, Johnson G, Lewis L, Locher A, Mealey A, Middleton L, Mellon D, Nigro L, Panskowitz J, Racey S, Roake B, Ross J, Row L, Schacter J, Weschler P (eds) (2000) Global ecosystems database version II: database, user’s guide, and dataset documentation. US Department of Commerce, National Oceanic and Atmospheric Administration, National Geophysical Data Center. http://www.ngdc.noaa.gov/seg/eco/cdroms/gedii_a/go.htm
Klein SL, Nelson RJ (1998) Adaptive immune responses are linked to the mating system of arvicoline rodents. Am Nat 151:59–67
Kosminskyi RB (1965) Feeding and reproduction of fleas of house mice under natural and experimental conditions. Zool Zh 44:1372–1375 (in Russian)
Krampitz HE (1980) Host preference, sessility and mating behaviour of Leptopsylla segnis reared in captivity. In: Traub R, Starcke H (eds) Fleas. Proceedings of the international conference on fleas, Ashton Wold, Peterbotough, UK, 21–25 June 1977. A.A. Balkema, Rotterdam, pp. 93–172
Krasnov BR, Shenbrot GI, Medvedev SG, Khokhlova IS, Vatschenok VS (1998) Habitat-dependence of a parasite–host relationship: flea assemblages in two gerbil species of the Negev Desert. J Med Entomol 35:303–313
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2001) The effect of temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J Med Entomol 38:629–637
Krasnov BR, Khokhlova IS, Shenbrot GI (2002a) The effect of host density on ectoparasite distribution: an example with a desert rodent parasitized by fleas. Ecology 83:164–175
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2002b) The effect of substrate on survival and development of two species of desert fleas (Siphonaptera: Pulicidae). Parasite 9:135–142
Krasnov BR, Khokhlova IS, Oguzoglu I, Burdelova NV (2002c) Host discrimination by two desert fleas using an odour cue. Anim Behav 64:33–40
Krasnov BR, Shenbrot GI, Khokhlova IS, Poulin R (2004a) Relationships between parasite abundance and the taxonomic distance among a parasite’s host species: an example with fleas parasitic on small mammals. Int J Parasitol 34:1289–1297
Krasnov BR, Shenbrot GI, Khokhlova IS (2004b) Sampling fleas: the reliability of host infestation data. Med Vet Entomol 18:232–240
Krasnov BR, Khokhlova IS, Burdelova NV, Mirzoyan NS, Degen AA (2004c) Fitness consequences of density-dependent host selection in ectoparasites: testing reproductive patterns predicted by isodar theory in fleas parasitizing rodents. J Anim Ecol 73:815–820
Krasnov BR, Shenbrot GI, Khokhlova IS, Degen AA (2004d) Relationship between host diversity and parasite diversity: flea assemblages on small mammals. J Biogeogr 31:1857–1866
Krasnov BR, Mouillot D, Shenbrot GI, Khokhlova IS, Poulin R (2005a) Abundance patterns and coexistence processes in communities of fleas parasitic on small mammals. Ecography 28:453–464
Krasnov BR, Burdelova NV, Khokhlova IS, Shenbrot GI, Degen AA (2005b) Pre-imaginal interspecific competition in two flea species parasitic on the same rodent host. Ecol Entomol 30:146–155
Krasnov BR, Stanko M, Miklisova D, Morand S (2006) Host specificity, parasite community size and the relation between abundance and its variance. Evol Ecol 20:75–91
Kucheruk VV (1983) Mammal burrows: their structure, topology and use. Fauna Ecol Rodents 15:5–54 (in Russian)
Lewis RE, Lewis JH (1990) An annotated checklist of the fleas (Siphonaptera) of the Middle East. Fauna Saudi Arabia 11:251-276
Linsdale JM, Davis BS (1956) Taxonomic appraisal and occurrence of fleas at the Hastings Reservation in Central California. Univ Calif Publ Zool 54:293–370
Lopez-Sepulcre A, Kokko H (2005) Territorial defense, territory size, and population regulation. Am Nat 166:317–329
Ludwig D (1999) Is it meaningful to estimate a probability of extinction? Ecology 80:298–310
Marshall AG (1981) The ecology of ectoparasite insects. Academic, London
Medvedev SG, Lobanov AL, Lyanguzov IA (2005) World database of fleas (Nov 2004 version). In: Bisby FA, Ruggiero MA, Wilson KL, Cachuela-Palacio M, Kimani SW, Roskov YR, Soulier-Perkins A, van Hertum J (eds) Species 2000 and ITIS catalogue of life: 2005 annual checklist. Species 2000, CD-ROM
Metzger ME, Rust MK (1997) Effect of temperature on cat flea (Siphonaptera: Pulicidae) development and overwintering. J Med Entomol 34:173–178
Mooring MS, Benjamin JE, Harte CR, Herzog NB (2000) Testing the interspecific body size principle in ungulates: the smaller they come, the harder they groom. Anim Behav 60:35–45
Morand S, Guégan J-F (2000) Distribution and abundance of parasite nematodes: ecological specialization, phylogenetic constraints or simply epidemiology? Oikos 88:563–573
Morris DW (1987) Ecological scale and habitat use. Ecology 68:362–369
Newton I (1998) Population limitation in birds. Academic, London
Poulin R (1999) Body size vs abundance among parasite species: positive relationships? Ecography 22:246–250
Poulin R (2005) Relative infection levels and taxonomic distances among the host species used by a parasite: insights into parasite specialization. Parasitology 130:109–115
Poulin R (2006) Variation in infection parameters among populations within parasite species: intrinsic properties versus local factors. Int J Parasitol 36:877–885
Rosenzweig ML (1981) A theory of habitat selection. Ecology 62:327–335
Shenbrot GI, Krasnov BR, Khokhlova IS, Demidova T, Fielden LJ (2002) Habitat-dependent differences in architecture and microclimate of the Sundevall’s jird (Meriones crassus) burrows in the Negev Desert, Israel. J Arid Environ 51:265–279
Smit FGAM (1962) Siphonaptera collected from moles and their nests at Wilp, Netherlands, by Jhr. W. C. van Heurn. Tijdschr Entomol 105:29–44
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Stanko M, Miklisova D, Gouy de Bellocq J, Morand S (2002) Mammal density and patterns of ectoparasite species richness and abundance. Oecologia 131:289–295
Stanko M, Krasnov BR, Morand S (2006) Relationship between host density and parasite distribution: inferring regulating mechanisms from census data. J Anim Ecol 75:575–583
Tompkins DM, Dobson AP, Arneberg P, Begon ME, Cattadori IM, Greenman JV, Heesterbeek JAP, Hudson PJ, Newborn D, Pugliese A, Rizzoli AP, Rosa R, Rosso F, Wilson K (2001) Parasites and host population dynamics. In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbiik H, Dobson AP (eds) The ecology of wildlife diseases. Oxford University Press, Oxford, pp. 45–62
Vatschenok VS (1988) Fleas—vectors of pathogens causing diseases in humans and animals. Nauka Publishing House, Leningrad (in Russian)
Acknowledgements
We thank two anonymous referees for their helpful comments on an earlier version of the manuscript. This study was partly supported by the Israel Science Foundation (grant no. 249/04 to B.R.K. and I.S.K.). This is publication no. 536 of the Mitrani Department of Desert Ecology and no. 213 of the Ramon Science Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Krasnov, B.R., Shenbrot, G.I., Khokhlova, I.S. et al. Is abundance a species attribute? An example with haematophagous ectoparasites. Oecologia 150, 132–140 (2006). https://doi.org/10.1007/s00442-006-0498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0498-9