Abstract
The knowledge of the reproductive biology of blackfin icefish Chaenocephalus aceratus in the southern Scotia Arc has been based, primarily on macroscopic observations of its maturation cycle, and lately, on histological analysis of ovaries in developing phase exclusively. Our study on reproduction of C. aceratus collected at Potter Cove (PC), South Shetland Islands (SSI), highlights the importance of histologic techniques to validate gonadal macroscopic maturity stages to estimate reliable reproductive parameters, in this work obtained from gravid females. Gonado-somatic index (GSI) of 7–21% (13.50 ± 4.20, n = 21), mature oocytes of 2.5–4.0 mm and total fecundity (TF) of 7372–17,212 oocytes/female (12,466 ± 2911, n = 13) were estimated. Although it is known that variations in the reproductive parameters of C. aceratus between areas of the Scotia Arc are linked to local environmental factors (i.e. hydrographic or biotic), we found close agreement between our results from PC and recent literature data for the South Georgia population, which also arose from the analysis of gravid females. Differently, lower GSIs and oocyte sizes, and higher TF reported lately for the offshore SSI population might be at least partially explained by the fact that these parameters were estimated from the analysis of developing ovaries. Based on our sampling and reproductive effort data, we suggest: (1) the spawning period of C. aceratus at the SSI might be more extended than previous belief, starting from late December until June; (2) the sheltered waters of PC might be a spawning site for C. aceratus, which highlights the role of nearshore areas as spawning grounds of notothenioids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctic icefishes, Channichthyinae, are unique among all vertebrates in their lack of hemoglobin, which is a distinctive polar adaptation (Eastman 1993). This subfamily contains 11 genera and includes 23 species that are endemic to the Southern Ocean and one species, Champsocephalus esox, which inhabits the southern Patagonian shelf (Duhamel et al. 2014).
The Scotia Sea or blackfin icefish Chaenocephalus aceratus (Lönnberg 1906) occupies the seasonal pack-ice zone in the Atlantic sector of the Southern Ocean. It is one of the most abundant fish species in shelf waters to 450 m and sporadically to a maximum of 770 m from the northern tip of the Antarctic Peninsula eastwards along the Southern Scotia Arc Islands (South Orkney Islands (SOI) and South Shetland Islands (SSI)) to South Georgia (SG) in the north and around Bouvet Island (Iwami and Kock 1990; Kock and Stransky 2000).
Resembling most icefishes in features and reproductive biology, it was reported that at the SSI C. aceratus females reach high Gonado-somatic index (22–28%) values and spawn large demersal eggs (4.4–4.9 mm) between April and June (Kock and Kellermann 1991; Kock 2005; Kock and Jones 2005). Although sexually mature males and gravid females are not regularly found synchronously at fishing grounds, they are known to form reproductive aggregations and to spawn egg masses guarded by males (Detrich et al. 2005). At SSI larval hatching lasts from July–August to December with a peak in November (Kock and Kellermann 1991; La Mesa and Ashford 2008). Hence, there is an extended period of eggs incubation before hatching that may last from 120 to 150 days (Kock and Kellermann 1991; Detrich et al. 2005).
The classification of gonads in fish based on the external appearance is a simple and rapid method although it has uncertain accuracy and may be too subjective (Kjesbu 2009). It is recognized that the use of histological techniques to separate immature or partially mature specimens from those in resting stage or postspawning condition are more precise than traditional macroscopic approaches (West 1990; Murua and Motos 1998; Saborido-Rey and Junquera 1998). For example, in the channichthyid Champsocephalus gunnari, mature ovaries (with oocytes in normal vitellogenesis) or in atretic condition (with oocytes in resorption) may be macroscopically indistinguishable, resembling a normal stage 2 of maturity in the scale of Kock and Kellermann (1991) (Everson 1984), a fact that was clarified by histological analysis (Macchi and Barrera-Oro 1995). Thus, it is important in determining the reproductive effort parameters and strategies in Antarctic fish, that gonadal maturation is also analyzed histologically (see also La Mesa et al. 2003; Meneghesso et al. 2017; Yates et al. 2018).
The knowledge of the reproductive biology of C. aceratus has been based primarily on macroscopic observations of its maturation cycle (Kock and Kellermann 1991 and references therein). Recently, histological descriptions have been carried out on a limited number of gravid specimens collected around SG (Militelli et al. 2015) and from females in developing phase (stage 3, in Kock and Kellermann 1991) collected in the Southern Scotia Arc (Riginella et al. 2016). As C. aceratus is a total spawner with determinate fecundity (Militelli et al. 2015) the accuracy of estimates of reproductive parameters such as fecundity and oocyte size at spawning should be obtained from the examination of gravid females in late vitellogenesis, or from further developed ovaries with oocytes in the hydration process (stage 4 in Kock and Kellermann 1991). By following this procedure, a potential overestimation of the number of eggs to be released during the breeding season is avoided, as indicated in Murua et al. (2003).
It has been reported that C. aceratus at SG moves to inshore areas to spawn (Kock 1981, 2005; Lisovenko 1988). Pre-spawning and post-spawning individuals have been captured along the shelf as deep as 100 m (Kock 1989; Kock et al. 2000). Yet it is uncertain if this species prefers specific spawning grounds, spawns all along the shelf in suitable areas and depths (Kock 2005), or a combination of both.
Potter Cove, in King George Island/Isla 25 de Mayo, South Shetland Islands, is an inshore site where C. aceratus occurs. It is a spawning locality for the bathydraconid dragon fish Parachaenichthys charcoti (Barrera-Oro and Lagger 2010; Novillo et al. 2018) and possibly for other inshore notothenioid species of the area including C. aceratus (Piacentino et al. 2018).
In this study, we present a macroscopic and histological analysis of the gonadal development of C. aceratus collected in summer at Potter Cove, focusing mainly on gravid females. Our aim is (1) to accurately determine the reproductive parameters for this species and (2) to clarify aspects of its life cycle in the SSI area, including possible spawning sites and reproductive potential.
Materials and methods
Sampling
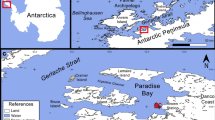
We examined 36 C. aceratus specimens caught at Potter Cove, near the Argentine scientific station “Carlini” (formerly known as “Jubany”, 62°14′S and 58°40′W) from late spring to late summer (November to March) in years 2009–2010, 2014–2015, 2016–2017 and 2017–2018. The sampling gear were trammel nets (length 25 m, width 1.5 m, inner mesh 2.5 cm, outer mesh 12 cm) set from rubber boats on rocky, macroalgae beds at 15–77 m depth (average, 52 m) at a site called Peñón de Pesca in the outer portion of the cove for 16–24 h (Fig. 1). The abiotic features and biotic components of this site as well as further details on the fishing procedures are given in Barrera-Oro et al. (2018). Collection data and the characteristics of the fish analysed are summarized in Table 1.
Macroscopic measurements
Total length (TL) and standard length (SL) of the fish were measured to the nearest 0.1 cm below, and total weight (TW) was recorded in g. After dissection, fish were sexed and their gonads and liver weighed to 0.1 g below. Macroscopic gonadal stage of maturity was evaluated based on the five-point scale for notothenioids (Kock and Kellermann 1991). In most of the fish (21 specimens) the gonads were removed and fixed in 10% buffered formalin for histological analysis and fecundity estimates. The specimens caught in seasons 09–10 and 14–15 were not used for histological study, because the first-caught were frozen after capture whereas in the second-caught only the initial gonadal macroscopic measurements were taken.
Reproductive investment was assessed by estimate of gonado-somatic index (GSI), fecundity and oocyte size (Kamler 1992). GSI and hepatosomatic index (HSI) were determined as the percentage of gonad weight and liver weight to the total fish weight, respectively. No differences in morphology, size and oocyte composition between the two ovaries were observed when inspecting them under magnification. Hence, we used the left ovary for fecundity estimations and the right for histological analyses.
Considering that most notothenioids are total spawners, yolky eggs belong to the only batch of eggs to be released in one single spawning event during the season. Thus, total fecundity (TF) was estimated in gravid females (stage 4) counting the total number of yolked oocytes by the gravimetric method (Murua et al. 2003), and then relative fecundity (RF) was calculated as total fecundity divided by total female weight (RF = TF/TW). Only mature ovaries with hydrated oocytes and absence of postovulatory follicles (POF) were used to estimate fecundity. Linear regression was performed to evaluate the relationship between total fecundity and fish TW–TL.
As no differences in oocyte sizes were found between anterior, middle and posterior sections of the ovary, mean size of yolked or hydrated oocytes was estimated by measuring the maximum diameter of 60 oocytes randomly selected from each of these zones. Likewise, the oocyte size frequency distribution was performed exclusively on gravid females.
Histological technique
For histological analysis, one section of the fixed right ovary or testicle was removed, dehydrated through increasing concentrations of ethanol and embedded in paraffin, following standardized procedures. Sections 7 µm thick were cut, mounted on slides and stained with Hematoxiline–Eosine technique.
To verify the macroscopic stage of gonad development, we analyzed the gonad sections by viewing with a Nikon Optiphot-2 microscope. The histological maturity stage was based on the description and the standardized terminology proposed by Brown-Peterson et al. (2011).
Results
Size and sex composition
Reproductive features were assessed in 36 C. aceratus adult specimens: 26 females ranged between 44.9–66.4 cm TL and 503–2715.22 g and 10 males ranged between 41.4–50.3 cm TL and 507.5–1034.1 g.
Macroscopic and histological analyses of gonadal development
Data on the macroscopic and histologic identification of the gonadal stage and on reproductive effort of the fish analysed are shown in Table 2.
Macroscopically, female were identified as follows: one immature (stage 1), one spent (stage 5), one developing (stage 3), two developing-gravid (stage 3–4) and 21 gravid (stage 4). Likewise, males were identified as: two resting (stage 2), two developed (stage 3), four resting-developed (stage 2–3) and two developed-ripe (stage 3–4).
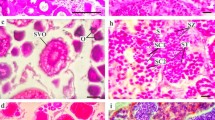
We obtained histological gonad sections from 14 females and 7 males, which are shown in Figs. 2, 3. The single female macroscopically considered as immature (stage 1, specimen 26) showed cortical alveoli stages which indicates that it was in early developing condition (stage 2). Three females that were macroscopically identified as mature or close to maturity (stage 4 or 3/4, specimens 18, 20, 21), were in developing condition (stage 3, Table 2), as inferred from the presence of oocytes in late vitellogenesis but with no signs of hydration (Fig. 2b). Likewise, two males (specimens 34, 35) that were macroscopically considered to be in resting and developed-ripe stage, respectively, were in spent condition (stage 5, Table 2), as evidenced from the presence of residual spermatozoa in lumina of lobules and in sperm ducts of the testicles (Fig. 3c).
Histologic sections of Chaenocephalus aceratus ovaries at different maturity stages: a early developing, with oocytes in primary growth and early cortical alveoli (scale bar 100 µm); b developing, with oocytes in vitellogenesis with yolk droplets, cortical alveoli, primary growth, and atresia (scale bar 100 µm); c gravid (stage 4), showing two main cohorts of oocytes: previtellogenic and hydrated oocytes (scale bar 500 µm); d spent, with postovulatory follicles and residual yolked oocytes at different atresia stages (scale bar 100 µm). PG primary growth, CA cortical alveoli, YO yolk droplets, At atresia, HO hydrated oocyte, POF postovulatory follicles
The early developing female showed oocytes in both primary growth and cortical alveoli stages (Fig. 2a), whereas developing females (stage 3) revealed signs of early vitellogenesis (e.g. cortical alveoli, yolk droplet accumulation) and atresia were present (Fig. 2b). In the case of gravid females, (stage 4) two cohorts of oocytes were easily distinguished: one comprised small sized previtellogenic or primary growth oocytes, and a larger batch was fully mature oocytes with abundant hydrated yolk globules and a thick chorion (Fig. 2c). Lastly, the single spent ovary (stage 5) contained postovulatory follicles and residual vitellogenic oocytes in different stages of atresia (Fig. 2d), indicating that spawning had occurred.
In males, abundant spermatozoa in lumina of lobules and distal spermatogonias were found in developed testes (stage 3) (Fig. 3a). Ripe testes (stage 4) showed spermatozoids uniformly distributed throughout the testis, and empty spermatocysts with some residual spermatozoa were characteristic of spent testicles (Fig. 3b).
Reproductive effort
All the specimens analysed had empty stomachs. In females, GSI values were 0.24% in one early developing (stage 2), between 2.06 and 3.70% in three developing (stage 3), between 6.75 and 21.18% in 21 gravid (stage 4) (X ± SD: 13.50 ± 4.20) and 2.82% in one spent (stage 5). Likewise, in males, GSI values were 0.43–1.81% in five immature (stage 2) or developed (stage 3), 1.68% in one mature (stage 4) and between 0.79 and 0.82% in two spent (stage 5) (Table 2).
HSI values ranged from 5.88 to 10.28% in gravid females and, although limited, these data constitute the only information available for this index in the literature.
Total fecundity was estimated from 13 gravid females (stage 4) between 54.6 and 66.4 cm TL and between 1215.0 and 2715.2 g (Table 2). Although the gravid specimens caught in season 09–10 were not used for histological analyses, they were included in fecundity estimation because there were no differences in total fecundity between seasons (ANOVA, F = 2.89, p > 0.05).
The number of mature hydrated oocytes varied from 7372 to 17,212 oocytes/female (X ± SD = 12,466 ± 2911, n = 13). Relative fecundity ranged from 5.00 to 8.96 oocytes/g (X ± SD = 6.28 ± 1.16, n = 13). There was a positive relationship between total fecundity and the variables TL and TW (Fig. 4). Previtellogenic oocytes ranged from 0.55 to 0.66 mm in diameter with a mean of 0.60 mm, whereas hydrated yolked oocytes ranged from 2.52 to 4.03 mm, with a mean of 3.52 mm (Fig. 5). The bimodal distribution in the oocyte size is shown in Fig. 6.
Discussion
It is known that in vertebrates, including fish, the use of histological techniques allows unequivocal identification of the gonadal maturity stages (West 1990). However, this methodology is not usually used for the analysis of a large numbers of samples because it requires elaborate laboratory equipment and is time consuming. In our study, on the blackfin icefish C. aceratus, the histological analysis of a selected number of gonads allowed us to accurately assess certain stages of maturity that were unclear from macroscopic examination (Table 2). Likewise, describing the exact gonadal stage is essential to reliably determine some reproductive features as fecundity. It is recognized that for total spawners, the optimal developmental stage to estimate fecundity is the pre-spawning phase, when the females are in late vitellogenesis (May 1967; Hunter et al. 1992; Kraus et al. 2000). In line with this premise, our sample of C. aceratus specimens collected at Potter Cove, consisting of mostly spawning females with ovaries in oocytes’ hydration process (stage 4), constituted reliable material for the estimation of the reproductive parameters of the species in the SSI area.
The histological analysis of the ovaries of C. aceratus confirmed the macroscopic characteristic of the two distinct cohorts of oocytes: one for the clutch consisted of large orange-hydrated oocytes in vitellogenic stage ready to be spawned in the upcoming spawning event, and a second clutch of smaller whitish oocytes in previtellogenic stage (Fig. 5). This finding is consistent with the description of “group synchronous ovaries” (Wallace and Selman 1981). The principle of bimodality in the oocyte size frequency distribution of the species (Fig. 6), is characteristic of most notothenioids (Kock and Kellermann 1991). In the histologic sections, gravid females showed large mature oocytes in the hydration process with a thick chorion, and a remnant clutch of primary growth oocytes that will continue developing over the course of the year until the next spawning season (Fig. 2c) (Everson et al. 1996). Postovulatory follicles and the resorption process are clearly distinguished in the spent female samples, supporting the observation that spawning had already occurred (Fig. 2d). With regard to males, the histology confirmed that most were in spent condition (Table 2; Fig. 3b), therefore, the spermatogenesis appeared to have been completed before the spawning period. This is typical of other notothenioids (e.g. Russo et al 2000; La Mesa et al. 2003, 2006) and can be associated with the effect of low temperature in spermatogenesis (Riginella et al. 2016).
Similar data on female C. aceratus reproductive biology in the Scotia Arc region reported in the literature over four decades can be observed in Table 3. The comparison of our results is mainly focused on those obtained in the most recent studies (2010s) where, as in this work, the macroscopic identification of the maturity stages was validated with histology and total and relative fecundity values were given not only in ranges but also with means (± standard deviation).
The reproductive effort parameters of total and relative fecundity and oocyte diameter obtained for C. aceratus at Potter Cove are similar to those reported by Militelli et al. (2015) for the SG population (Table 3). These oocyte diameters are higher than the analogous reported in Riginella et al. (2016) for SSI and SOI, probably because in this last study the measurements were from developing ovaries (stage 3), whereas in our and in the Militelli et al. (2015) studies measurements were obtained from oocytes in the hydration process (stage 4). The ranges of relative fecundity values given in Kock (1989) for Elephant Island and in Permitin (1973) for SG, are similar to those obtained in our study and the Militelli et al. (2015) study, but in the first two works mean values of this parameter were not provided for a more valid comparison.
To date, there is no accurate data on the range of GSI values linked to imminent spawning in C. aceratus elsewhere. Most of the females sampled at Potter Cove were gravid (stage 4), attaining GSIs from 6.7 to 21.2%, with a mean of 13.5 ± 4.2% (Table 3). Although the lower part of the confidence interval of the mean includes values that seem to be too low to reflect the proximity of the spawning event, the advanced gravid stage of the ovaries in the specimens was confirmed with histology (Fig. 2c). The empty stomachs observed in all these fish ensure that GSI estimations were not biased by the incidence of the stomach weights. As in other notothenioid species, fasting in specimens that are close spawning is a common behavior in C. aceratus (Ferrando et al. 2014; Le François et al. 2017). Fewer but larger oocytes in our more advanced gravid females (stage 4) may account for the similar mean GSI values reported in Riginella et al. (2016) for developing females (stage 3) at SSI (Table 3). Additional information on this index for C. aceratus in the Scotia Arc region is lacking. More evaluations of this index together with the microscopic analysis throughout C. aceratus reproductive phase may help to clarify the aforementioned uncertainties, as was observed for the nototheniid Patagonian toothfish Dissostichus eleginoides with a similar methodological approach developed (Yates et al. 2018).
The phenomenon of atresia was described for the developing ovaries of the mackerel icefish, C. gunnari as an isolated event or part of a generalized resorption process (Everson et al. 1991; Macchi and Barrera-Oro 1995). Atresia should be discounted from potential fecundity to determine true annual fecundity (Hunter et al. 1992; Murua et al. 2003). Although in developing ovaries of C. aceratus Riginella et al. (2016) did not observe the presence of atretic oocytes, we found evidence of oocyte resorption in this stage (Fig. 2b). Therefore, it is possible that the higher values of total and relative fecundity reported in Riginella et al. (2016) for SSI’ fish may be due to the fact that these parameters were estimated from the analysis of ovaries in stage 3, instead of gravid stage 4 (Table 3).
It has been suggested that in C. aceratus and other notothenioids at the southern Scotia Arc (SOI and SSI) there is low-population differentiation due to high-gene flow via larval dispersal (Papetti et al. 2009; Damerau et al. 2012). Thus, as suggested by Riginella et al. (2016), differences in reproductive effort observed between C. aceratus populations are likely due to different phenotypic responses to variations in the environmental and ecological conditions of these areas. Apparently, the strong influence of the cold water of the Weddell Sea on SOI shelves (Gordon et al. 2010) accounts for the distinct life history and reproductive traits of C. aceratus’ SOI population in comparison with the SSI population (Riginella et al. 2016). Warmer shelf waters, along with higher krill abundance at SSI in some years (Nicol et al. 2000), may be the reason why fish from SSI invest more in reproduction (= higher fecundity) than SOI fish (Riginella et al. 2016). Nevertheless although the total fecundity estimated in our study for C. aceratus at Potter Cove aligns with these premises, it is probable that the substantially higher values obtained of GSI and oocyte size in comparison with those reported for the SOI population, are at least partially explained by the fact that these parameters were attained from the analysis of gravid females in maturity stage 4.
Given the close agreement in data on reproductive effort for C. aceratus between our research at Potter Cove and that of Militelli et al. (2015) at SG, it is important to highlight that although there was a gap in the seasonal sampling time between areas, the results from both studies were obtained from the macroscopic and histologic analysis of gravid females. Therefore, the known variations in environmental factors such as hydrographic (e. g. higher water temperature, lower salinity and current complexity at SG) or biotic (higher productivity and energy flow at SG) between these two distinct areas of the Scotia Arc are not reflected in differences in the reproductive parameters of C. aceratus in these two studies. Likewise, the observation that fecundity in C. aceratus increases toward higher latitudes (Kock and Kellermann 1991), is not confirmed by our data or that of Militelli et al. (2015). In teleosts, complete histological analysis of samples collected over several years along the entire reproductive cycle would allow a better understanding of interannual variability of gonadal developmental timing and its relationship with environmental factors (Yates et al. 2018).
For notothenioids in general, including most of the Channichthyidae, the duration of the spawning period remains uncertain, and there are inter- and intraspecific variations between areas of occurrence (Kock and Kellermann 1991; Kock 2005). While it may last between 2 months in Chionodraco rastrospinosus and 3–3.5 months in Champsocephalus gunnari at SSI and SG respectively (Kock and Kellermann 1991), Channichthys rhinoceratus at Kerguelen Islands and the Patagonian Champsocephalus esox at Beagle Channel have an extended spawning period of about 9 months (Hureau 1966; Calvo et al. 1999). Although C. gunnari at South Georgia usually spawns from mid-March to May (Olsen 1955; Kock 1981; Everson et al. 2001), inter-annual variations in the onset of spawning in January in some years, and ending in June–July in others have been reported (Alekseeva and Alekseev 1997; Kock 2005). Therefore, within the local environmental context, seasonality, food availability and hydrographic conditions (i.e. water temperature) might be controlling the onset and duration of the spawning period in channichthyid species.
There are differences between our data and the literature regarding the spawning period of C. aceratus at the SSI. There is general agreement that this species spawns from April to June (Kock and Kellermann 1991; Kock 2005; Kock and Jones 2005). Although the sample analysed of C. aceratus from Potter Cove, an inshore site of the same Archipelago, are limited (n = 36), our results on reproductive effort (e.g. GSI, maturity stage and egg measurements), which were supported with histological validation of the advanced gonadal stages—gravid and spent—in females and males, suggests that the species may have spawned 3–4 months earlier in late December to March. Unfortunately, our sampling in the cove did not extend beyond March. Although investigations throughout the whole spawning period of the species elsewhere are lacking for comparison, the fish analyzed in our study were likely part of the first group that attained spawning condition within the reproductive season. From mid-March to early April, Riginella et al. (2016) caught a major proportion of specimens in developing stage 3, which might start spawning in April–June. Hence, we suggest that the reproductive period of C. aceratus at SSI might be more extended, starting from late December until June. If so, C. aceratus would not be a typical autumn–winter spawner, a characteristic that was thought to be common for the notothenioids of the Seasonal Pack Ice Zone and islands north of it (Kock and Kellermann 1991). A similar hypothesis on this feature was suggested for the bathydraconid Parachaenichthys charcoti also sampled at Potter Cove (Novillo et al. 2018).
Based on our sampling and reproductive effort data, we suggest that the sheltered waters of Potter Cove might be a spawning site for C. aceratus. The photograph documented in Detrich et al. (2005) off Bouvetoya Island shows a C. aceratus’ specimen—presumably male—at 140 m depth guarding an egg mass over a shallow depression on a muddy substrate with pebbles lacking of algae. Similarly, the inner Potter Cove, bordered by the Fourcade Glacier (Fig. 1), has a predominant soft-muddy bottom covered with glacial sediments and is devoid of algae (Quartino et al. 2001). There, a mass of eggs of Charcot’s dragon fish P. charcoti laid over a drop stone at 30 m depth was observed during scuba diving (Barrera-Oro and Lagger 2010). Differently, the outer Potter Cove is dominated by rocky bottom covered with macroalgae, with an abundant associated fauna of amphipods, other invertebrates and also fish, which might imply a significant predation over spawned fish eggs. Hence, we suggest that within Potter Cove, the inner zone would be the most appropriated for spawning of notothenioids.
In summary, our study on C. aceratus reproductive biology at Potter Cove highlights the importance of histologic techniques to validate gonadal macroscopic maturity stages to estimate reliable reproductive features. It also supports the hypothesis that near-shore sites in the Antarctic ecosystem might be playing a major role as spawning grounds of notothenioids. We hypothesize that prespawning C. aceratus may initiate spawning migration to inshore areas in Nov–Dec and that this behavior may last the entire reproductive season, but this aspect of the lifecycle of the blackfin icefish has not yet been documented.
References
Alekseeva EL, Alekseev FE (1997) Reproductive biology of mackerel icefish, Champsocephalus gunnari, from the region of South Georgia and Shag Rocks. Vopr Ikthiol 37(3):385–392. Transl as J Ichthyol 37(4):304–311
Barrera-Oro ER, Lagger C (2010) Egg-guarding behaviour in the Antarctic bathydraconid dragonfish Parachaenichthys charcoti. Polar Biol 33:1585–1587
Barrera-Oro E, Moreira E, Seefeldt MA, Valli Francione M, Quartino ML (2018) The importance of macroalgae and associated amphipods in the selective benthic feeding of sister rockcod species Notothenia rossii and N. coriiceps (Nototheniidae) in West Antarctica. Polar Biol 42:317. https://doi.org/10.1007/s00300-018-2424-0
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3:52–70
Calvo J, Morriconi E, Rae GA (1999) Reproductive biology of the icefish Champsocephalus esox (Gunther, 1861) (Channichthyidae). Antarct Sci 11:140–149
Damerau M, Matschiner M, Salzburger W, Hanel R (2012) Comparative population genetics of seven notothenioid fish species reveals high levels of gene flow along ocean currents in the southern Scotia Arc, Antarctica. Polar Biol 35:1073–1086
Detrich HW, Jones CD, Kim S, NorthAW Thurber A, Vacchi M (2005) Nesting behaviour of the icefish Chaenocephalus aceratus at Bouvetøya Island, Southern Ocean. Polar Biol 28:828–832
Duhamel G, Hulley PA, Causse R, Koubbi P, Vacchi M, Pruvost P, Vigetta S, Irisson JO, Moreméde S, Belchier M, Dettai A, Detrich HW, Gutt J, Jones CD, Kock KH, Lopez Abellan LJ, Van de Putte AP (2014) Biogeographic patterns of fish. In: Broyer C, Koubbi P, Griffiths HJ, Raymond B, d'Udekem d'Acoz C, Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G, Huettmann F, Post A, Ropert-Coudert Y (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 328–362
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic Press, San Diego
Everson I (1984) Fish biology. In: Laws RM (ed) Antarctic ecology, vol 2. Academic Press, London, pp 491–532
Everson I, Kock KH, Campbell S, Parkes G, Cielniaszek Z, Szlakowski J (1991) Reproduction in the mackerel icefish, Champsocephalus gunnari, at South Georgia Document WG-FSA-91/7. CCAMLR, Hobart, p 12
Everson I, Kock KH, Parkes G (1996) Ovarian development associated with first maturity in three Antarctic channichthyid species. J Fish Biol 49:1019–1026
Everson I, North AW, Paul A, Cooper R, Mc Williams NC, Kock KH (2001) Spawning locations of mackerel icefish at South Georgia. CCAMLR Sci 8:107–118
Ferrando S, Castellano L, Gallus L, Ghigliotti L, Masini MA, Pisano E, Vacchi M (2014) A demonstration of nesting in two Antarctic icefish (genus Chionodraco) using a fin dimorphism analysis and ex situ videos. PLoS ONE 9(3):e90512. https://doi.org/10.1371/journal.pone.0090512
Gordon AL, Huber B, McKee D, Visbeck M (2010) A seasonal cycle in the export of bottom water from the Weddell Sea. Nat Geosci 3:551556
Hunter JR, Macewicz BJ, Lo NCH, Kimbrell CA (1992) Fecundity, spawning, and maturity of female Dover Sole, Microstomus pacificus, with an evaluation of assumptions and precision. Fish Bull US 90:101–120
Hureau JC (1966) Biologie de Chaenichthys rhinoceratus Richardson et proble`m de sang incolore des Chaenichthyidae, poissons des mers antarctiques. Bull Soc Zool France 91:735–751
Iwami T, Kock KH (1990) Channichthyidae. In: Gon O, Hemstra PC (eds) Fishes of the Southern Ocean. Grahamstown, J.L.B. Smith Institute of Ichthyology, pp 381–399
Kamler E (1992) Early life history of fish: an energetic approach. Chapman & Hall, London, p 267
Kjesbu O (2009) Applied fish reproductive biology: contribution of individual reproductive potential to recruitment and fisheries management. Wiley, Hoboken
Kock KH (1981) Fischereibiologische Untersuchungen an drei antarktischen Fischarten: Champsocephalus gunnari Lonnberg 1905, Chaenocephalus aceratus (Lonnberg, 1906) und Pseudochaenichthys georgianus Norman, 1937 (Notothenioidei, Channichthyidae). Mitt Inst Seefisch Hambg 32:1–226
Kock KH (1989) Reproduction in fish around Elephant Island. Arch FischWiss 39:171–210
Kock KH (2005) Antarctic icefishes (Channichthyidae): a unique family of fishes. A review Part I. Polar Biol 28:862–895
Kock KH, Jones CD (2005) Fish stocks in the southern Scotia Arc region—a review and prospects for future research. Rev Fish Sci 13:75–108
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioids fish. Antarct Sci 3:125–150
Kock KH, Stransky C (2000) The composition of the coastal fish fauna around Elephant Island (South Shetland Islands, Antarctica). Polar Biol 23:825–832
Kock KH, Jones CD, Wilhelms S (2000) Biological characteristics of Antarctic fish stocks in the southern Scotia Arc region. CCAMLR Sci 7:1–41
Kraus G, Muller A, Trella K, Koster FW (2000) Fecundity of the Baltic cod: temporal and spatial variation. J Fish Biol 56:1327–1341
La Mesa M, Ashford JR (2008) Age and early life history of juvenile Scotia Sea icefish, Chaenocephalus aceratus, from Elephant Island and the South Shetland Islands. Polar Biol 31:221–228
La Mesa M, Caputo V, Rampa R, Vacchi M (2003) Macroscopic and histological analyses of gonads during the spawning season of Chionodraco hamatus (Pisces, Channichthyidae) of Terra Nova Bay, Ross Sea, Southern Ocean. Polar Biol 26:621–628
La Mesa M, Caputo V, Eastman JT (2006) Gametogenesis and reproductive strategies in some species of the Antarctic fish genus Trematomus (Nototheniidae) from Terra Nova Bay, Ross Sea. Polar Biol 29:963–970
Le François NR, Sheehan E, Desvignes T, Belzile C, Postlethwait JH, Detrich HW (2017) Characterization and husbandry of wild broodstock of the blackfin icefish Chaenocephalus aceratus (Lönnberg 1906) from the Palmer Archipelago (Southern Ocean) for breeding purposes. Polar Biol 40:2499–2516
Lisovenko LA (1988) Some new information on the reproduction of Chaenocephalus aceratus (Fam. Channichthyidae) of the region of the Island of South Georgia. J Ichthyol 28:130–135
Macchi G, Barrera-Oro E (1995) Histological study on the ovarian development of mackerel icefish (Champsocephalus gunnari) from the South Georgia Islands. CCAMLR Sci 2:35–49
May AW (1967) Fecundity of the Atlantic cod. J Fish Res Board Can 24:1531–1551
Meneghesso C, Riginella E, La Mesa M, Donato F, Mazzoldi C (2017) Age and reproduction in two Antarctic plunderfishes (Artedidraconidae) from the Weddell Sea. Polar Bio 40:13–24
Militelli MI, Macchi GJ, Rodrigues KA (2015) Maturity and fecundity of Champsocephalus gunnari, Chaenocephalus aceratus and Pseudochaenichthys georgianus in South Georgian and Shag Rocks Islands. Polar Sci 9:258–266
Murua H, Motos L (1998) Reproductive modality and batch fecundity of the European hake (Merluccius merluccius L.) in the Bay of Biscay. Calif Coop Res Rep 39:196–203
Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J Northwest Atl Fish Sci 33:33–54
Nicol S, Constable A, Pauly T (2000) Estimates of circumpolar abundance of Antarctic krill based on recent acoustic density measurements. CCAMLR Sci 7:87–99
Novillo M, Moreira E, Macchi G, Barrera-Oro E (2018) Reproductive biology in the Antarctic bathydraconid dragonfish Parachaenichthys charcoti. Polar Biol 41:2239–2248
Olsen S (1955) A contribution to the systematics and biology of chaenichthyid fishes from South Georgia. Nytt Mag Zool Oslo 3:79–93
Papetti C, Susana E, Patarnello T, Zane L (2009) Spatial and temporal boundaries to gene flow between Chaenocephalus aceratus populations at South Orkney and South Shetlands. Mar Ecol Prog Ser 376:269–281
Permitin YY (1973) Fecundity and reproductive biology of icefish (fam. Chaenichthyidae), fish of the family Muraenolepidae and dragonfish (Bathydraconidae) of the Scotia Sea (Antarctic). J Ichthyol 13:204–215
Piacentino G, Moreira E, Barrera-Oro E (2018) Early stages of notothenioid fish from Potter Cove, South Shetland Islands. Polar Biol 41:2607–2613
Quartino ML, Klöser H, Schloss IR, Wiencke C (2001) Biomass and associations of benthic marine macroalgae from the inner Potter Cove (King George Island, Antarctica) related to depth and substrate. Polar Biol 24:349–355
Riginella E, Mazzoldi C, Ashford J, Jones CD, Morgan C, La Mesa M (2016) Life history strategies of the Scotia Sea icefish, Chaenocephalus aceratus, along the southern Scotia Ridge. Polar Biol 39:497–509
Russo A, Angelini F, Carotenuto R, Guarino FM, Falugi C, Campanella C (2000) Spermatogenesis in some Antarctic teleosts from the Ross Sea: histological organisation of the testis and localisation of bFGF. Polar Biol 23:279–287
Saborido-Rey F, Junquera S (1998) Histological assessment of variations in sexual maturity of cod (Gadus morhua) at the Flemish Cap (north-west Atlantic). ICES J Mar Sci 55:515–521
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleost. Am Zool 21:325–343
West G (1990) Methods of assessing ovarian development in fishes: a review. Aust J Mar Fresh Res 41:199–222. https://doi.org/10.1071/MF9900199
Yates P, Ziegler P, Welsford D, McIvor J, Farmer B, Woodcook E (2018) Spatio-temporal dynamics in maturation and spawning of Patagonian toothfish Dissostichus eleginoides on the sub-Antarctic Kerguelen Plateau. J Fish Bio 92:34–54
Acknowledgements
We are grateful to C. Bellisio, O. Gonzalez, J. Piscicelli and F. Robino for their help in field activities and laboratory tasks and to M. Estrada and H. Brachetta for preparation of the histological sections. We thank three referees, K. Mintenbeck, A. Mandich, and V. Laptikhovsky, for their constructive comments. Funding was provided by Fondo para la Investigación Científica y Tecnológica (Grant No. PICTO0100-2010) and Instituto Antártico Argentino.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Novillo, M., Moreira, E., Macchi, G. et al. Reproductive effort in Chaenocephalus aceratus validated by gonadal histology: inshore sites serve as spawning grounds for some notothenioids. Polar Biol 42, 1959–1972 (2019). https://doi.org/10.1007/s00300-019-02571-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02571-8