Abstract
Information on the distribution and abundance patterns of trematodes are essential to reveal the ecology of host–parasite interactions. The Western Ghats of India, a biodiversity hotspot, is rich in freshwater fish diversity and endemism. Though there are several studies on various other aspects of fish ecology, studies on their parasitic fauna is meager. The objective of the present study is to explore the distribution and infection patterns of metacercariae of five species of trematodes in the freshwater fishes, Haludaria fasciata and Pseudosphromenus cupanus. The infection parameters were analyzed for each host and CART model was applied to analyze the environmental factors affecting parasite distribution patterns. All species of metacercariae showed an over-dispersed aggregate distributions. The classification tree models indicated that among the environmental factors considered, differences in host locality was the most influential factor in both fishes, followed at a greater distance by the factor seasonality. The parasite communities exhibited temporal and spatial differences in the infection pattern in response to seasonal and locational variations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater fishes are hosts to taxonomically diverse trematode parasites as both intermediate and definitive hosts. In the complex life cycles of helminth parasites, intermediate hosts often serve mainly as vehicles whose purpose is to take the parasite to their definitive hosts (Lafferty 1999). These parasite communities exhibit temporal and spatial changes in response to seasonal and locational variations in several biotic and abiotic environmental factors; these variations can be reflected in parasite species composition (Holmes 1987; Poulin 1998; Thieltges et al. 2011). The Western Ghats region of India, one of the biodiversity hotspots, is rich in freshwater fish diversity and endemism (Dahanukar et al. 2004, 2011; Raghaven et al. 2013). The melon barb, Haludaria fasciata (Jerdon, 1849) is an endemic freshwater fish inhabiting the rivers flowing through the Western Ghats (Chakraborty et al. 2017) and the spike tail paradise fish, Pseudosphromenus cupanus (Cuvier, 1831) contributes to the thriving aquarium industry of the Western Ghats regions (Raghaven et al. 2013).
It is essential to explore the parasite fauna and their population dynamics to reveal their effects on hosts such as: (a) production of a metabolic cost using host resources; (b) increasing the probability of mortality due to direct serious pathology, either by favoring new parasitic infections or by weakening the organism; (c) decreasing the reproductive rate and (d) causing changes in host behavior, including migratory movements (Mangel 2006; Poulin et al. 2012). Furthermore, knowledge on the ecological role of parasites and their biodiversity are essential to understand the functioning of any ecosystem. Essential data on the knowledge of the population parameters of parasites, in the host-parasite systems, as well as their spatial and temporal characteristics are scanty.
Study on the distribution and abundance patterns in nature and the causes and factors that determine them occupies a central role in the science of ecology (MacArthur 1972; Kingsland 1995; Krebs 2001). Knowledge on the shape of the frequency distributions of the variables under study in ecology not only serves as a statistical or phenomenological description of an event or model, but also informs us about the type of processes that cause it (Marquet 2009). In this way, the essential information that explains the range and the spatial pattern of distribution, the environmental factors that determine them, the dynamics of populations (e.g. of parasites and their hosts), epidemiological processes in the case of parasites, driving forces in evolutionary ecology, and finally the sampling strategy in data collection can be obtained (Li and Hsü 1951; Anderson and May 1978; Lester 1984; Krebs 1989; Schmidt and Roberts 2000; Southwood and Henderson 2000; Rosa and Puglieses 2002).
Parasites in general show an aggregate distribution among their hosts, so that heterogeneity in the number of parasites per host is the norm rather than the exception (Crofton 1971; Shaw and Dobson 1995; Poulin 2007). These distribution patterns are characterized by presenting statistical distributions with only positive values (count data), clearly skewed to the right, having a variance greater than the mean and the latter being non-constant, increasing as the mean value becomes greater (Shaw and Dobson 1995; Poulin and Morand 2000). One of the statistical distributions that best fits these characteristics is the negative binomial distribution, which has been widely used to represent over-dispersion and describe the distribution patterns of parasites (Poulin 2007). All these features are counterproductive for the application of classical linear statistical models based on the normal distribution (Dunn and Smyth 2018). Among the most used methods to describe and to model the infection patterns of parasites is the application of generalized linear models, GLMs, (McCullagh and Nelder 1989; Wilson and Grenfell 1997; Alexander 2012) and their extension when there is an abundance of zeros: the zero inflated models (O’hara and Kotze 2010; Chipeta et al. 2014; Rhodes 2015). However, when studying the influence of environmental or other factors on the levels of infestation observed, the variable selection process can be difficult and model-specific (Qian 2017). The classification and regression tree models (CART Breiman et al. 1984) and their multivariate extension (De'Ath 2002) are very useful for identifying variables that contribute to the response variable and for prediction and classification (De’Ath 2002; Qian 2017). Therefore, CART models have been applied successfully in various fields of ecology (De’Ath and Fabricius 2000; Roff 2006) including the studies on the influence of environmental factors on parasite infection levels (Vignon and Sasal 2010; Gazzinelli et al. 2017; Rossiter and Davidson 2018).
In the present study, the main objectives were to know the distribution pattern and the infection parameters of different species of metacercariae in H. fasciata and P. cupanus. CART models were used for study the influence of environmental factors such as the seasonality and difference in locality, on the infection patterns of metacercariae of melon barb and spike tail paradise fish. Special emphasis was given to the trematodes that showed a greater presence, such as Acanthostomum burminis (Bhalerao, 1926) Bhalerao, 1936, Haplorchoides mehrai Pande and Shukla, 1976 and Tetracotyle wayanadensis Jithila and Prasadan, 2018 in H. fasciata and A. burminis and Clinostomum complanatum (Rudolphi, 1819) in P. cupanus.
Materials and methods
Study area and sampling of fishes
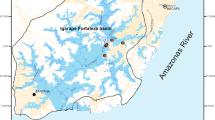
The Western Ghats of India with its geographical extension in the wet zone of Sri Lanka are considered as a biodiversity hotspot owing to its rich biodiversity and concentration of endemism. Ninety two specimens of H. fasciata and 106 specimens of P. cupanus, were collected from water bodies in different localities of the Wayanad region (lies between North 11′27′ and 15′58′ and East 75′47′ and 70′27′) of the Western Ghats (Fig. 1) during March 2017 to February 2018.
Collection of metacercariae
The collected H. fasciata and P. cupanus were brought alive to the laboratory and maintained in clean glass aquariums. Fishes were fed occasionally with fish meal. The scales, skin, gills, gill chambers and eyes of sacrificed fishes were examined under Labomed (Luxeo 4Z) stereozoom microscope for larval digeneans. Skin was removed, and the muscle tissues macerated to detect the metacercariae, if any. Internal organs were dissected out and placed in separate petri-dishes containing 0.75% saline, macerated and examined under the stereozoom microscope. The metacercariae of A. burminis, H. mehrai, T. wayanadensis, C. complanatum and Diplostomum ketupanense Vidyarthi, 1937 collected from H. fasciata and A. burminis, H. mehrai and C. complanatum from P. cupanus were carefully transferred to 0.75% saline in a petri-dish. Larvae were excysted either by rupturing the cyst wall with fine needles or by mounting them under cover glass and applying gentle pressure over it by fine needles. The excysted larvae were studied under a Nikon ECLIPSE Ni-U phase contrast research microscope (Japan) without stains or with vital stains like neutral red.
Quantitative analysis
For the study of infection patterns, samples collected during one year were considered. The analyses were performed using the data on host species and that of metacercariae obtained. Only the metacercariae obtained from more than one host specimen were considered for analyses, i.e. metacercariae of A. burminis, H. mehrai and T. wayanadensis of H. fasciata and A. burminis and C. complanatum of P. cupanus. Metacercariae of C. complanatum and D. ketupanense were obtained only from one specimen of H. fasciata, and therefore, their presence only was recorded. Similarly the presence of H. mehrai in P. cupanus was recorded.
Histograms were used to represent the distribution of the number of parasites per host, where the abundance of zeros and the skewed to the right were discerned. The infection parameters analyzed for each host and for the aforementioned parasites were: prevalence, mean intensity, median intensity and mean abundance (Bush et al. 1997; Reiczigel et al. 2019). In addition, the following aggregation indices were also analyzed: variance to mean ratio, Poulin's discrepancy index D and the parameter k of the negative binomial distribution (Wilson et al. 2002; Poulin 2007). These analyses were carried out following the methodology described by Reiczigel et al. (2019), using the free software accessible on the Quantitative Parasitology Web portal (http://www2.univet.hu/qpweb/qp10/index.php).
The levels of over-dispersion of different metacercariae were analyzed using the graphs of relationship of log mean abundance versus log mean variance. The negative binomial distribution, suitable to describe cases of over-dispersion in discrete variables where the variance is greater than the mean (Bolker 2008), which normally takes place in the distribution of parasites, was analyzed. The adjustment of the negative binomial distribution to the observed distribution of the trematodes per hosts was made using the R package 'fistdistrplus' (v. 1.1–1, Delignette-Muller et al. 2014). Goodness of fit was measured through psi squared tests carried out using the Quantitative Parasitology Web Portal above mentioned.
Analyses of environmental factors on the distribution of parasites
Influence of seasonal period (winter, summer, monsoon and post monsoon) and difference in collection localities were examined. To analyze their contribution on parasitic distribution, parasites having five or more sampled hosts only were considered. Boxplot was employed to explore the possible influence of seasonality and difference in locality on the distribution of parasites per host, especially when two or more samples were compared (Sokal and Rohlf 1995). Graphs for each factor in relation to each trematode species were constructed and used as exploratory tool.
CART model was applied to analyze the environmental factors affecting parasite distribution patterns. CART models are nonparametric statistical methods based on an algorithm known as recursive partitioning (Breiman et al. 1984). It was a step-by-step process by which a decision tree was constructed by either splitting or not splitting each node on the tree into two daughter nodes—a binary split (Izenman 2008). In the classification tree model the output variable was a binary-valued or discrete in the distribution of parasites. This process tends to produce over fitting, so for remedy it a pruning system was used based on the smallest prediction error obtained in a cross-validation procedure (Kabacoff 2015). The R package ‘rpart’ (v 4.1.15, Therneau et al. 2019) was used for the application of CART models. Following the structure of the R package rpart, the formula, Variable Y (parasites per host) ~ Factors (Season + Place + Type of water course) was used. Among the arguments of the formula, the definition of the “method” was "Poisson". This method was used when the variable Y (distribution of the number of parasites per host) is a vector or a two-column matrix. If it is a matrix, the first column was the observation times and the second was counts. Other argument referred to the “control parameters” was the complexity parameter. This parameter was set at 0.001, which implies that the split must decrease the overall lack of fit by a factor of 0.001 before being attempted. Therefore, it gave an indication of the most appropriate number of splits in the classification tree analysis. In the same way the program results showed the values of cross-validation with an indication of the error and also the percentage explanation of total variance in each split. Those information were used to define the appropriate number of splits in the model. The plots of classification tree models were obtained using the R package “rpart.plot” (v 3.0.8, Milborrow 2018).
Along with the use of software of the quantitative parasitology web portal, the rest of the statistical analyses and all the figures were made using R statistical software (R Core Team 2020 v 3.6.3).
Results
Distribution patterns
All species of metacercariae showed over-dispersed distributions with the variance greater than the mean (Table 1) and high abundance of zeros in both host fishes, H. fasciata and P. cupanus. In H. fasciata, the negative binomial distribution fits acceptably to the distributions of the number of parasites per host of the metacercariae A. burminis (Table 1, X2, p = 0.2717) and H. mehrai (Table 1, X2, p = 0.2316; Fig. 2a). In the case of T. wayanadensis the sample of infected fish was too small to check for negative binomial distribution. In P. cupanus, the negative binomial distribution fits acceptably with the distribution of the number of parasites per host in A. burminis (Table 2, X2, p = 0.1159) and C. complanatum (Table 2, X2, p = 0.1127; Fig. 2b).
Infection statistics and indices of aggregation
The metacercariae which showed the highest prevalence were A. burminis in H. fasciata (48.9%) and C. complanatum in P. cupanus (33%) (Table 1 and 2). Despite the relatively low prevalence of H. mehrai in H. fasciata (19.6%), its mean intensity was remarkable (8.44) as well as the range (1–32). In the case of remaining metacercariae, the average intensity barely exceeds the value of five as maximum, although the range was very wide in C. complanatum infection in P. cupanus (1–29). Mean abundance of infection was low for all parasite species observed in both hosts (Tables 1 and 2).
The variance to mean ratio was greater than one for all metacercariae infected in both H. fasciata and P. cupanus, confirming an over-dispersed (aggregated) distribution of these parasites. The metacercariae showed indices D values corresponded to aggregate distributions was higher than 0.85 in all cases, except A. burminis infection in H. fasciata which reached a value of 0.686. The k values obtained for the metacercarial infection in H. fasciata and P. cupanus were low and none exceeds the value of 0.4. The k with the lowest value was found for H. mehrai infection in H. fasciata (Tables 1 and 2).
Influence of environmental factors on distribution of metacercariae
Boxplots showed that seasonality had influence on the distribution of the number of parasites per host in the case of A. burminis in H. fasciata and P. cupanus (Fig. 3a, b) and C. complanatum infection in P. cupanus (Fig. 3c). Likewise, the distribution of the number of parasites per host was inclined by difference in locality in A. burminis and H. mehrai infection in H. fasciata (Fig. 3d, e) and to a lesser extent C. complanatum infection in P. cupanus (Fig. 3f).
The frequency distribution of Acanthostomum burminis against the factor variable season in Haludaria fasciata (a) and in Pseudosphromenus cupanus (b). c Frequency distribution of Clinostomum complanatum against the factor variable season in Pseudosphromenus cupanus. Frequency distribution of Acanthostomum burminis (d) and Haplorchoides mehrai (e) against the factor variable localities in Haludaria fasciata. Distribution of Clinostomum complanatum against the factor variable localities in Pseudosphromenus cupanus (f). Number of fish sampled was indicated on each boxplot
The classification tree models indicated that among the environmental factors considered, the difference in locality was most influential on distribution of parasites in both H. fasciata and P. cupanus, followed at a great distance by the factor seasonality. In the case of A. burminis infection in H. fasciata, the classification tree model resulted in four splits explaining the 58.6% of the total variability. The first split, based on the locality which explained 44% of the variability and the second split, based on seasonality with 8.6% of variability (Fig. 4). The infected fishes were more abundant in Kalpetta, Makkikolli and Venmani and with higher mean abundance during the summer season. The distribution pattern of H. mehrai in H. fasciata was also influenced principally by the difference in locality with Makkikolli and Palamukku showed highest infestation levels (Fig. 3e). In the case of C. complanatum infection in P. cupanus the classification tree model resulted in three splits explaining the 47.7% of the total variability mainly based on locality and seasonality factors. The first split, based on locality which explained 34.8% of the variability and the second split, seasonality, explained the 11.4% of the total variability (Fig. 5). The infected fishes were more abundant in Kakkancheri and Tharuvana with higher mean abundance during the monsoon season.
Classification tree plot of the distribution of the numbers of Acantostomum burminis in Haludaria fasciata in consideration with the environmental factors. The nodes include the name of the factor. In the terminal nodes the number of parasites with the number of host samples and the percentage were showed
Classification tree plot of the distribution of the numbers of Clinostomum complanatum in Pseudosphromenus cupanus taking in consideration the environmental factors. The nodes include the name of the factor. In the terminal nodes the number of parasites with the number of host samples and the percentage were showed
Discussion
Over dispersed distributions of helminth parasites are commonly observed within host populations (Shaw and Dobson 1995; Shaw et al. 1998) and are important for understanding many density-dependent processes in host-parasite interactions (Anderson and May 1978; Keymer 1982). The proximate causes of such aggregated or clumped frequency distributions are poorly understood, especially from natural systems (May and Southwood 1990; Jaenike 1994), but heterogeneity among hosts in the exposure to infective parasite stages is thought to be one important factor (Crofton 1971).
In the present study the infection of metacercariae in hosts showed over dispersed distribution, which confirms the general pattern of over dispersed distribution characteristic to the parasites (Crofton 1971; Shaw and Dobson 1995). When the data showed over dispersion or a certain degree of extra variation with respect to a Poisson distribution, it implies that the infection rate is not homogeneous in the population (Biggeri 2005). This is essential to understand the causes and consequences of the spatial distribution of parasites (Cohen et al. 2016).
The degree of aggregation of parasite populations has an influence on the stability of the parasite-host interactions, on the evolutionary biology of the parasites, on epidemiological aspects and on the characterization of the ecological processes in a given community and by extension in the ecosystem (Anderson and May 1978; Begon et al. 2006; Poulin 2007; Warburton and Vonhof 2018). Despite this importance, the causes that originate the widespread pattern of aggregation in parasites still remain diffuse (Grenfell and Gulland 1995; Tinsley et al. 2019) and it is difficult to arrive at a general theory in view of the great variety of cases and of possible explanations for the different parasite-host systems.
The index of discrepancy D, measures the departure between the observed parasite distribution and a hypothetical one in which all hosts harbor equal number of parasites (Poulin 1993, 2013) and the index varies between 0 (no aggregation) and 1 (maximum aggregation). The metacercariae showed indices D values, which corresponded to aggregate distributions, higher than 0.85 in all cases, except A. burminis infection in H. fasciata.
According to Poulin (2013) the heterogeneity in the infection levels in the hosts can be explained based on two broad criteria: the heterogeneity between the hosts in the exposure to the parasites and the susceptibility to infection. There are references putting the weight of reason on one or another criterion or on a combination of both (Lester 2012; Tinsley et al. 2019; Warburton and Vonhof 2018). However, the variance of parasite distribution in hosts is mostly constrained by the value of the mean (Poulin 2013). Thus, according to the meta-analysis studies of Shaw and Dobson (1995) and Poulin (2013), it was observed that the regression between log of the mean and the log of the variance of the number of parasites per host, showed a r2 of 0.87–0.88, which indicates that only 12% of the variability in the distribution of parasites remains under the explanation of other factors. In the present study, the five trematode populations analyzed showed an r2 of 0.87 (adjusted r2 = 0.83) which is similar or slightly lower than the values established in the previous reviews. This reflects that, in the present study, it is the abundance of parasites (the mean) which determines the level of aggregation observed and 17% of the variability would remain dependent on other factors (Fig. 6).
Relationship between the variance (in log) and the mean number of parasites per host (in log) across the trematode parasite populations infecting Haludaria fasciata and Pseudosphromenus cupanus. The dashed line is the fitted regression line (log variance = 2.0624 + 1.3084 * log mean; r2 = 0.87, adjusted r2 = 0.83 and s.e. of the slope = 0.2903). The solid line represents the 1:1 relationship above which parasites are aggregated
The distribution of parasites and their corresponding infection parameters are not static but dynamic and can change temporally and spatially (Poulin 2007). In the present study, seasonality plays a role in the distribution of A. burminis in H. fasciata and P. cupanus and C. complanatum infection in P. cupanus. The individual level heterogeneity, both inside the host and in the host’s environment, provides a mechanism behind patterns of parasite aggregation (Warburton and Vonhof 2018). Precisely, environmental factors can influence the changes in abundance and, therefore, in the aggregation pattern. Parasite distribution patterns are shaped by a suite of abiotic and biotic factors, which directly and/or indirectly influence both parasites and their hosts (Ostfeld et al. 2005; Paterson et al. 2019).
In lotic freshwater ecosystems, the combined influence of the unidirectional water flow and the mobility of the most mobile hosts are considered to be primary drivers that structure the distribution of parasites, especially those with complex life cycles such as trematodes (Blasco-Costa et al. 2013; Salgado-Maldonado et al. 2014; Paterson et al. 2019). According to Price (1990) attention needs to be given to host properties such as geographic range, abundance and reproduction because these factors drive the nature of parasite assemblage. In the present study, the distribution of metacercariae was mainly influenced by the differences in ecological parameters prevailed in different host localities. This is true in the case of obligate, multi-host parasites, like trematodes as the successful completion of their life cycles depend on the presence and abundance of suitable hosts (Blakeslee et al. 2011). Trematodes are highly specific to snail hosts (typically infecting only one or few species), but infection to second-intermediate and definitive hosts is often more general (Graczyk et al. 1997); even then, specific groups of host species are typically targeted. Therefore, the availability of suitable hosts explain the presence or absence of specific parasite species in an area (Blakeslee et al. 2011).
The present study represents the first approximation to the infection patterns by metacercariae in H. fasciata and P. cupanus. The authors consider this information as essential, to be more precise, to properly direct to make efforts in the formulation of new working hypotheses in relation to infection by parasitic trematodes in the freshwater fishes of the Western Ghats (India), an area of high ecological interest.
References
Alexander N (2012) Analysis of parasite and other skewed counts. Trop Med Int Health 17(6):684–693
Anderson RM, May RM (1978) Regulation and stability of host-parasite population interactions. I. Regulatory precesses. J Anim Ecol 47:219–247
Begon M, Townsed CR, Harper JL (2006) Ecology. from individuals to ecosystems, 4th edn. Blackwell Publishing, Hoboken
Biggeri A (2005) Negative binomial distribution. In: Armitage P, Colton T (eds) Encyclopedia of Biostatistics, vol 5, 2nd edn. Wiley, Chi Chester, pp 3625–3630
Blakeslee AMP, Altman I, Miller AW, Byers JE, Hamer CE, Ruiz GM (2011) Parasites and invasions: a biogeographic examination of parasites and hosts in native and introduced ranges. J Biogeogr. https://doi.org/10.1111/j.1365-2699.2011.02631.x
Blasco-Costa I, Koehlern AV, Martin A, Poulin R (2013) Upstream-downstream gradient in infection levels by fish parasites: a common river pattern? Parasitology 140:266–274
Bolker BM (2008) Ecological models and data in R. Princeton University Press, Princeton, p 396
Breiman L, Friedman JH, Olshen R, Stone CJ (1984) Classification and regression trees. Wadsworth International Group, Belmont, CA
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583
Chakraborty A, Rumki S, Koushik G (2017) An inventory of endemic fish species in India with notes on state-wise distribution and conservation measures. Int J Fish Aquat Stud 5(1):253–264
Chipeta MG, Ngwira BM, Simoonga C, Kazembe LN (2014) Zero adjusted models with applications to analyzing helminthes count data. BMC Res Notes 7:856. https://doi.org/10.1186/1756-0500-7-856
Cohen JE, Poulin R, Lagrue C (2016) Linking parasite populations in hosts to parasite populations in space through Taylor’s law and the negative binomial distribution. Proc Natl Acad Sci USA 114:47–56
Crofton HD (1971) A quantitative approach to parasitism. Parasitology 63:179–193
Dahanukar N, Raut R, Bhat A (2004) Distribution, endemism and threat status of freshwater fishes in the Western Ghats of India. J Biogeogr 31:123–136
Dahanukar N, Raghavan R, Ali A, Abraham R, Shaji C (2011) The status and distribution of freshwater fishes of the Western Ghats. In: Molur S, Smith KG, Daniel BA, Darwall WRT (Compilers) International Union for Conservation of Nature (IUCN) Gland, Switzerland and Zoo Outreach Organization (ZOO), Coimbatore, pp 21–48
De’Ath G (2002) Multivariate regression trees: a new technique for modeling species-environment relationships. Ecology 83(4):1105–1117
De’Ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81(11):3178–3192
Delignette-Muller M, Pouillot R, Denis J, Dutang C (2014) fitdistrplus: help to fit of a parametric distribution to non-censored or censored data. R package version 1.0–2
Dunn PK, Smyth GK (2018) Generalized linear models with examples in R. Springer, New York, p 562
Gazzinelli A, Oliveira-Prado R, Ferreira-Matoso L, Veloso BM, Andrade G, Kloos H, Bethong JM, Assunçao RM, Correa-Oliveira R (2017) Schistosoma mansoni reinfection: analysis of risk factors by classification and regression tree (CART) modeling. PLoS ONE 12(8):182–197
Graczyk TK, Fayer R, Cranfield MR (1997) Zoonotic transmission of Cryptosporidium parvum: implications for water-borne cryptosporidiosis. Parasitol Today 13(9):348–351
Grenfell BT, Gulland FMD (1995) Introduction: ecological impact of parasitism on wildlife host populations. Parasitology 111:3–14
Holmes JC (1987) The structure of helminth communities. Int J Parasitol 17:203–208
Izenman AJ (2008) Modern multivariate statistical techniques: regression, classification and manifold learning. Springer, New York, p 733
Jaenike J (1994) Aggregations of nematode parasites within Drosophila: proximate causes. Parasitology 108:569–577
Kabacoff RI (2015) R in action. Data analysis and graphics with R, 2nd edn. Manning Publications Co., Shelter Island, p 579
Keymer A (1982) Density-dependent mechanisms in the regulation of intestinal helminth populations. Parasitology 84(3):573–587
Kingsland SE (1995) Modeling nature. Episodes in the history of population ecology, 2nd edn. The University of Chicago Press, Chicago, p 306
Krebs CJ (1989) Ecological methodology. Harper Collins Publishers Inc, New York, p 654
Krebs CJ (2001) Ecology. The experimental analysis of distribution and abundance, 5th edn. Benjamin Cummings, an imprint of Addison Wesley Longman Inc., San Francisco, p 695
Lafferty KD (1999) The evolution of trophic transmission. Parasitol Today 15(3):111–115
Lester RJG (1984) A review of methods for estimating mortality due to parasites in wild fish populations. Helgolander Meeresunters 37:53–64
Lester RJG (2012) Over dispersion in marine fish parasites. J Parasitol 98(4):718–721
Li SY, Hsü HF (1951) On the frequency distribution of parasitic helminthes in their naturally infected hosts. J Parasitol 37:32–41
MacArthur RH (1972) Geographical ecology. Patterns in the distribution of species. Princeton University Press, Princeton, p 269
Mangel M (2006) The theoretical biologist’s toolbox. Quantitative methods for ecology and evolutionary biology. Cambridge University Press, Cambridge
Marquet PA (2009) Macro-ecological perspectives on communities and ecosystems. In: Levin SA (ed) The Princeton guide to ecology. Princeton University Press, Princeton, pp 386–394
May RM, Southwood TRE (1990) Introduction. In: Shorrocks B, Swingland IR (eds) Living in a patchy environment. Oxford University Press, Oxford, pp 107–125
McCullagh P, Nelder JA (1989) Generalized linear models, 2nd edn. Chapman & Hall/CRC, London, p 511
Milborrow S (2018) Plotting rpart trees with rpart.plot package. R package. https://CRAN.R-project.org/package=rpart.plot
O’hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122
Ostfeld RS, Glass GE, Keesing F (2005) Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol 20:328–336
Paterson RA, Knudsen R, Blasco-Costa I, Dunn AM, Hytterod S, Hansen H (2019) Determinants of parasite distribution in Arctic charr populations: catchment structure versus dispersal potential. J Helminthol 93:559–566. https://doi.org/10.1017/S0022149X18000482
Poulin R (1993) The disparity between observed and uniform distributions: a new look at parasite aggregation. Int J Parasitol 23:937–944
Poulin R (1998) Large-scale patterns of host use by parasites of freshwater fishes. Ecol Lett 1:118–128. https://doi.org/10.1046/j.1461-0248.1998.00022.x
Poulin R (2007) Evolutionary ecology of parasites, 2nd edn. Princeton University Press, Princeton, p 332
Poulin R (2013) Explaining variability in parasite aggregation levels among host samples. Parasitology 140:541–546
Poulin R, Morand S (2000) Parasite body size and interspecific variation in levels of aggregation among nematodes. J Parasitol 86(3):642–647
Poulin R, Closs GP, Lill AWT, Hicks AS, Herrmann KK, Kelly DW (2012) Migration as an escape from parasitism in New Zealand galaxiid fishes. Oecologia 169:955–963
Price PW (1990) Host populations as resources defining parasite community organization. In: Esch G, Bush A, Aho J (eds) Parasite communities: patterns and processes. Chapman and Hall, London, pp 21–40
Qian SS (2017) Environmental and ecological statistics with R, 2nd edn. CRC Press, Taylor & Francis Group, Boca Ratón, p 535
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Raghaven R, Dahanukar N, Tlusty MF, Rhyne AL, Kumar KK, Molur S, Rosser AM (2013) Uncovering an obscure trade: threatened freshwater fishes and the aquarium pet markets. Biol Conserv 164:158–169
Reiczigel J, Marozzi M, Fabian I, Rozsa L (2019) Biostatistics for parasitologists—a primer to quantitative parasitology. Trends Parasitol 35(4):277–281
Rhodes JR (2015) Mixture models for over dispersed data. In: Fox GA, Negrete-Yankelevich S, Sosa VJ (eds) Ecological statistics: contemporary theory and application. Oxford University Press, Oxford, pp 284–308
Roff DA (2006) Introduction to computer-intensive methods of data analysis in biology. Cambridge University Press, Cambridge, p 368
Rosa R, Puglieses A (2002) Aggregation, stability, and oscillations in different models for host-macro-parasite interactions. Theor Popul Biol 61:319–334
Rossiter W, Davidson HM (2018) Extrinsic and intrinsic predictors of variation in infection by Posthodiplostomum minimum MacCallum, 1921 (Trematoda) in sunfishes (Lepomis Rafinisque, 1819) from eastern Ohio. J Parasitol 104(3):202–209
Salgado-Maldonado G, Novelo-turcotte MT, Vazquez G, Caspetamandujano JM, Quiroz-Martinez B, Favila M (2014) The communities of helminth parasites of Heterandria bimaculata (Teleostei: Poeciliidae) from the upper Río La Antigua basin, east-central Mexico show a predictable structure. Parasitology 141:970–980
Schmidt GD, Roberts LS (2000) Foundations of parasitology, 6th edn. McGraw-Hill Higher Education (A Division of the McGraw-Hill Companies), Boston, p 670
Shaw DJ, Dobson AP (1995) Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology 111:111–133
Shaw DJ, Grenfell BT, Dobson AP (1998) Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117:597–610
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. W. H. Freeman and Company, New York, p 887
Southwood TRF, Henderson PA (2000) Ecological methods, 3rd edn. Blackwell Science Ltd, London, p 575
Therneau T, Atkinson B, Ripley B (2019) Rpart: recursive partitioning and regression trees. Mayo Foundation Rochester, MN, USA. https://cran.r-project.org/web/packages/rpart/index.html
Thieltges DW, Christian H, Dehling DM, Brändle M, Brandl R, Poulin R (2011) Host diversity and latitude drive trematode diversity patterns in the European freshwater fauna. Glob Ecol Biogeogr 20:675–682
Tinsley RC, Vineer HR, Grainger-Wood R, Morgan ER (2019) Heterogeneity in helminth infections: factors influencing aggregation in a simple host-parasite system. Parasitology 147(1):65–77. https://doi.org/10.1017/S003118201900129X
Vignon M, Sasal P (2010) Multiscale determinants of parasite abundance: a quantitative hierarchical approach for coral reef fishes. Int J Parasitol 40(4):443–451
Warburton EM, Vonhof MJ (2018) From individual heterogeneity to population-level over dispersion: quantifying the relative roles of host exposure and parasite establishment in driving aggregated helminth distributions. Int J Parasitol 48(34):309–318
Wilson K, Grenfell BT (1997) Generalized linear modelling for parasitologists. Parasitol Today 13(1):33–38
Wilson K, Bjornstad ON, Dobson AP, Merler S, Poglayen G, Randolph SE, Read AF, Skorping A (2002) Chapter 2: Heterogeneities in macroparasite infections: patterns and processes. In: Hudson PJ, Rizzoli A, Grenfell BT, Heersterbeek H, Dobson AP (eds) The ecology of wildlife diseases. Oxford University Press, Oxford, pp 6–44
Funding
This work was supported by Kerala State Council for Science, Technology and Environment (KSCSTE), Government of Kerala as a Research Fellowship (KSCSTE /972/2018-FSHP-MAIN Dated 23/01/2019).
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. P.J. J.: collection of fishes, isolation of parasites, concept of the manuscript and writing of the manuscript. P.A.: statistical analysis and writing of the manuscript. P.K.P.: concept of the manuscript and editing of the manuscript. All authors read, revised, and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jithila, P.J., Abaunza, P. & Prasadan, P.K. Distribution of different species of metacercariae in two freshwater fishes: Haludaria fasciata (Teleostei: Cyprinidae) and Pseudosphromenus cupanus (Teleostei: Osphromenidae). J Parasit Dis 46, 113–123 (2022). https://doi.org/10.1007/s12639-021-01421-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-021-01421-x