Abstract
Spawning pattern (assessed by seasonal changes in ovarian developmental stages) and type of fecundity (assessed by analysis of oocyte-size frequency distributions) of the round herring Etrumeus teres were studied in relation to ovarian growth and seasonal changes in the gonadosomatic (GSI), hepatosomatic (HSI) and liposomatic (LSI) index as well as the somatic condition of spawners (CS) in a spawning ground of southern Japan. Except for summer, mature and recently spawned ovaries occurred all year round. Oogonia and primary oocytes were present in all ovaries, and cortical alveoli stage (CA) oocytes occurred in all mature, hydrated and partially spent (PS) females (PS: females containing post-ovulatory follicles). Before hydration, a clutch of larger yolked oocytes, undergoing synchronous growth (range 0.7–1.1 mm), was present in mature ovaries which was completely separated from a more heterogeneous clutch of oogonia, primary and secondary oocytes (<0.150 mm) and oocytes in the CA stage (range 0.15–0.60 mm). As vitellogenesis progressed, the yolked clutch increased in size but the CA oocytes remained arrested. The latter entered into the secondary growth phase when hydration started in the advanced batch. Ovarian growth was isometric in all developmental stages, validating the use of GSI, which showed a consistent monthly evolution among years. Spawning stopped in summer (July and August) and peaked in winter and spring. HSI correlated positively with GSI on both a monthly mean basis (r = 0.76) and individual fish basis (liver weight explained 67–83% of the variability in ovary weight when females were grouped into 1-unit GSI intervals) suggesting a significant role of liver in vitellogenesis. LSI and CS also showed marked seasonal changes peaking from summer to middle autumn. Overall results suggest that E. teres is a multiple spawner with a group-synchronous ovarian development and indeterminate annual fecundity, with the three processes linked to an isometric growth of the ovary. We propose that such a reproductive pattern is an adaptation to produce batches of large pelagic eggs through a protracted spawning season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of spawning episodes during a reproductive season and the pattern of ovarian development normally determine the spawning strategy in teleosts. Fishes spawning only once develop their oocytes synchronously from oogonia to immature oocytes, vitellogenesis and final maturation (i.e. fishes with synchronous ovarian organization; Marza 1938; Wallace and Selman 1981). Other fishes spawn several times during the spawning period release clutches or batches of eggs at discrete intervals (Maddock and Burton 1998; Stequert et al. 2003) through either a group-synchronous or asynchronous oocyte development. Multiple-spawning fishes with group-synchronous ovaries have a discrete size distribution of oocytes with a distinctive gap between immature and advanced yolked oocytes prior to spawning in common. The existence of such a gap has been considered as indicative of determinate fecundity, i.e., all oocytes in the advanced batch will be released during the spawning season in successive batches (Marza 1938; Wallace and Selman 1981; Horwood and Walker, 1990; Kjesbu et al. 1990; Hunter et al. 1992; Hesp et al. 2004). For these fishes, the annual fecundity can be estimated from the stock of yolked oocytes at the onset of the spawning season. Conversely, multiple-spawning fishes with asynchronous oocyte development show a continuous size frequency distribution of oocytes in mature ovaries without a dominant population, except during hydration when hydrated oocytes appear clearly separated from non-hydrated oocytes. For these fishes, termed as indeterminate spawners, the annual fecundity can only be estimated by the product of batch fecundity and the number of spawnings per season (Marza 1938; Hunter and Goldberg 1980; Wallace and Selman 1981; Hunter and Macewicz 1985; Lowerre-Barbieri et al. 1996; Militelli and Macchi 2004).
When addressing the spawning strategy, it is also important to consider the ovarian growth as season progresses, because reproductive cycles are coupled with pronounced changes in gonadal size (Somarakis et al. 2004). A widely used method for such a purpose is the calculation of the gonadosmatic index (GSI). The latter should not be used without proper validation (DeVlamming et al. 1982; Somarakis et al. 2004). Validation of GSI requires to test for isometry, i.e., to confirm that the slope of the relationship: log(body weight) = log(a) + b log(ovary weight), is not significantly different from “1”, and this pattern does not change among ovarian developmental stages (Somarakis et al. 2004). Further justification for studying ovarian allometry in a given species rises from the evidence presented in a recent publication (Somarakis et al. 2004), that the pattern of ovarian allometry might reflect the pattern of oocyte growth (i.e., isometric vs. allometric ovarian growth could reflect group-synchronous vs. asynchronous oocyte development).
Round herring [Etrumeus teres (DeKay 1842)] is a commercially important clupeoid, distributed extensively in subtropical and tropical waters, with several separate populations in the Red Sea, eastern Africa, Japan, southern Australia, eastern Pacific, and western Atlantic Oceans. Around the Japanese archipelago, the round herring inhabits southern Japan with two distinctive stocks: the Tsushima warm current stock and the southern Pacific stock. Total annual catch of the round herring in Japanese waters has ranged from 20,000 to 60,000 tons in the last decade and the stock size of this species seems to be more stable than other clupeoids (Ishida et al. 2004; Ohshimo 2004). Despite its commercial importance and extensive geographic distribution, only few studies on its ecology and reproductive biology have been carried out to date (Chullarson et al. 1977; Hara 1977; Yamada 1994; Yanagawa 1996; Honda et al. 2002). These studies suggest that this species is likely to be a multiple-spawner, reaching first maturity at around 16 cm body length and 7 months of age, and that it has an asymptotic length from 22 to 24 cm and a life span from 2 to 4 years. Many aspects of its reproductive and life history traits remain still unknown, in particular, the spawning pattern and type of fecundity and how both processes are related to ovarian growth.
The aim of this study was to highlight the pattern of oocyte growth (i.e., asynchronous or group-synchronous) and the type of fecundity (i.e., determinate or indeterminate) in E. teres and their relationships to the ovarian growth pattern (i.e., allometric or isometric) as well as to the seasonal evolution of the gonadosomatic index (GSI), the hepatosomatic index (HSI), the liposomatic index (LSI) and the somatic condition of females.
Materials and methods
Biological data and reproductive parameters
Female E. teres were collected by angling and gill nets in Tosa Bay (southern Japan) onboard research vessels of the National Research Institute of Fisheries Science. Tosa Bay, located in the southwestern part of Shikoku Inland, is one the main spawning grounds for the round herring and for many other commercial important fishes (Hayashi et al. 1988; Honda et al. 2002).
A total of 1,933 females, ranging from 16 to 30 cm, were collected and analyzed over a period 56 months from September 1999 to September 2004 (Table 1). Angling samples consisted 90% of total catches and were taken at daytime from 11:00 to 17:00 hours. The remaining percentage corresponded to gill net samples taken at night from 22:00 to 24:00 hours. For each fish, total length (TL), total body weight (BW), eviscerated body weight (EBW), ovary weight (OW), liver weight (LW) and wet visceral fat weight (FW) were recorded. Visceral fat (adhered to the dorsal part of the abdominal cavity, and around the stomach and gut) was carefully removed using forceps and weighted using an analytical balance. All measurements of size and weight were made to the nearest 0.1 mm and 0.01 mg. The seasonal fluctuation in OW, LW, and FW were analyzed on a monthly basis using three indices calculated by the following general formula:
where i = gonadosomatic index (GSI), hepatosomatic index (HSI), liposomatic index, (LSI), MV = weight of the respective variable, i.e., OW, LW, or FW. Subsamples of fish were further analyzed in order to assess: (a) the histological appearance of the ovary and its relation to macroscopic classification, (b) ovarian growth, (c) pattern of oocyte development, and (d) seasonal fluctuations of reproductive and condition indices.
Histological appearance of the ovary and its relation to macroscopic classification
To determine the spawning pattern, ovaries of 1,090 females collected from January 2003 to September 2004 were classified into macroscopic maturity stages according to the criteria of Table 2 and fixed in 10% neutralized formalin. To evaluate the reliability of macroscopic classification histological sections were prepared and analyzed for a sub-sample of 280 females. Pieces of ovarian tissue were embedded in paraffin, sectioned to 6–9 μm and stained with Harris’s hematoxylin and eosin Y.
Ovarian growth
Ovarian growth was assessed in a random sample of 600 females collected from January 2003 to September 2004. All females were assigned to 1-mm intervals of mean oocyte diameter of the most advanced mode (MODAM, see below), i.e., 0.4–05; 05–06; 06–07; 0.8–09; 09–1, 1–1.1, and >1.2 mm. The traditional assumption for a valid GSI is that the slope from the relationship, log(BW) = log(a) + b log(OW), is not significantly different from unity in the different maturity stages (Somarakis et al. 2004). Hence, t tests were used to test for isometry (b = 1) and ANCOVA models to test for homogeneity of slopes and intercepts among MODAM classes (Sokal and Rohlf 1997).
Pattern of oocyte development
The pattern of oocyte development was assessed by size frequency distributions of oocytes in random samples of 200 oocytes per ovary. For this purpose, we first tested for differences in oocyte size between ovarian lobes and among three ovarian positions (anterior, middle, posterior). Since no significant differences were detected (two-way ANOVA, P > 0.05) a random portion was photographed under a stereo microscope with a digital camera under a constant light setting. Yolked oocytes were yellowish in mature ovaries (opaque under transmitted light) and easily recognizable from the unyolked oocytes (see Fig. 4c in “Results”). After suitable calibrations, the oocytes were measured using the Image-Pro software. The fixative affected the roundness of the oocytes in some females, particularly as development progressed. Hence, the average from the shortest and longest axis from 50 oocytes was considered as an individual measurement of the mean oocyte diameter of the most advanced mode (MODAM). MODAMs were used for further comparisons with the histological sections of the ovary tissues. The unyolked mode consisted of cortical alveoli oocytes (CA), which appeared hyaline under induced light, primary oocytes and oogonias. The latter two groups were very abundant and mostly <150 μm and hence were not included in the graphs to better illustrate the frequency of CA and yolked modes. It must be pointed out here that there was not a gap between CA oocytes and the less developed groups (see Fig. 3b in “Results”). The modal progression was studied by grouping females according to MODAM.
Seasonal fluctuations of reproductive and condition indices
The seasonal fluctuation in GSI, HSI, LSI and somatic condition was studied in females collected from January 2002 to September 2004, months in which a more comprehensive and continuous data set was available (March and May 2002 were not included due to small sample sizes). A previous evaluation of the TL–EBW relationships showed that the slope of the regression varied significantly among months. Hence, neither the Fulton condition factor nor the relative condition factors were appropriate to describe the condition of spawners as season progressed (see Cone 1989 for details). In consequence, the seasonal fluctuation in the somatic condition of fish was assessed through the index suggested by Patterson (1992) (Eq. 2):
where “EBW” is the eviscerated body weight, “a o” is the overall intercept, “a m” is the monthly effect, “L” is the total length, and “e i ” in the error term. In this analysis a common weighted mean slope was forced but data from different months were allowed a have a different intercept. Then, the residual error can be considered an index of fish condition irrespective of the length and month in which the samples were taken (see Patterson 1992 for details).
For a descriptive purpose least square means for GSI, HSI, and LSI from similar GLMs with month as a fixed effect were also estimated.
Integrated analysis
To test if the monthly fluctuations of maturity and condition indices over an annual cycle were not merely a result of differences in sampling size and data variation, an overall analysis pooling the entire data set of Table 1 was carried out. For this purpose, the following GLM model was applied for ovary, liver and fat weight:
where “MV” is the weight of the respective measured variable (i.e., OW, LW, or FW), “a o” is the overall intercept, “a m” is the monthly effect, “b y ” is the year effect, “c s ” is a dummy variable used as indicator of reproductive condition of ovaries (active and/or resting), and “EBW” is the eviscerated body weight. The model also includes the interactions between fixed factors (a m; b y ; and c s ) and the covariate (log EBW). A backward stepwise method was used to select the final model. The resulting monthly least square means (LSM) was used to assess the seasonal variation of OW, LW, and FW, and the Bonferroni test was used for multiple comparisons in LSMs over the annual cycle.
Similar analysis of the condition of spawners was made by using Eq. 1, but adding a year effect (b y ) with no interaction terms. Females with hydrated oocytes were excluded from all GLM analyses, because hydrated females were not collected consistently during all months.
Results
Maturity stages
Seasonal occurrence of the macroscopic maturity stages showed a protracted spawning season (Fig. 1). Maturing and recently spawned ovaries occurred almost all year round expect for summer; in particular, in August when reproduction of the entire population ceased. Oogonia and primary oocytes were present in all ovaries examined, and in resting ovaries some oocytes were in the initial phase of the cortical alveoli stage (Fig. 2). Oocytes in the late cortical alveoli stage were present in all maturing, hydrated, spawning and postspawning females (Table 2). Females classified macroscopically as “maturing” encompassed a wide range of oocytes, but almost all had central vesicle, 20% had POFs in late phase and 6% were in the migratory nucleus stage (MNS). MNS oocytes, however, were observed in all ovaries undergoing hydration, which also contained yellowish partially yolked oocytes. The spawning stages showed a low occurrence through the entire study period, and hence they were not included in further analyses.
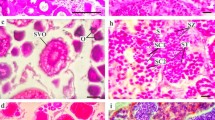
Etrumeus teres. Frequency of occurrence of oocyte developmental stages and other histological structures in the four macroscopic maturity stages for a random sample of females collected from April 2003 to March 2004. Og Oogonia; Po primary oocytes; Ca cortical alveoli oocytes; Py partially-yolked oocytes; Ay fully yolked oocytes; Mn migratory nucleus oocytes; Pf post ovulatory follicles present. P-spent postspawning
Ovarian allometry
EBW and OW were isometrically related in all MODAM classes, with slopes homogenous (ANOVA F (7, 559) = 0.64, P = 0.76) and not significantly different from 1. (all P > 0.05) (Table 3). The intercepts varied significantly among MODAM classes (ANOVA F (7,566) = 338.65, P < 0.001). All allometric equations were highly significant, with F values ranging from 59 to 441 (P < 0.001) and with female weight explaining more than 50% of the variability in ovary weight (Table 3).
Pattern of oocyte development
Figure 3a illustrates the modal progression of 60 females collected during January 2004, sequentially sorted by the mean oocyte diameter of the most advanced mode (MODAM). The onset of the modal progression corresponds to ovaries of pre-spawning or partially spawned females. Histological observations of partially spawned ovaries showed the occurrence of post-ovulatory follicles (Fig. 4a). As vitellogenesis progresses the larger clutch of yolked oocytes increases in size (Fig. 4b) but the smaller oocytes remain temporally detained. The less developed oocytes appeared hyaline under induced light (Fig. 4c) and corresponded to cortical alveoli oocytes, which are not fully separated from an undeveloped group encompassing oogonias and primary oocytes (Fig. 3b). Just when hydration begins the distinctive clutch of cortical alveoli oocytes seem to move to the true vitellogenesis stage, with ovaries in MNS stage ranging from 0.99 to 1.09 mm in MODAM. A fraction of the less developed mode turns yellowish (opaque under induced light; Fig. 4c). Histological examination confirmed that the yellowish opaque oocytes in hydrated ovaries were in early vitellogenesis. Hydration seemed to be gradual showing hyaline oocytes with different levels of yolk coalescence (Fig. 4d). Hydrated oocytes ranged from 1.1 to 1.5 mm, with the mean oocyte diameter ranging from 1.2 to 1.4 mm corresponding to an increase in egg volume from 1.78 to 2.1 times (i.e., 43–50%) due to hydration. In addition, the less advanced batch becomes bimodal. The distinctive gap found between the smaller oocytes and the clutch of larger yolked oocytes undergoing synchronous growth was also observed when mature ovaries were analysed in June 2003, a month representative of ovaries close to the resting period (Fig. 3c).
Etrumeus teres. a Modal progression (MP) of the frequency distribution of intraovarian oocytes sorted by the mean diameter of the most advanced mode of oocytes (MODAM) in 60 females E. teres collected in January 2004. Light grey areas ovaries with Py oocytes; dark grey areas ovaries with Ay; White areas hydrated ovaries. b Oocyte size frequency distribution in a maturing fish. Striped bars denote oogonia and oocytes in primary and secondary growth phase smaller than 0.15 mm that were excluded from MP analyses. Open bars denote cortical alveoli oocytes and filled bars yolked oocytes. c MP analysis in 30 mature females collected in June 2003, a month close to the resting period. Y axes were not drawn in MP graphs to make them easier to understand
Etrumeus teres. Histological sections of a postspawning (a) and mature (b) female along with intact sections under induced light of mature (c) and hydrated (d) ovaries. Po primary oocyte; Ca cortical alveoli stage; Py partially-yolked oocytes; Pof post-ovulatory follicles; Ay advanced yolked stage; hydrated oocyte with low (H1) and high (H2) levels of lipid coalescence. Scale bar = 0.5 mm
Seasonal variation in maturity and condition indices
Least square means of GSI showed seasonal fluctuations (Fig. 5a), during the protracted spawning season. Reproductive activity lasted for about 10 months, practically with no reproductive activity in August. HSI index showed similar seasonal variation (Fig. 5b) although somewhat more variable than GSI. LW and OW were positively regressed, but a great variability was observed for pooled data (Fig. 6 pooled) and also on a monthly and annual basis (results not shown here). However when females were grouped by 1-unit GSI interval, the LW explained from 67 to 83% of the variability in OW (Fig. 6, GSI 2–9) except for females with GSI <2 units (i.e., resting ovaries occurring mainly in July and August). The slopes of the regressions were not homogenous as ovary development progressed (ANCOVA interaction GSI code × LW; F (8,1230) = 52.19 P < 0.001). Mean monthly GSI and HSI were positively correlated, and the correlation was at a maximum when both the data from July and August were excluded and more than 30 observations by month were used (r = 0.76 F (1,20) = 26.27, P < 0.001).
Etrumeus teres. Seasonal and interannual fluctuations in least square mean (LSM) derived from three general linear models (GLM) with gonadosomatic index (GSI; a), Hepatosomatic index (HSI; b) and Liposomatic index (LSI; c) as dependent variable and month as a fixed factor. Condition of spawners (d) corresponds to monthly effects from a GLM of the form Log (EBW) I = ao + am + β log L i + e i where “EBW” is the eviscerated body weight, “ao” is the overall intercept, “am” is the monthly effect, “L” is the total length, and “e” in an error terms
LSM of LSI ranged from 0 to 2% showing a marked seasonal pattern (Fig. 5c) with a major peak in the accumulation of intra-visceral fat in summer, and with a small peak from May to June during 2004. The condition of the spawners evaluated through the monthly effect GLM model showed a somewhat more variable seasonal pattern than the LSI, (Fig. 5d), but coinciding in summer during the resting period. The model explained 97% of the variation in the eviscerated body weight.
Overall analysis of seasonal variation in maturity and condition indices
The results using pooled data showed the following three general patterns: (a) significant monthly fluctuations in LSM of OW, LW and FW (Table 4) closely matching to their respective ratio indices (i.e., GSI, HSI, and LSI; Fig. 7), (2) an inverse pattern between FW (LSI) with OW (GSI) and LW (LSI) for both the resting period of summer and the small peak of FW in autumn (Fig. 7), and (3) a significant but small contribution of the year effect on the modeled variability. The GLMs explained 83, 68 and 73% of the total variation in OW, LW and FW, respectively. Stepwise regression analyses showed that the effect of reproductive condition (main effect) was only highly significant for ovary weight owing to its marked differences between active and resting females (Fig. 8a), although the interaction was not statistically significant (Table 4). Final stepwise model for liver weight was similar to Eq. 3 but did not included reproductive condition as fixed factor (main factor and interaction were left out as part of the stepwise process: Table 4 and Fig. 8b), while for visceral fat only an additive model was enough with a m; b y ; and c s as main factors, due to the low correlation and high variance in the FW–EBW relationship (Table 4; Fig. 8c). The monthly effect of condition of spawners showed significant differences (F = 153.85, P < 0.001) following the same seasonal pattern as FW and LSI ratio (Table 5). The model accounted by 95% of the variability in eviscerated body weight, with only a 2% of the modeled variability accounted for by the year effect.
Etrumeus teres. Least square mean (LSM) of monthly effects of log-transformed ovary weight (OW), liver weight (LW) and visceral fat weight (FW) derived from a GLM using all females analyzed (see text). Vertical bars denote one standard error and different numbers in the base of each figure denote significant differences (P < 0.05: Bonferroni multiple comparison test). The secondary Y axis corresponds to LSM of ratio indices from a GLM model with raw GSI, HSI, and LSI as dependent variables, and month as a fixed factor
Etrumeus teres. Scatterplots illustrating the degree of association between eviscerated weight and ovary weight (OW; a), liver weight (LW; b) and visceral fat weight (FW; c) as related to the reproductive condition of females (i.e., active or resting). All regressions were statistically significant at P = 0.001
Discussion
Reproductive strategy
The following evidence support that E. teres is a multiple-spawner fish: (a) the protracted spawning season shown by the monthly evolution of gonad weight and somatic indices which imply that the population is reproductively active almost all year round except for summer; (b) the temporal pattern of occurrence of macroscopic and microscopic stages of ovaries; (c) the presence of post ovulatory follicles in partially spent ovaries, and (d) the dynamics of growth of intraovarian oocytes determining the type of fecundity (i.e., group synchronous spawner with indeterminate fecundity).
The gonadosomatic index has been widely used as a low cost indicator of reproductive condition in fishes. However, whereas some studies have recommended their use (Barbieri et al. 1996; Claramunt and Roa 2001), others have found that GSI is a poor indicator of spawning activity, particularly in multiple-spawners fishes with asynchronous ovarian organization (DeVlaming et al. 1982; Erickson et al. 1985; DeMartini and Lau 1999). For E. teres, however, ovarian growth was isometric, suggesting that GSI represent well the dynamics of ovarian development. Recently, Somarakis et al. (2004), found that ovarian development was also isometric in the Mediterranean sardine, Sardina pilchardus, which is a group-synchronous spawner with indeterminate fecundity (Ganias et al. 2003). Based on Sardina pilchardus and the results reported for the common snook, Centropomus undecimalis (a species with a similar reproductive strategy), Somarakis et al. (2004) suggested that a relationships could exist between the pattern of oocyte development and the pattern of ovarian allometry, i.e., isometric versus allometric ovarian development could reflect group-synchronous versus asynchronous oocyte development. The results of the present study on E. teres fully support this hypothesis.
A significant correlation was found across months between HSI and GSI. For most teleosts the liver is where the precursor of yolk (vitellogenin) is synthesized. Hence, the liver might enlarge during the female reproductive season as a response to vitellogenin needs (Wallace and Selman 1979). However, the relationship between liver and ovary weight is not always as strong and positive as could be expected (Yoneda et al. 1998). This is because the liver is also associated with storing reserves and the hepatosomatic index is also an indicator of recent feeding activity (Delahunty and DeVlaming 1980; Maddock and Burton 1998; Tomasini et al. 1999). For E. teres, however, a strong and positive correlation between liver weight and ovary weight was found, and correlation was stronger when both variables were ranked by GSI ranges. Although such correlation is mainly due to the influence of fish size on both organs, it can also reflect a synchronism between reproduction and vitellogenin uptake throughout the protracted spawning season. The increase in the slope of the OW–LW relationship in more advanced reproductive stages suggest that the ovary gains more weight compared to the liver, which is indicative that vitellogenin uptake stop and/or decrease in final oocyte maturation stages. This finding is further supporting the multiple-spawning strategy of E. teres.
Evidence supporting a group-synchronous ovarian organization with indeterminate fecundity
The consistent occurrence of maturing, hydrated and partially spent ovaries from autumn to the subsequent spring and the restricted occurrence of resting females to mainly summer months further argue that the round herring is a multiple-spawner with a protracted reproductive season. In addition, the presence of postovulatory follicles (Hunter and Goldberg 1980) in partially spent ovaries indicates that at least one batch of eggs has been spawned. Further evidence for multiple spawning is also provided by the analysis of the modal progression of the intra-ovarian oocytes. This analysis showed a distinctive gap between the less developed oocytes and advanced yolked oocytes in mature females. The occurrence of a hiatus is the common criteria used to classify determinate from indeterminate spawners (Horwood and Walker 1990; Hunter et al. 1992; Nichol and Acuna 2000); hence, a priori E. teres should be classified as a determinate spawner. However, we found that once oocytes begin hydration a new clutch of oocytes in early vitellogenesis (i.e., partially yolked oocytes) is very distinctive, even without histological examination. Furthermore, the less advanced mode (i.e., mainly composed of CA and Py oocytes) in the frequency distribution of oocytes of hydrated females was bimodal, contrasting with the unimodal feature of the less advanced mode (mainly composed of CA stage oocytes) of mature fish. In addition, CA stage oocytes were present during all year around, decreasing significantly only during the resting months. These three observations indicate that Py oocytes are indeed being recruited into vitellogenesis from cortical alveoli and primary oocytes to be spawned as subsequent batches. In addition, hydration seems to be gradual, with no evidence of bimodality in the frequency distribution of hydrated oocytes, which is indicative that all hyaline oocytes will be spawned simultaneously. All these observations suggest that E. teres has a group-synchronous ovarian development but indeterminate fecundity.
Most clupeoids are multiple spawners with indeterminate fecundity having more than one group of yolked oocytes co-existing in mature ovaries, and showing a continuous frequency distribution of oocyte size for mature ovaries (see Blaxter and Hunter 1982 and references there in). However, recruitment of a distinct clutch from pre-vitellogenic to vitellogenic stages has also been reported in other three clupeoids: the Brazilian sardine (Sardinella brasiliensis, Isaac-Nahum et al. 1988), a tropical anchovy (Encrasicholina heteroloba, Wright 1992), the bay anchovy (Anchoa mitchilli, Luo and Musick 1991), and the Mediterranean sardine, Sardina pilchardus. In addition, group-synchronous reproductive strategy with indeterminate fecundity has also been reported in several other fishes (e.g., in two species of sticklebacks (Gasterosteus aculeatus and Apeltes quadracus, Wallace and Sellman 1979), the American plaice Hippoglossoides platessoides (Maddock and Burton 1998), the common snook, Centropomus undecimalis (Taylor et al. 1998), the dusky grouper, Epinephelus marginatus (Marino et al. 2001); and the swordfish, Xiphias gladius (Taylor and Murphy 1992). It is reasonable to hypothesize that any reproductive strategy in fishes should be linked to the final output product, i.e., the egg size. In the case of round herring we found that the hydrated oocyte ranged from 1.2 to 1.4 mm in winter, which falls within the size range of planktonic eggs reported for this species. Certainly, comparison of intraovarian egg size among species can be spurious owing to the influence on egg size of fixatives, seasonal changes, and/or female size. Despite these limitations, however, the egg size of E. teres seems to be larger than for other multiple-spawning clupeoids with pelagic eggs (Table 6, see also Bagenal 1971). In addition, we also found that the percentage of increase in egg volume due to hydration varied from 35 to 51% which is lower than that of the four/five fold increase suggested for fishes with pelagic eggs (Wallace and Selman 1981; Craik and Harvey 1987). Unquestionably, the increase in volume due to hydration could be a limiting factor to produce large eggs due to the physical limitations of the abdominal cavity, particularly for relatively long-shaped small fishes such as E. teres.
The production of larger eggs intrinsically implies a reduction in batch fecundity. This seems to be true for E. teres, which exhibits lower batch fecundity (range 4,700–20,350 eggs; Plaza et al. unpublished data) in comparison to the Japanese sardine (Sardinops melanostictus; range 16,000–40,000 eggs; Morimoto 1998), when both species co-occur in the same spawning ground. Therefore, the adaptive strategy of developing series of clutches of eggs from smaller oocytes arrested at the cortical alveoli stage, seems to be advantageous to produce a series of larger eggs throughout a protracted spawning season.
Seasonality in maturity indices and condition of spawners
The analyses of maturity and condition of E. teres showed a protracted reproductive period extending from early autumn, continuing throughout winter and reaching the lowest level in summer, when population reached their best condition. However, the combined analysis (pooled data) also revealed a significant decrease in May in estimates of GW and LW as well as in their respective ratios (i.e., GSI, HSI), both of which matched the increase in fat weight and LSI. Although GLM removed variability associated to reproductive condition, eviscerated weight, and year, this finding is hard to explain and likely due to small sample size, spatial heterogeneity in the distribution of spawning, or another unknown effect.
One aspect to consider irrespective of fish size is that the intravascular fat seems to be an important reserve to support reproduction during the winter spawning for this species. For multiple-spawning clupeoids length of the spawning season depends upon the availability of forage (Hunter and Leong 1981; Blaxter and Hunter 1982), and likewise forage depends upon environmental variables in the spawning area. For the case of Tosa Bay, three peaks in chlorophyll a have been recognized, i.e., a minor peak in autumn, the main peak from winter to middle spring, and a secondary major peak in summer (Ichikawa and Hirota 2004). Hence, this species seems to match reproduction to the singular production pattern of this bay, i.e., a multiple-spawning pattern with a minor activity in autumn, the highest level in reproduction coinciding with the spring bloom in primary production, and the resting period matching the summer peak of chlorophyll a, so as to accumulate reserve for the subsequent spawning season. Furthermore, certainly there is a time lag between the peak in primary production and secondary production in coastal waters in middle latitude (Wyatt 1980), and most pelagic species rely on zooplankton rather than phytoplankton for reproduction (see Somarakis et al. 2006). Hence, cross-correlation analysis on a monthly basis between maturity and/or condition indices, and estimates of primary and secondary production would be useful to further evaluate these findings. Indeed, the adaptive choice in some species to use a given costal area as spawning and nursery ground could intrinsically imply that relatively stable and suitable environmental conditions occurs so as to maximize reproduction and survival of the progeny. The consistent seasonality and low percentage of variability accounted by the year effects in the GLMs of the maturity indices and condition of spawners suggest that such a selective mechanism could be operating for E. teres.
References
Bagenal TB (1971) The interrelation of the size of fish eggs, the date of spawning and the production cycle. J Fish Biol 3:207–219
Barbieri-Lowerre SK, Chittenden ME Jr, Barbieri LR (1996) The multiple spawning pattern of weakfish in the Chesapeake Bay and Middle Atlantic Bight. J Fish Biol 48:1139–1163
Blaxter JHS, Hunter JR (1982) The biology of the clupeoid fishes. In: Blaxter JH, Russel FS, Yonge M (eds) Advances in marine biology, vol 30. Academic, Dublin, pp 1–223
Chullarson D, Mako H, Oka M, Matsuyama Y (1977) Studies on the fisheries biology of the round herring in the western Sea of Japan. Bull Sekai Reg Fish Res Lab 50:37–71
Claramunt G, Herrera G (1994) A new method to estimate the fraction of daily spawning females and the number of spawning in Sardinops sagax in northern Chile. Sci Mar 58:169–177
Claramunt G, Roa R (2001) An indirect approach to estimate spawning fraction as applied to Sardinops sagax from northern Chile. Sci Mar 65:87–94
Cone RS (1989) The need of reconsider the use of condition indices in fishery science. Trans Am Fish Soc 118:510–514
Craik JCA, Harvey SM (1987) The causes of buoyancy in eggs of marine teleosts. J Mar Biol Ass UK 67:169–182
Delahunty G, De Vlaming VL (1980) Seasonal relationships of ovary weight, liver weight and fat stores with body weight in the goldfish, Carrasius auratus (L.). J Fish Biol 16:5–13
DeMartini EE, Lau BB (1999) Morphometric criteria for estimating sexual maturity in two snappers, Etelis carbunculus and Pristipomoides sieboldii. Fish Bull 89:9–18
DeVlaming V, Grossman G, Chapman F (1982) On the use of gonadosomatic index. Comp Biochem Physiol A 73:31–39
Erickson DL, Hightower JE, Grossman GD (1985) The relative gonadal index. An alternative for quantification of reproductive condition. Comp Biochem Physiol A 81:117–120
Ganias K, Somarakis S, Machias A, Theodorou A (2003) Pattern of oocyte development and batch fecundity in the Mediterranean sardine. Fish Res 67:13–23
Hara I (1977) Population studies on round herring—I: on round herring fished off the western coast of San’in district. Bull Jpn Soc Sci Fish 43:265–270
Hayashi M, Taniguchi N, Yamaoka K (1988) Quantitative analysis on fish larvae and juveniles caught by sardine drag net in Tosa Bay, Japan. Rep Mar Biol Inst Kochi Univ 10:83–92
Hesp SA, Potter IC, Hall NG (2004) Reproductive biology and protandrous hermaphroditism in Acanthopagrus latus. Environ Biol Fish 70:257–272
Honda H, Hirota Y, Mitani T, Uehara S, Sakaji H, Nashida K (2002) Seasonal changes in length-frequency distributions and maturity conditions of round herring Etrumeus teres, spawning in Tosa Bay and the occurrence period of the young fish. Fish Biol Oceanogr Kur 3:75–83
Horwood JW, Walker MG (1990) Determinacy of fecundity in sole (Solea solea) from the Bristol Channel. J Mar Biol Assoc UK 70:803–813
Hunter JR, Goldberg SR (1980) Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish Bull US 77:641–652
Hunter JR, Leong R (1981) The spawning energetics of female northern anchovy Engraulis mordax. Fish Bull US 79:215–230
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax, vol 36. NOAA-NMFS, Tech Rep, pp 79–94
Hunter JR, Macewicz BJ, Lo NCH, Kimbrel CA (1992) Fecundity, spawning, and maturity of female Dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish Bull 90:101–128
Isaac-Nahum VJ, Cardoso R del D, Servo G, Rosi-Wongtschowski CL del B (1988) Aspects of the spawning biology of the Brazilian sardine Sardinella brasiliensis (Steindachner, 1879), (Clupeidae). J Fish Biol 32:383–396
Ishida M, Mitani T, Uehara S, Honda H (2004) Stock assessment of the Pacific stock of the round herring Etrumeus teres. In: Stock assessment of the fisheries resources around Japan. Technical report. Fishery Agency and Fishery Research Agency, Tokyo, Japan, pp 471–481
Ichikawa T, Hirota Y (2004) Seasonal changes in primary productivity in Tosa Bay. Oceanogr Jpn 13:259–269
Kjesbu OS, Witthames PR, Solemdal P, Walker MG (1990) Ovulatory rhythm and a method to determine the stage of spawning in Atlantic Cod (Gadus morhua). Can J Fish Aquat Sci 47:1185–1193
Le Clus F (1979) Oocyte development and spawning frequency in the south west African pilchard Sardinops ocellata. Fish Bull S Afr 12:53–68
Lowerre-Barbieri SK, Chittenden ME, Barbieri LR (1996) The multispawning pattern of weakfish in the Chesapeake Bay and Middle Atlantic Bight. J Fish Biol 48:1139–1163
Luo J, Musick JA (1991) Reproductive biology of the bay anchovy in Chesapeake Bay. Trans Am Fish Soc 120:701–710
Maddock DM, Burton MPM (1998) Gross and histological observations of ovarian development and related condition changes in American plaice. J Fish Biol 53:928–944
Marino G, Azzuro E, Massari A, Finoia MG, Mandich A (2001) Reproduction in dusky grouper Epinephelus marginatus (Lowe 1834) from southern Mediterranean. J Fish Biol 58:909–927
Marza VD (1938) Histophysiologie de l’ovogenèse. Herman, Paris
Militelli MI, Macchi GJ (2004) Spawning and fecundity of king weakfish, Macrodon ancylodon, in the Rio de la Plata estuary, Argentina—Uruguay. J Mar Biol Assoc UK 84:443–447
Morimoto H (1998) Relationship between batch fecundity and egg size in Japanese sardine Sardinops melanostictus in Tosa Bay and off Kii Channel, southwestern Japan form 1990 to 1993. Fish Sci 64:220–227
Nichol GD, Acuna EI (2000) Annual and batch fecundity of the yellowfin sole, Limanda aspera, in the eastern Bering Sea. Fish Bull 99:108–122
Ohshimo S (2004) Stock assessment of the Pacific stock of the round herring Etrumeus teres. In: Stock assessment of the fisheries resources around Japan. Technical report. Fishery Agency and Fishery Research Agency. Tokyo, Japan, pp 482–497
Patterson KR (1992) An improved method for studying the condition of fish, with an example using Pacific sardine Sardinops sagax (Jenyns). J Fish Biol 40:821–831
Pina T, Esteves E, Andrade JP (2003) Gross and histological observation of ovarian development in twaite shad, Alosa fallax fallax, from the rivers Mira and Guadiana (Portugal). Sci Mar 67:313–322
Sokal RR, Rohlf FJ (1997) Biometry, 3rd edn. W. H. Freeman, San Francisco 887 p
Somarakis S, Ganias K, Tsepes G, Koutsikopoulos (2004) Ovarian allometry and the use of gonadosomatic index: a case of study in the Mediterranean sardine, Sardina pilchardus. Mar Biol 146:181–189
Somarakis S, Ganias K, Slapatis A, Koutsikopolus C, Machias S, Papaconstantinou C (2006) Spawning habitat and daily egg production of sardine (Sardina pilchardus) in the eastern Mediterranean. Fisher Ocenogr 15:281–292
Stequert B, Menard F, Marchal E (2003) Reproductive biology of Vinciguerria nimbaria in the equatorial waters of the eastern Atlantic Ocean. J Fish Biol 62:1116–1136
Taylor RG, Murphy M (1992) Reproductive biology of swordfish Xiphias gladius in the Straits of Florida and adjacent waters. Fish Bull US 90:809–816
Taylor RG, Grier HJ, Whittington JA (1998) Spawning rhythms of common snook in Florida. J Fish Biol 53:502–520
Tomasini JA, Collart D, Quignard JP (1999) Reserve management strategy for the sand smelt from brackish lagoons in southern France. J Mar Biol Assoc UK 79:145–151
Wallace RA, Selman K (1979) Physiological aspects of oogenesis in two species sticklebacks, Gasterosteus aculeatus L. and Apeltes quadracus (Mitchill). J Fish Biol 14:551–564
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte size in teleosts. Am Zool 21:325–343
Wright PJ (1992) Ovarian development, spawning frequency and batch fecundity in Encrasicholina heteroloba (Ruppell, 1858). J Fish Biol 40:833–844
Wyatt T (1980) The growth season in the sea. J Plank Res 2:81–91
Yamada H (1994) Ecology of round herring Etrumeus teres in Kumano-nada Sea. Bull Jpn Soc Fish Oceanogr 58:286–291
Yanagawa S (1996) Batch fecundity and spawning period of the round herring in Tosa Bay. In: Report of stock assessment of fishery resource by prefectures in southwestern Japan, vol 4, pp 43–53
Yoneda M, Tokimura M, Fujita H, Takeshita N, Takeshita K, Matsuyama M, Matsuura S (1998) Reproductive cycle and sexual maturity of the anglerfish Lophiomus setigerus in the East China Sea with a note on specialized spermatogenesis. J Fish Biol 53:164–178
Acknowledgments
This study was carried out while GP was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science (No. P 03522). We express our gratitude to the captain and crews of the research vessel Kotakamaru for their assistance in the field. Special thanks to Mrs. Kazu Sumikawa for assistance in Laboratory analysis and Joel O’Dea for providing constructive comments on an early draft of this MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Nishida.
An erratum to this article is available at http://dx.doi.org/10.1007/s00227-007-0876-9.
Rights and permissions
About this article
Cite this article
Plaza, G., Sakaji, H., Honda, H. et al. Spawning pattern and type of fecundity in relation to ovarian allometry in the round herring Etrumeus teres . Mar Biol 152, 1051–1064 (2007). https://doi.org/10.1007/s00227-007-0756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0756-3