Abstract
Coral reef conservation management policy often focuses on larval retention and recruitment of marine fish with scant data available on important, less motile reef-building species such as corals. To evaluate the concept of population connectivity in corals, we tested whether broadcast spawning reproduction per se confers the same degree of dispersal to two sister species, Montastraea annularis (Anthozoa: Scleractinia; Ellis and Solander 1786) and M. faveolata (Ellis and Solander 1786), both dominant taxa in reefs of the northern Caribbean. Genetic analyses of ten nuclear DNA loci (seven microsatellite and three single-copy RFLP) reveal strikingly different patterns of population genetic subdivision for these closely related, sympatric species, in spite of likely identical dispersal abilities. Strong population genetic structure typified the architecture of M. annularis, whereas M. faveolata populations were principally genetically well mixed. A higher level of clonality was observed in M. annularis potentially because of a susceptibility to physical fragmentation. Clonality did not, however, significantly contribute to population genetic structure or low-level Hardy–Weinberg and linkage disequilibria observed in some populations. The lack of consistent association between reproductive mode and dispersal reinforces the perspective that population connectivity is not so much a function of predictable marine population source and sink relationships as is due to a more complex interface of oceanic currents interacting with and amplifying stochastic fluctuations in larval supply and settlement success. Our results support others promoting an overall ecosystem approach in marine protected area design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stony corals are the foundation of highly diverse marine ecosystems, providing structure, habitat, and primary productivity over vast areas of tropical near-shore habitat. Globally, many coral reef ecosystems are on a declining trajectory, as bleaching, disease, pollution, siltation from terrestrial run-off, fishing, and a variety of other natural and anthropogenic pressures, singly and in combination, take their toll (Gardner et al. 2003; Pandolfi et al. 2003; Bellwood et al. 2004; Jones et al. 2004). Degradation frequently is noted for Caribbean coral reefs (Hughes 1994; Hughes and Tanner 2000; Gardner et al. 2003) with a growing indication that the Great Barrier Reef system of Australia also is deteriorating (Bellwood et al. 2004). A current focus of coral reef conservation is on estimating the degree of ecological interconnectivity of spatially disjunct reef systems (e.g., Bode et al. 2006; Cowen et al. 2006). Understanding the scale of dispersal for reef organisms will, in theory, provide a spatial context for drawing marine reserve boundaries as either protecting locally self-propagating populations or as encompassing larger-scale areas that rely on regionally separated larval pools. The actual degree of population connectivity in organisms capable of long distance dispersal, however, has been the subject of considerable debate (Roberts 1997; Jones et al. 1999; Swearer et al. 1999; Cowen et al. 2000, 2006; Rocha et al. 2002; Palumbi 2003; Taylor and Hellberg 2003). Levels of genetic subdivision indicative of local larval retention have been observed for a number of species with pelagic larvae, contradicting paradigms that pelagic larvae are effective long distance dispersers (Hamm and Burton 2000; Taylor and Hellberg 2003; Cowen et al. 2006).
Inferring degrees of connectivity may be particularly difficult for taxa within the reproductively-protean order Scleractinia because its members are known to outcross (Knowlton et al. 1997; Szmant et al. 1997), inbreed (Knowlton and Jackson 1993), hybridize (Knowlton et al. 1997; Szmant et al. 1997; Vollmer and Palumbi 2002) and propagate clonally (Veron 2000), all of which contribute in differing ways to population subdivision. Sedentary as adults, corals rely on free-floating larvae for dispersal and recruitment, and as expected, brooding species of coral, generally characterized as dispersal-challenged, have lower levels of gene flow than species with mass-spawning gametic phases (Hellberg 1994, 1996; Ayre and Hughes 2000). The degree to which corals with higher dispersal potentials are genetically structured, however, is not clear. When considering the population structure of only mass-spawning species, genetic mixing among reefs has been moderate to high and often if present, the resulting genetic subdivision is generally distance-dependent (Hellberg 1996; Ayre and Hughes 2000; Mackenzie et al. 2004). In other cases, however, even proximate sites within reefs exhibit genetic differentiation, particularly for highly clonal, broadcast-spawning species (Ayre and Hughes 2000). In the Caribbean basin, populations of Acropora palmata cluster into two major regions, one eastern and one western, between which no recent genetic interchange has occurred (Baums et al. 2005). Even patterns of population subdivision within a single mass-spawning species (Plesiastrea versipora) are not consistent, with highly restricted gene flow along the southeast Australian coast contrasting to genetic homogeneity over a similar range in the Ryukyu Archipelago of Japan (Rodriguez-Lanetty and Hoegh-Guldberg 2002). Clearly, no consensus view of dispersal and population subdivision in coral species has yet emerged.
Members of the Montastraea annularis species complex (Anthozoa: Scleractinia; M. annularis and M. faveolata [Ellis and Solander 1786] and M. franksi [Gregory 1895]) are generally slow growing and long-lived, and show low rates of sexual recruitment (Knowlton et al. 1997; Hughes and Tanner 2000). Ecologically, these boulder corals provide the structural reef integrity throughout much of the Caribbean and consequently are important conservation targets. As such, they are one of the most extensively studied reef-building corals in the western Atlantic (Knowlton et al. 1997; Szmant et al. 1997; Hughes and Tanner 2000; Budd and Pandolfi 2004; Fukami et al. 2004; Levitan et al. 2004). Reports that reef communities are shifting from these framework-building genera (e.g., Montastraea spp. and Acropora spp.) to non-framework building taxa (e.g., Agaricia spp. and Porites spp.) (Edmunds and Carpenter 2001; Knowlton 2001; Cho and Woodley 2002) make it particularly important that baseline genetic information for putatively declining species (such as Montastraea spp.) be garnered quickly. Montastraea spp. are hermaphroditic with gametes that are synchronously mass spawned annually in the late summer (Sammarco and Andrews 1988; Szmant 1991). Planulae larvae develop within 24 h of spawning and can remain at the surface for up to 96 h before settling (Wellington and Fitt 2003). Broadcast spawning and pelagic larva confer upon Montastraea spp. an expected ability to disperse widely. Previous studies in these corals have focused on their potential for hybridization because of their similar biology, overlapping spawning periods and the morphological diversity present among and within each of the species (Knowlton et al. 1992; Knowlton et al. 1997; Szmant et al. 1997; Fukami et al. 2004; Levitan et al. 2004). Although the three species, M. annularis, M. faveolata and M. franksi, exhibit similar spawning schedules over a 4–8 day period typically following the full moon in August, multiple isolating mechanisms appear to maintain the sympatry of these species (Levitan et al. 2004). For example, M. franksi begins spawning a day earlier than M. annularis and M. faveolata, and, if in a given day, all three are spawning, M. franksi precedes the other two species by at least 2 h. This temporal lead for M. franksi is particularly important with respect to its relationship with M. annularis, because laboratory-mating studies indicate a substantial degree of compatibility between gametes of these two species. M. annularis and M. faveolata, on the other hand, spawn nearly synchronously, but fertilization trials have shown that their gametes are predominantly, but not completely, incompatible (Knowlton et al. 1997; Szmant et al. 1997; Levitan et al. 2004). Until the issue of hybridization in these corals formalizes, the species designations are at best tentative, but for our purposes, evaluating such closely related taxa with similar range distributions and mass-spawning reproductive strategies allows us to analyze the interdependence of dispersal potential and hydrodynamics on reef connectivity.
Here, we use a combination of ten polymorphic nuclear DNA markers constituted by three single-copy restriction-fragment-length polymorphisms (RFLP) and seven microsatellite, to test for associations between gene flow and reproductive strategy in populations of the dominant Caribbean coral reef species, M. annularis and M. faveolata. By including and comparing two marker types, we can minimize any marker-specific effects that could potentially distort an accurate picture of population connectivity. Although mitochondrial DNA often is used in population genetics studies of animals, it evolves much too slowly in coral to be useful at the population or even species levels (Shearer et al. 2002). Single-copy RFLP gene markers have predominantly been used in gene mapping and domestic animal and plant breeding studies (Paterson et al. 1988; Martin et al. 1989). These markers, however, also have proven effective in population genetics of natural populations for sorting out anomalies or uncovering hidden molecular variation not detected by other means (Karl and Avise 1992; Karl et al. 1992; Cattell and Karl 2004). Microsatellites are the current favorite tool of population biologists and as highly polymorphic loci are particularly useful for genotyping reproductively complex organisms such as corals because unique genets usually can be identified with a high degree of accuracy and precision. As one facet of a multidisciplinary and comprehensive evaluation of the state of Caribbean coral reefs, this genetic component can aid management authorities in matters concerning connectivity, heritable bleaching susceptibility, and transplantation strategies geared to maximize genetic diversity, and to maintain genetically distinct populations.
Materials and methods
DNA markers
Seven microsatellite and three anonymous single-copy nuclear DNA markers were isolated as described previously (Severance et al. 2004a, b). For the single-copy locus analyses, RFLP of polymerase chain reaction (PCR) amplification products were used to genotype individuals. In this di-allelic system, individuals were scored as either homozygous for the presence of, homozygous for the absence of, or heterozygous for cleavage at an endonuclease recognition site. Microsatellite genotypes were determined by the size of fluorescently labeled PCR fragments that were size sorted on an ABI 377 automated sequencer (Iowa State University Sequencing Facility). PCR conditions for amplifying field samples using both sets of markers were reported previously (Severance et al. 2004a, b).
Field samples
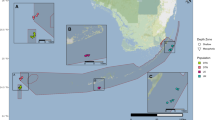
Using SCUBA, a total of 127 M. annularis and 152 M. faveolata samples were collected in water at depths from 0 to 25 m at four locations in the Western Atlantic (Table 1; Fig. 1). Unlike with fast-growing branching corals, collecting samples from the massive boulder-like corals is more threatening to the health of the coral; consequently utmost care was taken to minimize damage to sampled colonies. The number of samples collected at any one location was highly restricted by the governing marine authorities and by the number of colonies present at any given site. With respect to the latter, we collected fewer M. annularis individuals simply because they were less abundant at some sites and completely absent at others. Approximately 2–4 cm2 sections of M. annularis and M. faveolata were removed with a hammer and chisel from the basal portion of the colony and placed in labeled plastic zip-lock bags. Samples were stored on ice immediately following the dive and placed in 90% ethanol for longer term storage as soon as feasible. Multiple sites separated by less than 10 km were sampled within each geographic location. At all sites, only one sample per coral head was collected and to the extent possible, only nonadjacent coral heads were collected to minimize multiple recording of the same individuals or clone mates. Likewise, our sampling methods specifically targeted colonies that were morphologically unambiguously either M. annularis or M. faveolata. Consequently, any colony of questionable taxonomic identity was avoided and the potential of collecting hybrids (Szmant et al. 1997) was minimized. Details concerning collection sites including the reefs sampled, depth, longitude and latitude coordinates, and the number of samples taken from each location are summarized in Table 1.
Major geographic locations sampled in this study. Site abbreviations are as in Table 1
DNA extraction
DNA was extracted using a modified Chelex protocol (Walsh et al. 1991). Colony fragments of approximately 4 mm2 were placed in 500 μL of 5% Chelex and boiled for 15 min. Samples were vortexed for 15 s and centrifuged for 5 min at 15,000 rpm. The supernatant was removed, placed in a new tube and extracted three times with 25:24:1 phenol:chloroform:isoamyl alcohol and once with 24:1 chloroform:isoamyl alcohol. The solution was incubated for 1 h at 65°C and then overnight at room temperature with a 4 M lithium chloride solution. The mixture was centrifuged for 30 min and the pellet resuspended in 50 μL of 1x TE (10 mM Tris–HCl, pH 7.5, 5 mM EDTA). Maintaining the stability of the DNA isolated in this way proved challenging; therefore, amplifications were performed as soon as possible after DNA isolation.
Population genetic analyses
Tests for conformance to Hardy–Weinberg genotype frequency equilibrium (HWE) expectations and for significant deviations from linkage equilibrium were performed using ARLEQUIN v. 2.00 (Schneider et al. 2000). Population sample pairwise F ST estimates for single-copy RFLP data and significance tests also were performed using ARLEQUIN v. 2.00. F ST values were estimated based on a count of the number of each allele (Weir and Cockerham 1984; Michalakis and Excoffier 1996; Schneider et al. 2000) and significance levels estimated by permuting genotypes between populations for 3,024 iterations and the probability (P) reflecting the proportion of permutations leading to an F ST value equal to or larger than the observed. For microsatellite loci, sample pairwise Rho values, an unbiased estimator of Slatkin’s R ST (Slatkin 1995), and significance tests were calculated using RSTCALC version 2.2 (Goodman 1997). We chose Rho because there is an explicit consideration of mutation and microsatellite loci clearly do not evolve under the infinite allele model assumed with F ST. Estimates of Rho were calculated across all loci and tested for significance based on 1,000 bootstraps, each with 1,000 permutations. All population genetic analyses were done separately for each set of molecular markers and with and without clonal replicates included (see text beyond). To minimize the possibility that null allele effects were contributing to the observed population genetic structure, analyses were also performed with and without the dinucleotide locus, MS2-17, which seemed to amplify some individuals less efficiently than the other markers. Sample sites within reefs were pooled if sample pairwise comparisons of F ST and Rho values were not significantly different from zero. Isolation by distance was tested using a linear correlation coefficient of log transformed geographic distance versus Nei’s D (Nei 1972), δμ2 (Goldstein et al. 1995), and chord distance (Cavalli-Sforza and Edwards 1967) which were calculated using the program MICROSAT (Minch 1995). Geographic distances were estimated as the most direct aquatic route between sites.
For each population, multi-locus genotypes, including microsatellite and single-copy loci, were sorted in Microsoft Excel and unique genotypes were identified. Evaluating clonality using microsatellite markers offers distinct advantages over other marker based systems because the high mutation rate at these loci generates a large number of alleles per locus (Sunnucks et al. 1996; Gomez and Carvalho 2000; Reusch et al. 2000). The microsatellites used here averaged approximately 21 alleles per locus (Severance et al. 2004a), indicating that they are likely to be highly sensitive in the identification of clonal genotypes. Nevertheless, we tested the probability that each identical genotype could be the result of sexual reproduction using MLGSIM (Stenberg et al. 2003). Corresponding critical P values were calculated using 10,000,000 simulations. Significant differences between species in the number of clonal genotypes relative to non-clonal genotypes were tested by chi-square analyses.
Results
Hardy–Weinberg equilibrium
For both species, significant deviations from HWE were present and all but one were heterozygote deficits. In M. annularis, 13 of the 44 locus-by-population tests (29.5%) deviated significantly before sequential Bonferroni correction. Seven of these were for the loci MS12 and MS2-17, which were two of the most diverse loci with 29 and 27 alleles, respectively, (Severance et al. 2004a). In M. faveolata, 9 of the 49 tests (18.4%) deviated significantly before sequential Bonferroni correction. Seven of these were for MS12 and MS2-17 where 24 and 29 alleles were seen in this species. No other locus showed consistent deviations across samples. The Dry Tortugas samples of both M. annularis and M. faveolata deviated at seven and three of the ten loci, respectively. This result persisted when clonal genotypes were removed and when sites within the Dry Tortugas were analyzed separately. No other sample had a preponderance of loci that deviated from HWE expectations. All significant differences remained so after sequential Bonferroni correction (P = 0.01) (Rice 1989).
Population genetic structure
Sample pairwise F ST and Rho estimates between collection sites within locations were not statistically different from zero so they were pooled except for the Yucatan locations. Here, M. annularis individuals from the two YC1 sites (YC1a and YC1b) were indistinguishable but different from YC2, so we pooled the YC1 sites but not YC1 with YC2. Although the M. faveolata samples from these sites were not genetically statistically differentiated, they were kept separate to facilitate interspecific comparisons. The resulting microsatellite Rho and single-copy F ST values for pairwise comparisons of major locations are shown in Tables 2 and 3. In M. annularis, significant inter-reef genetic subdivision was indicated for eight of the ten microsatellite and for seven of the ten single-copy pairwise location comparisons (Table 2). The average pairwise population Rho was 0.11 ± 0.07 (SD) and \( \ifmmode\expandafter\bar\else\expandafter\=\fi{F}_{{{\text{ST}}}} \, = \,0.38\, \pm \,0.29. \) Conversely, for M. faveolata there was little to no significant among location differentiation with only one each of the ten pairwise Rho or F ST values significantly different from zero (Table 3). The average Rho (0.01 ± 0.02) and \( \ifmmode\expandafter\bar\else\expandafter\=\fi{F}_{{{\text{ST}}}} {\text{ }}(0.03\, \pm \,0.03) \) for M. faveolata also were significantly less than the values estimated for M. annularis (P ≤ 0.01; 1-tailed t test; Sokal and Rohlf 1995). F ST values of the single-copy markers were generally larger than the Rho values of the microsatellites, which was not surprising given the mutational mode differences between these two types of loci. Nevertheless, both sets of markers reflected the same pattern of interspecific differences (i.e., M. annularis was more genetically structured than M. faveolata), thus verifying that the observed structure is not a marker-specific phenomenon. Similarly, removal of the possible null-allele-associated locus MS2-17 from the analyses did not significantly change resulting Rho values in these comparisons. There was no significant association between genetic and geographic distance even for M. annularis where most pairwise tests of population differentiation were significant (data not shown).
It is possible that ascertainment bias from developing the markers with M. annularis may account for at least some of the difference seen between the species. We do not, however, believe that this is the case here. We have shown that these loci are highly polymorphic (Severance et al, 2004a, b) and that the degree of variability is nearly identical in both species (see supplemental material A and B). Further, if ascertainment bias existed, we would expect to see a higher heterozygosity and lower F ST in the species from which the markers were developed (i.e., M. annularis) when, in fact, we observe just the opposite.
Clonality
Individuals that possessed an identical set of alleles (i.e., genotype) at all ten nuclear loci were considered clones. Most individuals appear to have been produced by sexual reproduction as evidenced by a preponderance of unique genotypes in the sample (Table 4). Identical multi-locus genotypes, however, were observed in all populations (except YC2) from both species. No sharing of identical genotypes occurred between populations in major geographic regions. By using the program MLGSIM (Stenberg et al. 2003), we estimated the probability that any of the observed putative clonal genotypes was actually the result of sexual reproduction was less than 10−15. This probability was significant compared to the simulated critical values at P ≤ 0.05. We conclude, therefore, that the identical genotypes observed were generated via asexual reproduction.
Over all samples, the total percent of clonally produced individuals in M. annularis (x = 18.6%) was significantly higher than that observed in M. faveolata (x = 9.2%; P ≤ 0.025; χ2 test). The magnitude of asexual reproduction varied among populations (Table 4). For M. annularis, Puerto Rico and the Dry Tortugas had the highest percentage of clonal individuals (21.4 and 25.8%, respectively). In general, the M. faveolata populations had few clones, except for the LK sample where 14.6% of the sampled individuals were clonally derived (Table 4). Although clonality did not represent the predominant mode of reproduction among our populations, we felt compelled to reanalyze our genetic structures excluding clonemates, especially with respect to M. annularis, since this species was associated with both higher levels of genetic subdivision and clonality. The degree of significant population genetic subdivision remained unchanged following re-analysis (Tables 2, 3).
Linkage disequilibria
Nonrandom associations between loci revealed that significant associations of microsatellite alleles occurred in most populations of both species (data not shown). No locus pairs, however, consistently were linked, suggesting that the deviations are not due to physical linkage. For M. faveolata, none of the 15 possible single-copy dilocus comparisons and only 11 of the 105 (10.5%) possible microsatellite dilocus comparisons showed statistically significant linkage (P ≤ 0.01). For M. annularis, zero of the 15 single copy and 33 of the 105 (31.4%) microsatellite dilocus comparisons were statistically significantly linked (P ≤ 0.01). Two populations, the Dry Tortugas and the Lower Keys, accounted for 27 of the significant microsatellite disequilibria (16 and 11, respectively). These populations also indicated relatively higher frequencies of clonal reproduction. Surprisingly, Puerto Rico, despite a high frequency of clones, had only three di-locus comparisons, which were in disequilibrium. Reanalysis of linkage disequilibria with clonal individuals removed resulted in a decrease of the number of linked loci for the Lower Keys population from 11 to 7 but no change for the Dry Tortugas population. Reanalysis without pooling sample sites within regions did not change the number of linked loci.
Discussion
In our study, two sister species of mass-spawning Scleractinian coral exhibited very different population genetic structures, indicating that common reproductive mode need not imply common patterns of gene flow. Whereas sites within reefs were generally undifferentiated, populations of M. annularis among major geographic regions showed significant interpopulation genetic divergence. This is characteristic of a restriction in gene flow among the northern Caribbean populations examined in our analysis. To the contrary, M. faveolata populations were genetically indistinguishable throughout the same range in spite of nearly identical larval dispersal ability. These results demonstrate that realized dispersal, at least for Montastraea spp. cannot be accommodated under a single model of larval exchange. In reference to geography or larval strategy, the genetic patterns revealed here do not correspond to either a strictly open model of larval dispersal mediated by oceanic currents, or a closed model of restricted dispersal and local recruitment. Spawning strategy or putative larval dispersal ability simply are not reliable predictors of the potential for panmixia, and other ecological or evolutionary processes must underlie the discordant genetic patterns. It is conceivable that even if two species were identical in all important respects, as long as there is non-synchronized variance around mean life history parameters among species, confluence of this variance with environmental variance can result in a benefit to one species without necessarily benefiting the other. In other words, even in the absence of differences in larval biology, M. faveolata can have a good spawning and settlement year, whereas M. annularis does not. Given the rarity of sexual recruitment and the longevity of the species, the effect of any chance differences is likely to persist for a long time. Collectively, this and other studies (e.g., Ayre and Hughes 2000, 2004; Miller and Ayre 2004) reinforce that coral population structure, like coral life-history, is complex and likely influenced by numerous factors including geographic scale and micro- and macro-physicalities specific to individual reefs.
The dominant mode of reproduction for Montastraea spp. is considered to be sexual (Szmant 1991), and therefore, the finding of clonality in both species in the present study is noteworthy. Clonality in these species contrasts with previous allozyme studies where all M. annularis and M. faveolata samples from Curaçao and Panama had unique genotypes (sample sizes were 25 and 43, respectively; Knowlton et al. 1992; Van Veghel and Bak 1993). The degree of clonality, therefore, is more likely a consequence of extrinsic influences rather than an inherent aspect of the organism’s mode of reproduction. For example, asexual propagation via polyp expulsion during periods of unfavorable conditions has been reported in Favia favus in the Red Sea and Oculina patagonia in the Mediterranean Sea (Kramarsky-Winter et al. 1997). Given the declining population sizes and health of Caribbean corals (Gardner et al. 2003), this asexual survival strategy cannot be ruled out, although its occurrence in Montastraea spp. has not been documented. A more likely explanation and one that accounts for the differences observed between the species, however, may be physical colony fragmentation. The importance and association of clonality via colony fragmentation and disturbance has been well documented for coral (Tunnicliffe 1981; Hughes et al. 1992; Coffroth and Lasker 1998). Fragmentation and disturbance also may explain the differences in the apparent rates of clonality seen among the Montastraea morphotypes. Unlike the broad-based M. faveolata, the more columnar and sometimes top-heavy growth form of M. annularis is particularly predisposed to physical fragmentation (Edmunds 1994), and lobes could be broken off and scattered by storms. A similar growth form was believed to have enabled a now extinct organ-pipe M. annularis-like morphotype to colonize regions of high illumination in shallow water habitats, but predisposed it to fragmentation during times of disturbance (Pandolfi et al. 2002). Hurricanes can cause substantial damage to coral reefs, and because coral reefs in the Caribbean have been subjected to considerable storm activity over the past few decades (Rogers et al. 1991; Rogers 1992; Gardner et al. 2003), such disturbances likely are a proximate force for colony fragmentation in M. annularis. In addition, concerns about changes in ocean chemistry due to global warming resulting in carbon-dioxide-induced coral skeletal dissolution (Kleypas et al. 1999; Hughes et al. 2003) are particularly relevant to this species, as this predicted climate change would act to compound an already structurally compromised physical morphology of M. annularis.
During the initial colonization of these reefs, it is possible that they were settled by cohorts of larvae from geographically subdivided and genetically differentiated subpopulations resulting in admixture. Reefs would be similarly admixed if, over the course of the history of a reef, self-recruitment (i.e., larvae recruiting back to reefs from which they were spawned) was low and larval sources for particular locations frequently changed depending on hydrodynamic conditions (Wolanski 1994). Our observed deviations from Hardy–Weinberg genotypic frequency equilibrium and linkage equilibrium in some populations are consistent with mixing of genetically differentiated populations resulting in admixture (e.g., Dry Tortugas). We do not, however, see indications of widespread departures from equilibrium and therefore do not believe it is a dominant feature of underlying evolutionary processes occurring in these Montastraea species. Nevertheless, it is interesting that the population most affected by both Hardy–Weinberg and linkage disequilibria was the Dry Tortugas M. annularis population, even when the factors of clonality and null-allele effects were removed from the analyses. This location is considered to reside in an oceanographic hotspot (Lee et al. 1994; Lee and Williams 1999) that is particularly subjected to temporal micro- and meso-scale current fluctuations.
Large and small-scale oceanic water circulation patterns can interpose between random events and the biology of a species and may account for the contrasting patterns of genetic connectivity observed in this study. Water flow in the Caribbean basin is complex with current patterns characterized by the presence of eddies, meanders, and transient gyres that can act as mechanisms for larval access to and retention in inshore settlement sites (Yeung and McGowan 1991; Lee et al. 1992, 1994; Criales and Lee 1995; Lee and Williams 1999; Limouzy-Paris et al. 1997; Yeung and Lee 2002, Cowen et al. 2006). Figure 2 illustrates the complexity of surface current movement as tracked by 294 drift buoys released from 1978 to 2003 and archived and analyzed by the National Oceanographic and Atmospheric Administration, Atlantic Oceanographic and Meteorological Laboratory program (Gyory et al. 2005). This plot shows a considerable number of areas devoid of tracks as well as numerous eddies where buoys are temporarily entrained in local vortices (e.g., Fig. 2 bold track). Similarly, Murphy and Hurlburt (1999) using linear and non-linear simulation models have demonstrated how decaying rings broken off from the North Brazil Current during retroflection are advected through the Lesser Antilles and form anticyclonic eddies. These eddies transit the Caribbean, often intensifying greatly along the way, before emerging into the Gulf of Mexico through the Yucatan Channel approximately 10 months after entering the basin. Larvae entrained in these eddies would only be able to settle if or when these cohesive water masses next encounter suitable habitat. A linear relationship of geographic distance between reefs and degree of genetic connectivity, therefore, would not be expected. With our data, correlations of genetic [(δμ)2 and Nei’s D] with geographic distances were not significant (data not shown) for either M. annularis where most pairwise tests of population differentiation were significant or for M. faveolata where genetic homogeneity indicative of widespread dispersal would presumably reflect isolation-by-distance mechanisms. Given a somewhat limited geographical sampling, however, we may lack the power to detect isolation-by-distance, if present.
Spaghetti plot tracks for 294 near-surface drift buoys from 1978 to June 2003. This figure is based on, and modified from, the National Oceanographic and Atmospheric Administration, Atlantic Oceanographic and Meteorological Laboratory Drifting Buoy Data Assembly Center data (see Gyory et al. 2005). All of the buoys were released in the southeastern Caribbean (i.e., lower right of the figure) and the principle direction of flow is to the northwest (i.e., upper left of the figure). The bold path corresponds to the track of buoy 09526392 from late March to early November 1996. During the August spawning season of Montastraea spp., the buoy spent the first three weeks in the loop indicated with an arrow
With respect to our genetic data in the context of ongoing oceanographic research in this locale (Yeung and McGowan 1991; Lee et al. 1992, 1994; Criales and Lee 1995; Lee and Williams 1999; Limouzy-Paris et al. 1997; Yeung and Lee 2002), we posit that genetic connectivity among reefs due to ocean currents will be significantly influenced by factors peculiar to each species, spawning season, spawning year, and geographical location as well as inherent biological factors. The heterozygote deficiencies of the Dry Tortugas, for example, could very well be the result of localized eddies resulting in enhanced settlement of related recruits in this region for M. annularis but not for M. faveolata. The lack of divergence among M. faveolata populations or the presence of divergence among M. annularis may simply be a result of chance timing of larval entrainment in eddies either enhancing or retarding inter-reef dispersal. Since these are long-lived organisms as adults (a typical one-meter diameter, adult colony is at least a century old [Hughes and Tanner 2000]), the genetic signal of historical events also will be detected far into the future. Taken together, we believe that the highly variable and sometimes counterintuitive inferences drawn from studies of population connectivity in corals, Caribbean and Pacific alike, are a reflection of the variable hydrodynamic regime in which the species live.
The inability of species to realize a level of population connectivity commensurate with intrinsic dispersal potential can be attributed to a complex web of interactions (Hedgecock 1986; Connell et al. 1997). The population genetics of these two Montastraea spp. indicate that connectivity must be gauged on a species by species basis even for sympatric, closely related taxa with seemingly identical life history characteristics. This conclusion holds important implications for the conservation and management of natural marine systems. The establishment of marine reserves as a fisheries management and conservation tool is fast replacing classical, target-species catch and effort quota approaches, but debate centers on the placement, size, and arrangement of marine protected areas (MPAs) (Ogden 1997; Roberts and Schmidt 1997). In theory, MPA design and management strategies preserve processes acting at ecological scales (i.e., dispersal and recruitment among present-day populations); yet genetically defined connectivity encompasses evolutionary scales (Leis 2002). The results of this research emphasize that indirect assessments based solely on presumed dispersal potential or even population genetic structure may be misleading. Considerations such as the frequency of clonal reproduction or temporal variation in reproductive success may be more important than larval dispersal potential in defining the health or connectivity of coral reefs. As such, the results add additional support for the need for a total ecosystem approach to the design of marine protected areas. Accurately defining distinct species groups or habitats as open (Roberts 1997) or closed (Cowen et al. 2000) may be impossible at anything but the species level. Delineating marine reserves, therefore, may be as data intensive as traditional single-species catch quotas.
References
Ayre DJ, Hughes TP (2000) Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54:1590–1605
Ayre DJ, Hughes TP (2004) Climate change, genotypic diversity and gene flow in reef-building corals. Ecol Lett 7:273–278
Baums IB, Miller MW, Hellberg ME (2005) Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol Ecol 14:1377–1390
Bellwood DR, Hughes TP, Folke C, Nystrom M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bode M, Bode L, Armsworth PR (2006) Larval dispersal reveals regional sources and sinks in the Great Barrier Reef. Mar Ecol Prog Ser 308:17–25
Budd AE, Pandolfi JM (2004) Overlapping species boundaries and hybridization within the Montastraea “annularis” reef coral complex in the Pleistocene of the Bahama Islands. Paleobiol 30:396–425
Cattell MV, Karl SA (2004) Genetics and morphology in a Borrichia frutescens and B. arborescens (Asteraceae) hybrid zone. Am J Bot 91:1757–1766
Cavalli-Sforza LL, Edwards AWF (1967) Phylogenetic analysis models and estimation procedures. Evolution 3:550–557
Cho LL, Woodley JD (2002) Recovery of coral reef at Discovery Bay, Jamaica and the role of Diadema antillarum. Proc Nin Intl Coral Reef Symp 1:331–338
Coffroth MA, Lasker HR (1998) Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution 52:379–393
Connell JH, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Mon 67:461–488
Cowen RK, Luiza KM, Sponaugle S, Paris CB, Olson DB (2000) Connectivity of marine populations: open or closed? Science 287:857–859
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
Criales MM, Lee TN (1995) Larval distribution and transport of penaeoid shrimps during the presence of the Tortugas Gyre in May-June, 1991. Fish Bull USA 93:471–482
Edmunds PJ (1994) Evidence that reef-wide patterns of coral bleaching may be the result of the distribution of bleaching-susceptible clones. Mar Biol 121:137–142
Edmunds PJ, Carpenter RC (2001) Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc Natl Acad Soc USA 98:5067–5071
Ellis J, Solander D (1786) The natural history of many curious and uncommon zoophytes. Benjamin White and Son, London, p 208 63 pls
Fukami H, Budd AF, Levitan DR et al (2004) Geographic differences in species boundaries among members of the Montastraea annularis complex based on molecular and morphological markers. Evolution 58:324–337
Gardner TA, Cote IM, Gil JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
Goldstein DB, Linares AR, Cavalli-Sforza LL, Feldman MW (1995) Genetic absolute dating based on microsatellites and the origin of modern humans. Proc Natl Acad Sci USA 92:6723–6727
Gomez A, Carvalho GR (2000) Sex, parthenogenesis and genetic structure of rotifers: microsatellite analysis of contemporary and nesting egg bank populations. Mol Ecol 9:203–214
Goodman SF (1997) RSTCALC: a collection of computer programs for calculating unbiased estimates of genetic differentiation and gene flow from microsatellite data and determining their significance. Mol Ecol 6:881–885
Gregory JW (1895) Contributions to the paleontology and physical geology of the West Indies. Q J Geol Soc Lond 51:255–312
Gyory J, Mariano AJ, Ryan EH (2005) The Caribbean current ocean surface currents. http://www.oceancurrents.rsmas.miami.edu/caribbean/caribbean_2.html
Hamm DE, Burton RS (2000) Population genetics of black abalone, Haliotis cracherodii, along the central California coast. J Expl Mar Biol Ecol 254:235–247
Hedgecock D (1986) Is gene flow from pelagic larval dispersal important in the adaptation and evolution of marine invertebrates? Bull Mar Sci 39:550–564
Hellberg ME (1994) Relationships between inferred levels of gene flow and geographic distance in a philopatric coral, Balanophyllia elegans. Evolution 48:1829–1854
Hellberg ME (1996) Dependence of gene flow on geographic distance in two solitary corals with different larval dispersal capabilities. Evolution 50:1167–1175
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean reef. Science 265:1547–1551
Hughes TP, Tanner JE (2000) Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81:2250–2263
Hughes TP, Ayre D, Connell JH (1992) The evolutionary ecology of corals. TREE 7:292–295
Hughes TP, Baird AH, Bellwood DR et al (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jones GP, Milicich MJ, Emslie MJ, Lunow C (1999) Self-recruitment in a coral reef fish population. Nature 402:802–804
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Pro Natl Acad Sci USA 101:8251–8253
Karl SA, Avise JC (1992) Balancing selection at allozyme loci in oysters: implications from nuclear RFLPs. Science 256:100–102
Karl SA, Bowen BW, Avise JC (1992) Global populations structure and male-mediated gene flow in the green turtle (Chelonia mydas): RFLP analyses of anonymous nuclear DNA regions. Genetics 131:163–173
Kleypas JA, Buddemeier RW, Archer D et al (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Knowlton N (2001) The future of coral reefs. Proc Natl Acad Sci USA 98:5419–5425
Knowlton N, Jackson JBC (1993) Inbreeding and outbreeding in marine invertebrates. In: Thornhill NW (ed) The natural history of inbreeding and outbreeding. University of Chicago Press, Chicago, pp 200–249
Knowlton N, Weil E, Weigt LA, Guzman HM (1992) Sibling species in Montastraea annularis, coral bleaching, and the coral climate record. Science 255:330–33
Knowlton N, Mate JL, Guzman HM, Rowan R, Jara J (1997) Direct evidence for reproductive isolation among the three species of the Montastraea annularis complex in Central America (Panama and Honduras). Mar Biol 127:705–711
Kramarsky-Winter E, Fine M, Loya Y (1997) Coral polyp expulsion. Nature 387:137
Lee TN, Williams E (1999) Mean distribution and seasonal variability of coastal currents and temperature in the Florida Keys with implications for larval recruitment. Bull Mar Sci 64:35–56
Lee TN, Rooth C, Williams E et al (1992) Influence of Florida Current, gyres and wind-driven circulation on transport of larvae and recruitment in the Florida Keys coral reefs. Cont Shelf Res 12:971–1002
Lee TN, Clarke ME, Williams E, Szmant AF, Berger T (1994) Evolution of the Tortugas gyre and its influence on recruitment in the Florida Keys. Bull Mar Sci 54:621–646
Leis JM (2002) Pacific coral-reef fishes: the implications of behavior and ecology of larvae for biodiversity and conservation, and a reassessment of the open population paradigm. Enviro Biol Fish 65:199–208
Levitan DR, Fukami H, Jara J et al (2004) Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58:308–323
Limouzy-Paris CB, Graber HC, Jones DL, Ropke AW, Richards WJ (1997) Translocation of larval coral reef fishes via sub-mesoscale spin-off eddies from the Florida current. Bull Mar Sci 60:966–983
Mackenzie JB, Munday PL, Willis BL, Miller DJ, Van Oppen MJH (2004) Unexpected patterns of genetic structuring among locations but not color morphs in Acropora nasuta (Cnidaria; Scleractinia). Mol Ecol 13:9–20
Martin B, Nienhuis J, King G, Schaefer A (1989) Restriction fragment length polymorphisms associated with water use efficiency in tomato. Science 243:1725–1728
Michalakis Y, Excoffier L (1996) A generic estimation of population subdivision using distances between alleles with special reference to microsatellite loci. Genetics 142:1061–1064
Miller KJ, Ayre DJ (2004) The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity 92:557–568
Minch E (1995) Microsat Version 1.4. Stanford University, Stanford
Murphy SJ, Hurlburt HE (1999) The connectivity of eddy variability in the Caribbean Sea, the Gulf of Mexico, and the Atlantic Ocean. J Geophy Res 104:1431–1453
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Ogden JC (1997) Marine managers look upstream for connections. Science 278:1414–1415
Palumbi SR (2003) Population genetics, demographic connectivity, and the design of marine reserves. Ecol Apps 13:S146–S158
Pandolfi JM, Lovelock CE, Budd AF (2002) Character release following extinction in a Caribbean reef coral species complex. Evolution 56:479–501
Pandolfi JM, Bradbury RH, Sala E et al (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Paterson A, Lander E, Hewitt J, Peterson S, Lincoln S, Tanksley S (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction length polymorphisms. Nature 335:721–726
Reusch TBH, Stam WT, Olsen JL (2000) A microsatellite-based estimation of clonal diversity and population subdivision in Zostera marina, a marine flowering plant. Mol Ecol 9:127–140
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Roberts CM (1997) Connectivity and management of Caribbean coral reefs. Science 278:1454–1457
Roberts CM, Schmidt KF (1997) ‘No-take’ zones spark fisheries debate. Science 277:489–491
Rocha LA, Bass AL, Robertson DR, Bowen BW (2002) Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae). Mol Ecol 11:243–252
Rodriguez-Lanetty M, Hoegh-Guldberg O (2002) The phylogeography and connectivity of the latitudinally widespread scleractinian coral Plesiastrea versipora in the Western Pacific. Mol Ecol 11:1177–1189
Rogers CS (1992) A matter of scale: damage from Hurricane Hugo (1989) to U.S. Virgin Islands reefs at the colony, community, and whole reef level. Proc Sev Intl Coral Reef Symp 1:127–133
Rogers CS, McLain L, Tobias C (1991) Effects of Hurricane Hugo (1989) on a coral reef in St. John. Mar Ecol Proc Ser 78:189–199
Sammarco PW, Andrews JC (1988) Localized dispersal and recruitment in Great Barrier Reef corals: The Helix experiment. Science 239:1422–1424
Schneider S, Roessli D, Excoffier L (2000) Arlequin version 2.000: A software for population genetics data analysis. Genetics and Biometry Laboratory - University of Geneva, Geneva
Severance EG, Szmant AM, Karl SA (2004a) Microsatellite loci isolated from the Caribbean coral, Montastraea annularis. Mol Ecol Notes 4:74–76
Severance EG, Szmant AM, Karl SA (2004b) Single-copy gene markers isolated from the Caribbean coral, Montastraea annularis. Mol Ecol Notes 4:167–169
Shearer TL, van Oppen MJH, Romano SL, Worheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Sokal RR, Rohlf JF (1995) Biometry. 3rd edn. WH Freeman, San Francisco
Stenberg P, Lundmark M, Saura A (2003) MLGsim: a program for detecting clones using a simulation approach. Mol Ecol Notes 3:329–331
Sunnucks P, England PR, Taylor AC, Hales DF (1996) Microsatellite and chromosome evolution of parthenogenic Sitobio aphids in Australia. Genetics 144:747–756
Swearer SE, Caselle JE, Lea DW, Warner RR (1999) Larval retention and recruitment in an island population of a coral-reef fish. Nature 402:799–802
Szmant AM (1991) Sexual reproduction by the Caribbean reef corals Montastraea annularis and M. cavernosa. Mar Ecol Prog Ser 74:13–25
Szmant AM, Weil E, Miller MW, Colon DE (1997) Hybridization within the species complex of the Scleractinian coral Montastraea annularis. Mar Biol 129:561–572
Taylor MS, Hellberg ME (2003) Genetic evidence for local retention of pelagic larvae in a Caribbean reef fish. Science 299:107–109
Tunnicliffe V (1981) Breakage and propagation of the stony coral, Acropora cervcornis. Proc Natl Acad Sci USA 78:2427–2431
Van Veghel MLJ, Bak RPM (1993) Intraspecific variation of a dominant Caribbean reef building coral Montastraea annularis: genetic, behavioral and morphometric aspects. Mar Eco Prog Ser 92:255–265
Veron JEN (2000) Corals of the World. Australian Institute of Marine Science, Townsville
Vollmer SV, Palumbi SR (2002) Hybridization and the evolution of reef coral diversity. Science 296:2023–2025
Walsh HE, Metzger DA, Higuchi R (1991) Chelex 100 medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10:506–513
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wellington GM, Fitt WK (2003) Influence of UV radiation on the survival of larvae from broadcast-spawning reef corals. Mar Biol 143:1185–1192
Wolanski E (1994) Physical oceanographic processes of the Great Barrier Reef. CRC Press, Boca Raton
Yeung C, McGowan MF (1991) Differences in inshore-offshore and vertical distribution of phyllosoma larvae of Panulirus, Scyllarus, and Sylloarides in the Florida Keys in May-June 1989. Bull Mar Sci 49:699–714
Yeung C, Lee TN (2002) Larval transport and retention of the spiny lobster, Panulirus argus, in the coastal zone of the Florida Keys, USA. Fish Ocean 11:286–309
Acknowledgements
We thank D. Hagman and E. Weil for providing samples from Mexico and Puerto Rico, respectively; A. Bass, C. Curtis, J. Garey, M. Garvey, K. Hayes, C. Lund, K. Overholtzer, L. Robbins, T. Schwartz and the staff at the Florida Keys National Marine Sanctuary for help in obtaining samples; A. Szmant for generously providing gamete bundles used for the genomic library construction; I Baums, B. Bowen, M. Craig, N. Knowlton, C. Puchulutegui, L. Rocha, A. Szmant, M. Zacks and anonymous reviewers for comments on this and previous drafts. Financial support for this work was provided by the Fred and Helen Tharp Foundation and Florida Sea Grant/Aylesworth Foundation grants to E.G.S. and by Florida Institute of Oceanography and National Science Foundation Grant in Systematics DEB 98-06905 to SAK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Nishida, Tokyo
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Severance, E.G., Karl, S.A. Contrasting population genetic structures of sympatric, mass-spawning Caribbean corals. Mar Biol 150, 57–68 (2006). https://doi.org/10.1007/s00227-006-0332-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0332-2