Abstract
Information about the genetic population structure of the Atlantic spotted dolphin [Stenella frontalis (G. Cuvier 1829)] in the western North Atlantic would greatly improve conservation and management of this species in USA waters. To this end, mitochondrial control region sequences and five nuclear microsatellite loci were used to test for genetic differentiation of Atlantic spotted dolphins in the western North Atlantic, including the Gulf of Mexico (n=199). Skin tissue samples were collected from 1994–2000. Significant heterozygote deficiencies in three microsatellite loci within samples collected off the eastern USA coast prompted investigation of a possible Wahlund effect, resulting in evidence for previously unsuspected population subdivision in this region. In subsequent analyses including three putative populations, two in the western North Atlantic (n=38, n=85) and one in the Gulf of Mexico (n=76), significant genetic differentiation was detected for both nuclear DNA (R ST=0.096, P≤0.0001) and mitochondrial DNA (Φ ST=0.215, P≤0.0001), as well as for all pair-wise population comparisons for both markers. This genetic evidence for population differentiation coupled to known biogeographic transition zones at Cape Hatteras, North Carolina and Cape Canaveral, Florida, USA, evidence of female philopatry, and preliminary support for significant genetic differences between previously documented morphotypes of Atlantic spotted dolphins in coastal and offshore waters all indicate that the biology and life history of this species is more complex than previously assumed. Assumptions of large, panmictic populations might not be accurate in other areas where S. frontalis is continuously distributed (e.g., eastern Atlantic), and could have a detrimental effect on long-term viability and maintenance of genetic diversity in this species in regions where incidental human-induced mortality occurs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Atlantic spotted dolphin, Stenella frontalis, is a small delphinid that is endemic to the Atlantic Ocean. In the western North Atlantic, its distribution includes warm temperate and tropical waters from northern New England to the Gulf of Mexico and the Caribbean, and southward to the coast of Venezuela (Perrin et al. 1987). Two distinct morphotypes of the Atlantic spotted dolphin have been described within this range: a larger, more heavily spotted form found over continental shelf waters, and a smaller, less spotted form found in more pelagic offshore waters, the Caribbean, and around oceanic islands (Perrin et al. 1987). Although Atlantic spotted dolphins are common in the Gulf of Mexico and in western North Atlantic waters off the eastern coast of the USA, little is known of the life history, migratory patterns, or population dynamics of this species.
The Marine Mammal Protection act (MMPA) requires annual assessments of the status of S. frontalis stocks in USA waters. In this context, “stock” is analogous to the commonly used definition of the biological population and is defined by the MMPA as a “group of the same species or smaller taxa in a common spatial arrangement that interbreed when mature” [16 USC 1362 § 3 (11) (1972)]. Currently, Atlantic spotted dolphins in USA waters are managed as two separate units designated as Gulf of Mexico and western North Atlantic stocks, respectively (Waring et al. 2000), although these delineations were not based on direct knowledge of population structure, but rather were developed to simplify management because these two areas support very different commercial fisheries (G. Waring, personal communication).
Population structure hypotheses for Atlantic spotted dolphins in the western North Atlantic and Gulf of Mexico would be most biologically meaningful if they were formulated based on identifiable barriers to gene flow among dolphin populations. However, well-known physical oceanographic barriers to gene flow (e.g., currents, physio-chemical water properties) that affect many marine species are unlikely to affect movements of highly mobile cetaceans. Instead, population-level differences in cetaceans have historically been attributed to a variety of life history characteristics and behavioral differences, including feeding behavior (Hoelzel 1994), sex-related dispersal (Palumbi and Baker 1994; Lyrholm et al. 1999), and separate breeding seasons (Valsecchi et al. 1997), among other factors. Although some knowledge of S. frontalis life-history characteristics was obtained from a long-term study of free-ranging Atlantic spotted dolphins in the northern Bahamas (Herzing 1997), no comparable information is available for spotted dolphins in the Gulf of Mexico or other areas of the western North Atlantic.
Genetic population structure studies have played an increasingly integral role in conservation and management of cetacean species (Rosel et al. 1994; Rosel et al. 1995; Baker et al. 1998; García-Martínez et al. 1999). Microsatellite DNA markers and mitochondrial DNA (mtDNA) sequences have been particularly useful in population-level studies of cetaceans (e.g., Baker et al. 1994; Valsecchi et al. 1997; Brown Gladden et al. 1999; Rosel et al. 1999a; Escorza-Treviño and Dizon 2000). When used in tandem, these non-coding, hypervariable markers provide insight into both biparental and maternal inheritance patterns.
The present study uses nuclear and mtDNA markers to test the null hypothesis of one panmictic population of S. frontalis in western North Atlantic waters Footnote 1 and the Gulf of Mexico, with the alternative hypothesis that these areas are genetically distinct, as was suggested for coastal bottlenose dolphin [Tursiops truncatus (Montagu 1821)] populations (Dowling and Brown 1993). Ultimately, the results of genetic analyses could be used in combination with information on abundance and incidental fishery-related spotted dolphin mortality to assess the status of Atlantic spotted dolphin populations, and, in turn, to provide a biological basis for future management of this species. In this way, the evolutionary potential of each identified population can be conserved.
Materials and methods
Sample collection and DNA extraction
Skin tissue samples were collected from 199 individual spotted dolphins, S. frontalis (G. Cuvier 1829), from 1994–2000 (Fig. 1). Of these, 123 were collected in waters off the eastern coast of the USA in the western North Atlantic and 76 were collected in the Gulf of Mexico. Tissue was collected from live dolphins in the field (n=193) using pole spears, cross bows or biopsy dart guns, and from beach-cast individuals on the east coast (n=4) and Gulf of Mexico coast (n=2). Skin tissues were preserved in either 20% dimethyl sulfoxide saturated with NaCl or frozen at −80°C until extraction.
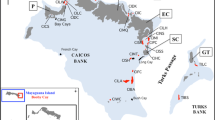
Stenella frontalis. Map of sample collection locations in the Mid-Atlantic Bight (MAB, filled triangles), South Atlantic Bight (SAB, filled circles), and Gulf of Mexico (GOM, filled squares). Samples were separated into Mid-Atlantic Bight and South Atlantic Bight populations at 35°. n number of individuals sampled in a region. Isobaths plotted from 20 to 100 m in 20-m increments, and at 200 m
Total genomic DNA was extracted from approximately 25 mg of tissue using standard proteinase K digestion and phenol–chloroform organic extraction protocols followed by ethanol precipitation, as described by Rosel and Block (1996). Phase lock gel tubes (Eppendorf) were used for organic phase extraction. DNA quality was assessed through visualization on a 1% agarose gel stained with ethidium bromide, and the DNA concentration was quantified using a fluorometer.
Sex determination
To investigate sex-related differences in dispersal, the sex of biopsied individuals was determined using a multiplex polymerase chain reaction (PCR) protocol modified from Bérubé and Palsbøll (1996). This protocol uses primers (ZFYX0582F, ZFY0767R, and ZFX0923R) specific to the ZFX and ZFY genes on the sex-determining chromosomes, producing two amplification bands for male samples and one for female samples when visualized on an agarose gel (Bérubé and Palsbøll 1996). The cycling profile consisted of initial denaturation at 92°C for 30 s, followed by 35 cycles of 92°C, 50°C and 72°C for 45 s each. For most samples, a pit-stop PCR approach (Lopes et al. 1999) was used to prevent preferential amplification of the ZFX band by adding the reverse primer ZFX0923R after the sixth amplification cycle. All PCR products were visualized on a 2.0% agarose gel stained with ethidium bromide, and compared to a positive male control.
Microsatellites
Amplification of five dinucleotide repeat microsatellite loci (Ttr04, Ttr11, Ttr19, Ttr34 and Ttr48; Rosel et al. 2005) was accomplished via PCR in 25 μl reaction volumes. Each reaction contained 50–100 ng DNA (samples with mostly low molecular weight DNA were an exception, where 130–350 ng DNA were used), and the following reagent concentrations: 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.3 μM forward and reverse primers, 0.25 mM dNTPs, and 1.25 U Taq DNA polymerase (Invitrogen Life Technologies). Primer concentrations and/or Taq polymerase concentrations were doubled for samples that yielded poor quality, low molecular weight DNA. Locus Ttr04 was amplified with an initial denaturation at 94°C for 30 s, followed by 30 cycles of 94°C for 20 s, 62°C for 20 s, and 72°C for 40 s, with a final extension step of 72°C for 10 min. Loci Ttr11 and Ttr19 were co-amplified with a cycling profile that differed from that of Ttr04 only by a 2°C decrease in annealing temperature. Loci Ttr34 and Ttr48 were also co-amplified with the following cycling profile: initial denaturation at 94°C for 30 s, followed by 27 cycles of 94°C, 58°C, and 72°C for 30 s each with a final extension step at 72°C for 10 min.
Microsatellite data were collected on an ABI Prism 310 Genetic Analyzer (Applied Biosystems, Inc.) and scored by GeneScan version 3.1 fragment analysis software using the internal lane size standard GS-500 (Applied Biosystems, Inc.). Allele sizes were binned using a graphical approach described by Rosel et al. (1999a) and with a microsatellite binning program provided by B. Amos (personal communication). For each putative population, genotypic linkage disequilibrium tests for independent assortment of microsatellite loci and tests of deviation from Hardy-Weinberg equilibrium were run using the Markov chain method for P value estimation with 10,000 dememorization steps, 1,000 batches and 10,000 iterations per batch as implemented by GenePop version 3.2a (Raymond and Rousset 1995). Microsatellite diversity estimates were calculated as average heterozygosity (Arlequin version 2.0, Schneider et al. 2000) and total number of alleles per population (GenePop version 3.2a, Raymond and Rousset 1995).
Microsatellite population differentiation was estimated as F ST using Arlequin version 2.0 (Schneider et al. 2000), which considers allele frequency differences between populations, and R ST using R STCalc version 2.2 (Goodman 1997), which uses both frequency differences as well as a microsatellite mutation model to estimate population differentiation. R ST was calculated by averaging variance components over loci. The significance of each statistic’s deviation from zero was tested via 10,000 random permutations of the data as implemented in Arlequin or R STCalc. Sequential Bonferroni corrections (Holm 1979) for multiple tests were applied using an initial α value of 0.05.
Based on the Hardy-Weinberg test results, evidence for inbreeding and further population subdivision within the western North Atlantic was investigated. Wright’s inbreeding coefficient F IS was calculated using Arlequin version 2.0 with 10,000 permutations (Schneider et al. 2000). Two approaches were used to test for population subdivision. First, western North Atlantic samples were iteratively separated into two groups divided north and south of each degree of latitude from 29°N to 40°N, and then allele frequencies of each population pair were compared to Hardy-Weinberg expectations. Measures of F ST (population subdivision) and F IS (apparent population inbreeding) were also calculated for all iteratively separated population pairs using Arlequin version 2.0 (Schneider et al. 2000). Secondly, evidence for differentiation between putative morphotypes was examined using sampling depth as a proxy for morphotype, where biopsies from continental shelf waters (≤200 m) were designated as “coastal” and those from deeper waters beyond the shelf break were designated as “offshore” (Perrin et al. 1994). Depths were obtained from field data (when available) or from bathymetric depth grids from the Coastal Relief Model, volume 2, provided by the NOAA National Geophysical Data Center (Boulder, CO, USA) for use in Arcview, version 8.0 (ESRI). Coastal and offshore allele frequencies were compared to Hardy-Weinberg expectations, and measures of population subdivision were calculated as F ST using Arlequin version 2.0 (Schneider et al. 2000).
MtDNA
A 430-base pair (bp) fragment of mtDNA, including 65 bases of flanking tRNA and a 365-bp portion of the variable 5’ end of the control region, was amplified by PCR using primers L15824 and H16265 (Rosel et al. 1999b). Each 50-μl reaction contained: 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.3 μM forward and reverse primers, 0.15 mM dNTPs, 2.5 U Taq DNA polymerase (Invitrogen Life Technologies), and 40–100 ng DNA (samples with mostly low molecular weight DNA were an exception, where 100–250 ng DNA was added). Some amplification reactions also contained 8 μg bovine serum albumin (no. A4503, Sigma Chemical) to facilitate amplification of extractions yielding poor DNA quality. The cycling profile consisted of initial denaturation at 94°C for 30 s, followed by 30 cycles of 94°C, 55°C, and 72°C for 30 s each. After amplification, 5 μl of PCR product was visualized on a 1% agarose gel stained with ethidium bromide. The remaining PCR products were then purified by PCR band extraction from 0.8% SeaPlaque GTG low melting point agarose gels (FMC) stained with ethidium bromide. The gel bands were melted for 10 min at 65°C and then digested overnight at 40°C with approximately 0.075 U of agarase (Sigma Chemical) μl−1 of PCR product.
All PCR products were cycle sequenced in both forward and reverse directions and purified by ethanol precipitation following sequencing protocols from Applied Biosystems, Inc. for use with the ABI Prism BigDye Terminator Ready-reaction Mix version 2.0, with two exceptions: BigDye reaction mix concentration was 0.5×, and 2.5× cycle sequencing buffer (200 mM Tris–HCl, 5 mM MgCl2, pH 9.0) was used in place of water. Sequence data were collected on an ABI 310 Genetic Analyzer (Applied Biosystems, Inc.), edited in Sequence Navigator version 1.0.1 (Applied Biosystems, Inc.), and aligned by eye using SeqPup version 0.6f (Gilbert 1996). A consensus sequence of the forward and reverse sequence for each sample was used for mtDNA analyses.
MtDNA diversity measures included nucleotide diversity (π) and haplotype diversity (h) (Nei 1987) calculated using Arlequin version 2.0 (Schneider et al. 2000). The Tukey-Kramer procedure (Dunnet 1980) was applied to test for significant differences in mitochondrial diversity between populations with α=0.05. Population differentiation was tested using an analysis of molecular variance (AMOVA) (Excoffier et al. 1992) to compute F ST using allele frequencies, and Φ ST using the Tamura–Nei distance algorithm (Tamura and Nei 1993) and a gamma correction with α=0.5. The significance of both F ST and Φ ST was tested against a null distribution constructed by 10,000 random permutations of the data (Schneider et al. 2000). As with microsatellite comparisons, sequential Bonferroni corrections for multiple tests (Holm 1979) were applied using an initial α value of 0.05. A median-joining network of all unique haplotypes was constructed to visualize phylogeographic relationships among haplotypes and populations using Network version 3.1.1.1 (Bandelt et al. 1999).
Results
Microsatellite DNA
All microsatellite loci conformed to Hardy-Weinberg expectations in the Gulf of Mexico, but in the western North Atlantic sample, two loci differed significantly from expected genotype frequencies (P≤0.001, Table 2). Subsequent tests for heterozygote deficiency revealed that these two loci, as well as another (Ttr19), were deficient in the number of expected heterozygotes. To help explain these deviations from Hardy-Weinberg expectations, we tested for evidence of further population subdivision (i.e., a Wahlund effect) and for inbreeding within the western North Atlantic (see discussion).
Iterative separation of western North Atlantic samples by latitude revealed that all microsatellite loci conformed to Hardy-Weinberg equilibrium expectations when the western North Atlantic samples were separated into two groups at either 35° or 36°N (Fig. 2). Populations separated at these two points also exhibited the highest levels of population subdivision and lowest F IS values (Fig. 2). Because F ST and F IS values were comparable for populations separated at 35° and 36°N, the former was chosen as a point of differentiation based on its proximity to a documented biological transition zone at Cape Hatteras, North Carolina (Briggs 1974). This point separates the western North Atlantic into two putative populations, hereafter referred to as Mid-Atlantic BightFootnote 2 (n=38), which includes waters from Georges Bank to Cape Hatteras, North Carolina, and South Atlantic Bight2 (n=86), which extends from Cape Hatteras, NC to the southern tip of Florida.
When western North Atlantic samples were partitioned according to depth, the number of microsatellite loci that were deficient in the expected number of heterozygotes was reduced from three to one (P≤0.0002). An AMOVA including all five loci revealed that individuals sampled in continental shelf waters (≤111 m) were significantly different than those collected in waters beyond the shelf break (≥306 m; no samples were collected between 111 and 306 m depth) for both microsatellite data (F ST=0.1444, P<0.0001) and mtDNA sequences (F ST=0.0622, P≤0.0399). However, all offshore samples (n=24) were collected north of 35°N (i.e., in the Mid-Atlantic Bight). Although subsequent partitioning of Mid-Atlantic Bight samples into coastal and offshore groups at the same depths as above greatly reduced the size of the coastal sample (n=12), significant differentiation remained for both microsatellites (F ST=0.1082, P<0.0001), and mtDNA sequences (F ST=0.0617, P≤0.0001).
Wright’s inbreeding coefficient (F IS) was significant for the western North Atlantic as a whole (F IS=0.0885, P≤0.001). When western North Atlantic samples were partitioned according to latitude and depth, significant inbreeding was detected in the offshore Mid-Atlantic Bight population only (F IS=0.0563, P≤0.0271), but this estimate was not significant after corrections for multiple tests.
Based on patterns revealed by these analyses, our alternative hypothesis changed, and we tested for population subdivision among three putative populations: two within the western North Atlantic, designated Mid-Atlantic Bight and South Atlantic Bight, and one in the Gulf of Mexico. Microsatellite linkage tests for the three putative populations were not significant after sequential Bonferroni corrections for multiple tests and, as mentioned above, all loci were in Hardy-Weinberg equilibrium.
Sample composition
The sex of 187 of 193 biopsied dolphins was successfully determined using the multiplex pit-stop PCR protocol, giving a total of 193 samples of known sex when including the six strandings whose sex was determined using morphological techniques (n=116 female, n=77 male; Table 1). The total number of samples was significantly biased towards females (P≤0.0050) at 60.1%; this percentage was consistent within putative populations except the Mid-Atlantic Bight, where females constituted 55.6% of the individuals.
Two sample pairs had identical microsatellite genotypes across all five loci. One pair was identified as the same sex, shared identical mtDNA haplotypes, and both samples were collected from the same group in the field; one of these duplicate samples was removed from all analyses. The other comparison revealed identical microsatellite genotypes at all five loci shared by a sample from the South Atlantic Bight and a sample from the Gulf of Mexico, but the sex and mitochondrial haplotype of the two samples differed. Therefore, both samples in the latter comparison were retained in the data set.
Genetic diversity
All five loci were used to genotype 196 S. frontalis samples from the western North Atlantic and Gulf of Mexico, with the exception of two samples that could not be amplified at locus Ttr19, and one sample that was not amplified at locus Ttr11. The number of alleles in all samples at each microsatellite locus ranged from 7 to 15, with an average of 11 alleles per locus. On average, levels of observed heterozygosity (Ho) were highest in the Mid-Atlantic Bight samples and lowest in the Gulf of Mexico samples (Table 2).
Polymerase chain reaction amplification and sequencing was accomplished for 196 Atlantic spotted dolphin samples, resulting in a 329-bp alignment of the variable 5’ end of the mitochondrial control region. The alignment contained 30 transitions and 1 transversion substitution. Thirty-four S. frontalis haplotypes were identified (Genbank accession no’s DQ060054-DQ060064). Of the 34 total haplotypes, 13 were unique to the Mid-Atlantic Bight samples, 5 were found only in the South Atlantic Bight, and 7 were unique to the Gulf of Mexico samples, leaving only 9 haplotypes that were shared by two or more putative populations.
For all sequences from all three populations combined, the mean number of pair-wise differences between sequences was 4.246 (SE±2.127), haplotype diversity was 0.9007 (SE±0.0120), and nucleotide diversity was 0.0147 (SE±0.0080) (Table 3). According to Tukey-Kramer tests, the estimated haplotype diversity for the Mid-Atlantic Bight population was significantly higher than the South Atlantic Bight and Gulf of Mexico estimates; haplotype diversity within the South Atlantic Bight was significantly lower than estimates for the other two populations (Table 3). Nucleotide diversity estimates were significantly higher in the Mid-Atlantic Bight as compared to the Gulf of Mexico; all other comparisons were non-significant.
Population differentiation
Significant genetic differentiation was revealed overall among the three groups for both microsatellite DNA (F ST=0.0578, P≤0.0001; R ST=0.0964, P≤0.0001), and mtDNA (F ST=0.1070, P≤0.0001; Φ ST=0.2147, P≤0.0001), as well as for each pair-wise population comparison (Table 4). Generally, mtDNA differentiation estimates were higher than microsatellite estimates. However, mtDNA and microsatellite DNA markers differed with respect to the relative levels of specific pair-wise population differentiation comparisons. For example, the South Atlantic Bight and Gulf of Mexico were the most differentiated populations according to mtDNA results, and least differentiated according to microsatellite population differentiation estimates.
When mtDNA control region sequences were analyzed separately by sex, some F ST and Φ ST values were no longer significant after corrections for multiple tests. Generally, mtDNA population differentiation estimates for females were about twice as large as corresponding male comparisons (Table 4). In contrast, microsatellite population differentiation estimates were comparable for males and females when analyzed separately.
Generally, haplotypes in the median-joining network that are most common and shared by all three populations occupy more internal positions within the network, while haplotypes unique to one population typically occupy more external positions (Fig. 3). Although haplotypes from each population extend throughout the network, there is a subtle shift in position related to geography, with most Mid-Atlantic Bight haplotypes occurring on the left side of the network (50% of all Mid-Atlantic Bight haplotypes are within two mutations of node A), Gulf of Mexico haplotypes extending primarily from the center (73% of all Gulf of Mexico haplotypes are within two mutations of node B), and South Atlantic Bight haplotypes concentrated on the right side of the diagram (61% of all South Atlantic Bight haplotypes are within two mutations of node C).
S. frontalis. Median-joining network of Atlantic spotted dolphin mtDNA haplotypes. Circle diameter is proportional to haplotype frequencies, and proportion of each population within each haplotype is shaded: white Mid-Atlantic Bight; gray South Atlantic Bight; black Gulf of Mexico. Length of lines is proportional to number of mutations between haplotypes, with hatch marks indicating total number of mutations on lines where more than one mutation is present. Nodes labeled a, b, and c refer to positions discussed in text. Stars indicate missing intermediate haplotypes
Discussion and conclusions
Here, we provide the first data on genetic population structure of S. frontalis in the western North Atlantic and the Gulf of Mexico. The AMOVA analyses for both mtDNA and microsatellite data confirm significant genetic differentiation between Gulf of Mexico and western North Atlantic populations of Atlantic spotted dolphins. These data also illustrate the importance of testing the assumptions upon which population genetics analyses are based, and how these tests can reveal clues to the presence of cryptic population structure. Heterozygote deficiencies found in three microsatellite loci within the western North Atlantic population prompted us to consider alternate population structure hypotheses within this region.
Several factors can cause heterozygote deficiencies, including non-random mating, selection, null alleles, inbreeding, and population substructure (a Wahlund effect). Although we cannot rule out non-random mating or selection in this study, three independent loci with significant heterozygote deficiencies found only in the western North Atlantic makes the null allele hypothesis unlikely. Instead, this pattern is more likely explained by inbreeding or the Wahlund effect, a phenomenon which causes a reduction in the observed frequency of heterozygotes when two or more isolated subpopulations are tested as one panmictic population (Wahlund 1928).
Iterative separation by latitude tests support a Wahlund effect as the most likely explanation of heterozygote deficiency, with evidence of population differentiation at Cape Hatteras, NC. While inbreeding could not be eliminated as a possible cause of heterozygote deficiency in the western North Atlantic, evidence for inbreeding may in fact be an artifact of population substructure, as revealed by a general decreasing trend in the inbreeding estimate as the point of population differentiation approaches Cape Hatteras, NC in iterative tests (Fig. 2). Although preliminary evidence suggests further subdivision of the Mid-Atlantic Bight samples into coastal and offshore groups, this pattern was not considered in further analyses for several reasons. Heterozygote deficiency remains when samples are partitioned by depth, and increased sample sizes within the mid-Atlantic Bight and offshore samples from the South Atlantic Bight are needed to obtain reliable estimates of genetic variance as related to depth and morphotype. Unfortunately, the absence of offshore samples in the South Atlantic Bight makes depth a confounding variable for tests that simultaneously attempt to partition the total genetic variance within the western North Atlantic into depth and latitudinal components.
Genetic diversity, population structure and phylogeographic inference
Overall, mtDNA and nuclear genetic diversity estimates in the Mid-Atlantic Bight, South Atlantic Bight, and Gulf of Mexico were within the range of genetic diversity values for other cetaceans (Baker et al. 1994; Rosel et al. 1994; Lyrholm et al. 1999; Rosel et al. 1999a; Escorza-Treviño and Dizon 2000). Interestingly, both mtDNA and nuclear diversity estimates were highest in the Mid-Atlantic Bight population, despite the small sample size (n=38). In fact, although approximately one-third as many samples were collected in the Mid-Atlantic Bight when compared to the South Atlantic Bight, this group harbored nearly twice as many mtDNA haplotypes, the majority of which were unique to this population. In addition, all haplotypes within the Mid-Atlantic Bight were relatively rare, belonging to four or fewer individuals. This high genetic variability within a small sample size suggests an inadequate survey of genetic variability in this region.
Both mtDNA and microsatellite DNA data reveal significant genetic differentiation among Mid-Atlantic Bight, South Atlantic Bight, and Gulf of Mexico populations. Generally, population differentiation estimates that take both frequency differences and distance measures into account (Φ ST and R ST) were higher than those based solely on frequency differences between populations (F ST), suggesting that populations have been separated long enough for mutational processes to contribute to genetic differentiation, in addition to genetic drift. This conclusion is further supported by the median-joining network analysis, which reveals a complex picture of interrelatedness among the three populations. Numerous reticulations among mtDNA haplotypes shared by all three populations in more internal positions reveal a high degree of homoplasy and interrelatedness among several ancestral haplotypes. The emergence of new haplotypes unique to each of the three populations from each of these shared ancestral haplotypes reflects a recent expansion and rapid divergence from the ancestral population (Slatkin and Hudson 1991), a theory supported by the star-like phylogeny of rare haplotypes extending from nodes A and C. However, the shift in population contribution to haplotypes across the network suggests that these populations have been separated long enough to acquire not only haplotype frequency differences, but phylogeographic differences as well.
In this study, data from maternally inherited mtDNA sequences generally revealed higher levels of population differentiation than biparentally inherited microsatellites. This could be partially explained by the mode of inheritance of these markers. The haploid nature and matrilineal inheritance pattern of mtDNA reduce the effective population size to one-fourth that of nuclear genes (Birkey et al. 1983), allowing changes in population allele frequencies to accumulate faster in mtDNA than in nuclear gene lines, resulting in higher mtDNA population differentiation estimates. In addition, a pattern of female philopatry coupled with male dispersal could amplify the differences in the amount of genetic differentiation detected in this study by nuclear and mtDNA markers. This pattern has been documented in several cetaceans (Baker et al. 1998; Lyrholm et al. 1999; Rosel et al. 1999a; Escorza-Treviño and Dizon 2000), and is supported for Atlantic spotted dolphins when mtDNA population results are separated by sex: F ST estimates for females were about twice those of males for all significant population comparisons. In contrast, overall microsatellite F ST values for males (F ST=0.0589) and females (F ST=0.0562) differed by only 0.27% when sexes were analyzed separately, perhaps reflecting the homogenizing effects of males that migrate and breed with neighboring populations. These results suggest that S. frontalis exhibits some degree of sex-biased dispersal.
Mitochondrial DNA sequences revealed that the South Atlantic Bight and Gulf of Mexico groups were most differentiated, while microsatellite DNA data showed that these groups were least differentiated. Even more perplexing, the Mid-Atlantic Bight and Gulf of Mexico samples were least differentiated according to mtDNA data. Intuitively, the pattern revealed by the microsatellite data makes the most biological sense. The amount of differentiation between two populations is affected by many factors, including the distance between the populations and barrier permeability, both of which affect the amount of migration between populations. It is expected that as a result of their proximity, more individuals would be exchanged between the South Atlantic Bight and Gulf of Mexico than between the Mid-Atlantic Bight and the Gulf of Mexico, ultimately allowing more gene flow between the South Atlantic Bight and Gulf of Mexico groups. Although this expectation was not supported by the single mtDNA marker, it was supported by the five independent microsatellite markers, even when each locus was analyzed separately. These incongruencies could simply reflect the random nature of lineage sorting of individual genes. Regardless of which populations are most closely related, all three are significantly divergent.
Genetic isolation mechanisms
Although dispersal of cetacean species is not likely to be restricted by oceanographic currents or other hydrological barriers, Dowling and Brown (1993) hypothesized that prey distribution might influence bottlenose dolphin distribution in the western North Atlantic and Gulf of Mexico, whereby physical barriers to prey dispersal have an indirect effect on gene flow among genetically differentiated coastal bottlenose dolphin populations. Hoelzel (1994) supported this hypothesis, stating that the distribution of cetaceans in tropical and temperate waters is often dictated by prey distribution.
Atlantic spotted dolphins feed on cephalopods, benthic invertebrates, and a wide range of small fish from several taxonomic groups (Perrin et al. 1987, 1994). There are two well-known biogeographic barriers in the transitional areas separating the three putative populations of Atlantic spotted dolphins (e.g., Schwartz 1989). Morphological and life history differences have been found in fish species north and south of Cape Hatteras, North Carolina (White and Chittenden 1977; Wilk et al. 1980; Ross 1988), and genetic differences have been documented in fish and invertebrate species separated at Cape Canaveral, Florida (Bert 1986; Saunders et al. 1986; Avise et al. 1987; Bowen and Avise 1990; Reeb and Avise 1990; Staton and Felder 1995). Both points mark the northern- and southern-most distribution limits for several marine species (Briggs 1974; Schwartz 1989). The biogeographic patterns observed at Cape Hatteras and Cape Canaveral reflect contemporary barriers of temperature and hydrography (Schwartz 1989), while faunal differences at Cape Canaveral could also be explained by historical climatic changes leading sea level fluctuation and hydrographic shifts during the Pleistocene glaciation (Avise 1992) or earlier glacial episodes (Bert 1986). These disjunctions suggest that both modern and historical barriers to dispersal of fish and invertebrates species could create differences in prey composition and availability for the putative populations of Atlantic spotted dolphins.
Management implications
Analyses of variation within mtDNA and nuclear DNA give strong genetic support for independent management of western North Atlantic and Gulf of Mexico stocks of S. frontalis. In addition, mtDNA and microsatellite data also provide evidence for genetic separation of dolphins within the western North Atlantic into two stocks with a provisional point of differentiation near Cape Hatteras, which should be considered in future management of Atlantic spotted dolphins. Evidence for population subdivision is particularly relevant for the Mid-Atlantic Bight population, which is not recognized as a distinct management stock, yet historically has suffered the highest incidental mortality rates in the western North Atlantic (Waring et al. 2000).
Because morphological data were not available for the individuals sampled in this study, we could not verify whether the western North Atlantic samples came from the coastal or offshore form (Perrin et al. 1987) (the offshore form is not known to occur in the Gulf of Mexico). But preliminary results using water depth as a proxy for morphotype suggest that genetic differences between coastal and offshore Atlantic spotted dolphins in the western North Atlantic might exist, and should be investigated more thoroughly. Interestingly, sampling depths and geographic locations revealed that all western North Atlantic biopsies designated as the offshore morphotype (samples in depths ≥ 306 m) were from the Mid-Atlantic Bight (although the offshore form of S. frontalis is seen in deeper waters of the South Atlantic Bight, these waters have not yet been sampled). Given this data set, we cannot determine the relative contribution of sampling depth (i.e., morphotype) to the genetic differentiation found between Mid-Atlantic Bight and South Atlantic Bight populations. However, coastal Mid-Atlantic Bight samples are still significantly differentiated from the (coastal) South Atlantic Bight population (microsatellites F ST=0.1082, P≤0.0001; mtDNA F ST=0.0622, P≤0.0399), indicating there are potentially both morphotypic and biogeographical components to genetic structure of S. frontalis in the western North Atlantic.
Stratification by depth results are somewhat speculative, given the small sample size of both groups from the Mid-Atlantic Bight. Additionally, sampling depth might not be strictly correlated with morphotype. Yet genetic support for these morphotypic differences is not unlikely; genetic differences have been found between morphologically different nearshore and offshore populations of two other delphinid species: the bottlenose dolphin (Tursiops truncatus) (Hoelzel et al. 1998), and common dolphin (Delphinus delphis) (Rosel et al. 1994), the latter of which is now considered two species (Rice 1998). Further investigation of the genetic differences between coastal and offshore morphotypes and variability within offshore morphotypes of Atlantic spotted dolphins throughout the western North Atlantic are critical for accurate stock delineation in this region.
The unexpected within-ocean basin structure found in this study suggests that we must revise our expectations of large, panmictic populations of this species. Since Atlantic spotted dolphins are common in the eastern Atlantic as well, and are often caught incidentally in purse-seine fisheries in this region (Perrin et al. 1994), studies of population structure in the eastern Atlantic should also be undertaken to ensure diversity within the species is not lost.
Notes
In this manuscript, the term “western North Atlantic” refers to oceanic waters off the eastern coast of North America excluding the Gulf of Mexico, Caribbean Sea, and other associated partially enclosed bodies of water, in an attempt to remain consistent with Atlantic spotted dolphin stock boundaries in the western North Atlantic
Although “Mid-Atlantic Bight” and “South Atlantic Bight” are not officially recognized oceanographic terms (see Richards 1999), their conventional use closely approximates the distribution of Atlantic spotted dolphins in coastal USA waters of the western North Atlantic
References
Avise JC (1992) Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos 63:62–76
Avise JC, Reeb CA, Saunders NC (1987) Geographic population structure and species differences in mitochondrial DNA of mouthbrooding marine catfishes (Ariidae) and demersal spawning toadfishes (Batrachoididae). Evolution 41:991–1002
Baker CS, Slade RW, Bannister JL, Abernethy RB, Weinrich MT, Lien J, Urban J, Corkeron P, Calmabokidis J, Vasquez O, Palumbi SR (1994) Hierarchical structure of mitochondrial DNA gene flow among humpback whales Megaptera novaeangliae, worldwide. Mol Ecol 3:313–327
Baker CS, Medrano-Gonzalez L, Calmabokidis J, Perry A, Pichler F, Rosenbaum H, Straley JM, Urban-Ramirez J, Yamaguchi M, Ziegesar OV (1998) Population structure of nuclear and mitochondrial DNA variation among humpback whales in the North Pacific. Mol Ecol 7:695–707
Bandelt H-J, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bert TM (1986) Speciation in western Atlantic stone crabs (genus Menippe): the role of geological processes and climatic events in the formation and distribution of species. Mar Biol 93:57–170
Bérubé M, Palsbøll P (1996) Identification of sex in cetaceans by multiplexing with three ZFX and ZFY specific primers. Mol Ecol 5:283–287
Birkey CW Jr, Maruyama T, Fuerst P (1983) An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics 103:513–527
Bowen BW, Avise JC (1990) Genetic structure of Atlantic and Gulf of Mexico populations of sea bass, menhaden, and sturgeon: influence of zoogeographic factors and life-history patterns. Mar Biol 107:371–381
Briggs JC (1974) Marine zoogeography. McGraw-Hill, New York
Brown Gladden JG, Ferguson MM, Friesen MK, Clayton JW (1999) Population structure of North American beluga whales (Delphinapterus leucas) based on nuclear DNA microsatellite variation and contrasted with population structure revealed by mitochondrial DNA variation. Mol Ecol 8:347–363
Dowling TE, Brown WM (1993) Population structure of the bottlenose dolphin (Tursiops truncatus) as determined by restriction endonuclease analysis of mitochondrial DNA. Mar Mamm Sci 9:38–155
Dunnet CW (1980) Pairwise multiple comparisons in the homogeneous variance, unequal sample size case. J Amer Statist Assoc 75:789–795
Escorza-Treviño S, Dizon AE (2000) Phylogeography, intraspecific structure and sex-biased dispersal of Dall’s porpoise, Phocoenoides dalli, revealed by mitochondrial and microsatellite DNA analyses. Mol Ecol 9:1049–1060
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes. Application to human mitochondrial DNA restriction data. Genetics 131:479–491
García-Martínez J, Moya A, Raga JA, Latorre A (1999) Genetic differentiation of the striped dolphin Stenella coeruleoalba from European waters according to mitochondrial DNA (mtDNA) restriction analysis. Mol Ecol 8:1069–1073
Gilbert DG (1996) Seqpup: a biosequence editor and analysis platform, version 0.6f. Bionet Software, <news://4rb7hr$6rc@usenet.ucs.indiana.edu> See also ftp://iubio.bio.indiana.edu/molbio/seqpup/
Goodman SJ (1997) R ST Calc: a collection of computer programs for calculating estimates of genetic differentiation from microsatellite data and determining their significance. Mol Ecol 6:881–885
Herzing DL (1997) The life history of free-ranging Atlantic spotted dolphins (Stenella frontalis): age classes, color phases, and female reproduction. Mar Mamm Sci 13:576–595
Hoelzel AR (1994) Genetics and ecology of whales and dolphins. Annu Rev Ecol Syst 25:377–399
Hoelzel AR, Potter CW, Best PB (1998) Genetic differentiation between parapatric `nearshore’ and `offshore’ populations of the bottlenose dolphin. Proc R Soc Lond (Ser B) 265:1177–1183
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Statist 5:65–70
Lopes RF, Senna J, Chies JM, Rodrigues JL (1999) Pit-stop PCR: an approach to increase final product yield of multiplex PCR. Biotechniques 26:638–639
Lyrholm T, Leimar O, Johanneson B, Gyllensten U (1999) Sex-biased dispersal in sperm whales: contrasting mitochondrial and nuclear genetic structure of global populations. Proc R Soc Lond (Ser B) 266:347–354
Marine Mammal Protection Act of 1972 (2005) 16 USC § § 1361–1421h
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Palumbi SR, Baker CS (1994) Contrasting population structure from nuclear intron sequences and mtDNA of humpback whales. Mol Biol Evol 11:426–435
Perrin WF, Mitchell ED, Mead JG, Caldwell DK, Caldwell MC, VanBree PJH, Dawbin WH (1987) Revision of the spotted dolphins, Stenella spp. Mar Mamm Sci 3:99–170
Perrin WF, Caldwell DK, Caldwell MC (1994) Atlantic spotted dolphin Stenella frontalis (G. Cuvier, 1829). In: S.H. Ridgway, R. Harrison (eds) Handbook of marine mammals, pp 173–190
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Reeb CC, Avise JC (1990) A genetic discontinuity in a continuously distributed species: mitchondrial DNA in the American oyster, Crassostrea virginica. Genetics 124:397–406
Rice DW (1998) Marine mammals of the world. Allen Press, Inc., Lawrence, KS
Rosel PE, Block BA (1996) Mitochondrial control region variability and global population structure in the swordfish, Xiphias gladius. Mar Biol 125:11–22
Rosel PE, Dizon AE, Heyning JE (1994) Genetic analysis of sympatric morphotypes of common dolphins (genus Delphinus). Mar Biol 119:159–167
Rosel PE, Dizon AE, Haygood MG (1995) Variability of the mitochondrial control region in populations of the harbour porpoise, Phocoena phocoena, on interoceanic and regional scales. Can J Fish Aquat Sci 52:1210–1219
Rosel PE, France SC, Wang JY, Kocher TD (1999a) Genetic structure of harbour porpoise Phocoena phocoena populations in the northwest Atlantic based on mitochondrial and nuclear markers. Mol Ecol 8:S41–S54
Rosel PE, Tiedemann R, Walton M (1999b) Genetic evidence for limited trans-Atlantic movements of the harbor porpoise Phocoena phocoena. Mar Biol 133:583–591
Rosel PE, Forgetta V, Dewar K (2005) Isolation and characterization of twelve polymorphic microsatellite markers in bottlenose dolphins (Tursiops truncatus). Mol Ecol Notes (in press) doi: 10.1111/j.1471-8286.2005.01078x
Ross SW (1988) Age, growth, and mortality of Atlantic croaker in North Carolina, with comments on population dynamics. Trans Am Fish Soc 117:461–473
Saunders NC, Kessler LG, Avise JC (1986) Genetic variation and geographic differentiation in mitochondrial DNA of the horseshoe crab, Limulus polyphemus. Genetics 112:613–627
Schneider S, Roessli D, Excoffier L (2000) Arlequin: a software for population genetics data analysis. Genetics and Biometry Lab, Dept. of Anthropology, University of Geneva, Geneva
Schwartz FJ (1989) Zoogeography and ecology of fishes inhabiting North Carolina’s marine waters to depths of 600 meters. In: George RY, Hulbert AW (eds) North Carolina coastal oceanography symposium. US Dept. of Commerce, National Oceanic and Atmospheric Administration, Oceanic and Atmospheric Research, Office of Undersea Research, Rockville, MD, pp 335–374
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Staton JL, Felder DL (1995) Genetic variation in populations of the ghost shrimp genus Callichirus (Crustacea: Decapoda: Thalassinoidea) in the western Atlantic and Gulf of Mexico. Bull Mar Sci 56:523–536
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Valsecchi E, Palsbøll P, Hale P, Glockner-Ferrari D, Ferrari M, Clapham P, Larsen F, Mattila D, Sears R, Sigurjonsson J, Brown M, Corkeron P, Amos B (1997) Microsatellite genetic distances between oceanic populations of the humpback whale (Megaptera novaeangliae). Mol Biol Evol 14:355–362
Wahlund S (1928) Composition of populations from the perspective of the theory of heredity. Hereditas 11:65–105
Waring GT, Quintal JM, Swartz SL (2000) US Atlantic and Gulf of Mexico marine mammal stock assessments. NOAA Technical Memorandum, NMFS-NE-162
White ML, Chittenden ME Jr (1977) Age determination, reproduction, and population dynamics of the Atlantic croaker, Micropogonias undulatus. Fish Bull 75:109–123
Wilk SJ, Smith WG, Ralph DE, Sibunka J (1980) Population structure of summer flounder between New York and Florida based on linear discriminant analysis. Trans Am Fish Soc 109:265–271
Acknowledgements
This work was made possible by the people who provided samples, including A. Hohn, W. Hoggard, W. Jones, K. Mullin, and T. Martinez (NMFS-SEFSC); B. Griffin (Mote Marine Laboratory), J. Nicolas (NMFS-NEFSC), and W. McFee (NOS). The authors would like to thank Bill Amos (Cambridge University) for use of his microsatellite binning program. Special thanks also go to Sarah Kingston (NMFS-SEFSC) for confirming species identification of several samples using nuclear AFLP markers, Anna Sellas (NMFS-SEFSC) for her work that made depth stratification possible using Arcview, and to Krystal Tolley (University of Stellenbosch) and two anonymous reviewers for suggestions that improved this manuscript. Contributions to sample depth analyses made by Keith Mullin (NMFS-SEFSC) were also much appreciated. All biopsy samples were collected under US Marine Mammal Protection Act Permit numbers 738, 917, and 779–1339 issued by the US National Marine Fisheries Service. Funding for this study was provided by the National Marine Fisheries Service and the Lerner-Gray Fund for Marine Research. This is contribution number 277 of the Grice Marine Laboratory, College of Charleston, Charleston, SC. This publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the National Oceanic and Atmospheric Administration (NOAA). No reference shall be made to NOAA, or this publication furnished by NOAA, to any advertising or sales promotion which would indicate or imply that NOAA recommends or endorses any proprietary product mentioned herein, or which has its purpose as an interest to cause the advertised product to be used or purchased because of this publication. All experiments carried out in conjunction with this research comply with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P.Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Adams, L.D., Rosel, P.E. Population differentiation of the Atlantic spotted dolphin (Stenella frontalis) in the western North Atlantic, including the Gulf of Mexico. Marine Biology 148, 671–681 (2006). https://doi.org/10.1007/s00227-005-0094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0094-2