Abstract
The effect of the thermo-hydro-mechanical (THM) densification in a closed system on the set recovery and thermal stability of lime (Tilia cordata) wood was evaluated. The THM densification process consists of four steps: plastification, densification, post-treatment and cooling. The THM-densified wood samples were subjected to three different post-treatment temperatures, and for each one, three different periods were used. Comparison between THM-densified wood sample without and with post-treatment at different temperatures and times was made. The set recovery tests showed that the post-treatment at higher temperatures and times improves the dimensional stability of the samples suggesting a permanent fixation of the transversal compression, but also reduces their thermal stability, by having lower temperatures corresponding to the maximum decomposition rate, lower values for the mass loss at the end of decomposition stage and lower values for the kinetic parameters. The samples with post-treatments at lower temperatures showed high set recovery values and exhibited higher values for the temperatures corresponding to the maximum decomposition rate, lower values for the mass loss at the end of the stage and higher activation energies and reaction orders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood is a natural raw material widely used in construction, furniture, packaging industry, etc. Due to its polymeric structure, it is susceptible to humidity, temperature and biological agents. Wood contains three major components: cellulose (a linear polymer built up of anhydro–d–glucopyranose units linked by β(1–4) glycosidic linkages), lignin (a three-dimensional crosslinked aromatic polymer formed from phenyl propane units, with/without methoxyl groups bonded to the aromatic ring, and linked together by β–O–4 aryl ether or carbon–carbon linkages) and hemicelluloses (mainly composed of glucose, mannose, galactose, xylose and arabinose), which are branched polymers with a significantly lower molecular mass compared with cellulose (Chang et al. 2010; Assor et al. 2009).
Due to the environmental concerns regarding the use of certain preservatives and in order to improve environmentally friendly, more resistant wooden materials, research has been carried out in recent years on methods of mechanical and thermal modification of wood. The basic concept is the heating and compressing of wet wood in order to improve its physical and mechanical properties and change its shape. These methods are usually called densification methods. Under hot saturated steam and at or above the glass transition temperature of amorphous polymers of wood, large deformation occurs and wood is re-shaped in the transversal direction. The drawback of this technique is the shape memory effect, which appears when densified wood is re-moistened and heated (Navi and Heger 2004). A review on densification of wood is given by Kutnar and Sernek (2007).
In order to prevent the shape memory effect, different chemical, physical and mechanical post-treatments are used. Post-treatment of densified wood through the use of saturated or unsaturated steam, temperature and compression has been shown to reduce completely the shape memory effect (Navi and Heger 2005). As a result, wood can be modified to a desired form and functionality, and this modification can be defined as a process that improves wood properties resulting in a new material, which does not present any environmental hazard greater than unmodified wood (Hill 2006).
During wood densification and particularly thermo-hydro-mechanical (THM) post-treatments, the individual wood components undergo chemical degradations depending on the processing parameters such as temperature, wood moisture content and processing time. Over the last two decades, many research works have been reported on the chemical degradation of TH wood aiming to correlate between this degradation of wood components and the macroscopic properties of TH wood. This has provided, on the one hand, useful insights into the design of new wood-based material and contributed to an improved predictability of the macroscopic behaviour of THM wood. On the other hand, it has strengthened the confidence of engineers and architects in THM wood and boosted the use of THM wood in conventional fields of application, such as the construction sector.
Thermogravimetry (TG/DTG) has proved to be a useful technique for elucidating the thermal decomposition/history of various biomass materials. Many researchers have used this technique to evaluate the differences between different wood species (Grønli et al. 2002; Shebani et al. 2008; Popescu et al. 2011) or to quantify the modifications appearing on the thermally treated wood (Korosec et al. 2009).
The three-dimensional nature and microstructure of wood are variables, which have an important role on their effects on devolatilisation behaviours. Thus, the individual chemical components of wood behave differently with respect to the thermal degradation. During the thermal decomposition processes of wood, small molecules are eliminated, some components begin to break down chemically at low temperatures (degradation at low rate occurs in lignin and hemicelluloses) and usually a charred mass remains (Helsen and Van den Bulck 2000; Beall 1986).
In a complex material, such as wood, the thermogravimetric processes of all components overlap. Usually, two main zones are observed and are associated with the devolatilisation of the water and the main components (complex curve due to the overlapping). The predominant process in the second zone is that of cellulose, which has a high rate of mass loss in its decomposition interval (Teng and Wei 1998; Orfao et al. 1999).
The aim of this study is to determine the effect of THM treatment on the set recovery and to evaluate the devolatilisation/thermal degradation behaviours of THM-densified lime (Tilia cordata Mill.) wood by thermogravimetry/derivative thermogravimetry analysis in order to establish whether or not the treatment has improved thermal stability of the material.
Materials and method
Materials
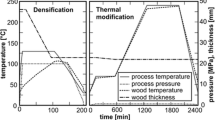
Defect-free lime (Tilia cordata Mill.) wood samples of 30 × 30 × 150 mm³ (L × T × R) were subjected to THM densification treatments along the radial direction under steam-saturated conditions at a temperature of 140 °C in a multi-parameter THM reactor, which has been developed before (Heger et al. 2003; Navi and Heger 2004) to densify and post-treat wood specimens with high-temperature steam. The process involves four steps: plastification (wood specimens were heated and kept at 140 °C in saturated steam conditions for 15 min)—homogenisation of the moisture content and temperature of the samples; densification (the specimens were kept at 140 °C, and a controlled mechanical pressure of 18 MPa was applied, time varied between 11 and 15 min); post-treatment (different temperatures and time—see Table 1—were applied after the treatment ended); and cooling. The diagram of THM processing of lime wood is shown in Fig. 1.
The density of the untreated wood samples is about 0.52 g/cm3, while the density of the THM-densified samples is between 1.35 and 1.38 g/cm3.
Methods
Set recovery tests were carried out at 70 °C. Specimens of ~50 mm radial and 10 mm tangential direction were cut from the treated wood samples. Swelling-contraction cycle tests were carried out using hot water at 70 °C for swelling and oven dried at 70 °C for contraction. For the THM-densified samples, seven swelling/drying cycles were used, each one with a period of 24 h (12 h swelling–12 h drying).
Additionally, powdered wood samples were sieved from different parts of the specimen, homogenised, and then, the fraction with average diameter less than 200 μm was retained for thermogravimetric analysis.
Thermogravimetric analysis (TGA) was carried out under constant nitrogen flow (200 ml/min) at a heating rate of 10 °C/min using a Mettler Toledo TGA/SDTA 851 balance. The heating scans were performed on 3–5 mg of sample in the temperature range 25–700 °C. The kinetic parameters were evaluated by integral methods using VERSATILE commercial programme, which gives kinetic parameters by various methods.
Results and discussion
The main reason for THM densification of wood is to improve some of its mechanical and physico-chemical properties. The specimens before and after THM densification, from the transversal plane, are shown in Fig. 2. The density of the compressed wood depends on the final pressure, the temperature used during the densification and post-treatment, time of the post-treatment and wood species.
The value of the compression set was observed to be approximately 65 %.
Set recovery of THM-densified wood
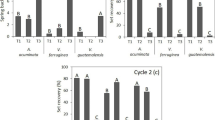
The degree of set recovery of differently treated (THM densified) lime wood samples during soaking and drying is presented in Fig. 3a, b, respectively.
The samples with no post-treatment (S0) or with low time and temperature post-treatment (S3, S4) show a very high set recovery. The samples subjected to post-treatment at 180 °C for 15 (S8) and 20 (S9) minutes have the same behaviours with the sample subjected to a post-treatment at 160 °C for a period of 80 (S6) minutes. These three samples show the lowest set recovery (Fig. 3), suggesting a permanent fixation of transversal compression set. All the samples reached a plateau after the second or third soaking—drying cycle.
Higashihara et al. (2000) suggested that the elastic strain energy stored in the semi-crystalline microfibrils and lignin of wood is the principal reason of compression set recovery. The low amount of set recovery of post-treated THM-densified wood may be due to the breaking of the bonds in-between the crosslinked structures responsible for the memory effect, development of new chemical bonds in the cell wall, coupled with softening of lignin, and some formation of covalent bonds in the compressed position (Inoue et al. 2008; Kutnar and Kamke 2012).
Thermogravimetric analysis
In Fig. 4, the TG/DTG curves of the untreated and THM-densified treated (S0) lime wood samples are represented. The DTG curves show the two decomposition steps (first one assigned to dehydration and elimination of low molecular compounds and second one to decomposition of the wood components).
From the thermograms (Figs. 4, 5), several parameters can be evaluated for each step of mass loss: T i—onset temperature, T m—temperature corresponding to the maximum rate of mass loss, T sh—shoulder temperature and T f—temperature corresponding to the end of stage, the amount of desorbed water and low molecular compounds (up to 150 °C), the mass loss of the process of thermal degradation (as a percentage from the initial mass) and the residual mass at 700 °C. The range of temperatures in which degradation occurs was estimated from the DTG curves, and the weight loss percentage was read from the TG plots.
The first step takes place from 25 to 124 °C for the untreated lime wood sample, with the temperature corresponding to the maximum rate of mass loss at 69 °C and the weight loss at the end of the stage of 3.93 % (Fig. 4, Table 2). For the THM-densified wood sample, this step develops on a wider range of temperatures (from 25 to 143 °C); the temperature corresponding to the maximum rate of mass loss is at 79 °C and the weight loss at the end of the stage of 5.91 %. The increase in mass loss for the first step suggests an increase in the amount of compounds, which decompose at lower temperatures.
The majority of mass is lost in the second step, which corresponds to the thermal degradation of wood components. This step involves a complex process, which consists of several overlapping reactions and/or successive processes of decomposition (Fig. 4). Because the temperature intervals of the wood components (hemicelluloses, cellulose and lignin) decomposition partially or totally overlap each other, the hemicelluloses and/or amorphous cellulose decomposition step usually appears as a shoulder instead of a well-defined peak, followed by an maximum associated with cellulose decomposition with a rapid decay and a long tail associated with the lignin decomposition (wide range of temperatures).
This step takes place from 152 to 400 °C (Table 2) for the untreated lime wood, with a temperature corresponding to the maximum rate of mass loss of 355 °C (Table 2), and a mass loss of about 66 % (Table 2). For the same sample, the temperature corresponding to the shoulder is at 304 °C (Table 2). The THM-densified wood (S0) shows a narrower range of temperatures for the second step compared with untreated sample, between 196 and 409 °C (Table 2). The temperature for the maximum decomposition rate is 360 °C (Table 2), while the temperature for the shoulder is almost the same as for untreated wood, at 302 °C (Table 2). Here, the mass loss at the end of the stage is about 72 % (Table 2) from the total mass of the sample. The increase in the onset temperature for the treated sample and decrease in the shoulder located at 302 °C (Fig. 4) suggest modification of the wood components, especially hemicelluloses structure, during the applied treatment.
Generally, it is known that during hydro-thermal treatment of wood, acetic acid is released by the cleavage of acetyl groups linked as ester groups to the hemicelluloses. Further, in the succeeding reactions, in the presence of water, the acid-catalysed hydrolysis of the ester and also the depolymerisation of the carbohydrates occur (Tjeedsma and Militz 2005; Windeisen et al. 2007; Fang et al. 2012). The increase in the mass loss percentage for the first step and the reduction in the width for the second step suggest the occurrence of the above-mentioned reactions in the THM-densified lime wood sample.
Figure 5 represents the comparison between THM-densified lime wood sample (S0) and THM-densified and post-treated samples (S1–S9) at different temperatures and times. The thermogravimetric data are shown in Table 2.
The samples are separated in three series according to the temperature of post-treatment. In Fig. 5a, the TG/DTG curves of the S0 (no post-treatment) and post-treated wood samples at 140 °C for a period of 150, 180 and 210 min are shown. Figure 5b presents the TG/DTG curves of the S0 and post-treated wood samples at 160 °C for a period of 30, 60 and 80 min, while Fig. 5c represents the TG/DTG curves of the S0 and post-treated wood samples at 180 °C for a period of 10, 15 and 20 min.
For the first step, the temperature corresponding to the maximum mass loss and to the end of the stage decreases (from the S0 sample with no post-treatment) with increasing time of the post-treatment for the first two series of samples (Fig. 5a, b, Table 2) and remains constant for the last series of samples (Fig. 5c, Table 2). The mass losses (at the end of the stage) decrease for the first two series compared to the sample S0 and increase for the third series of samples. The decrease in mass loss and also temperature of the end of the stage (for the samples S1–S3 and S4–S6) compared to the samples S0 suggests the formation of more hydrophobic structures after the post-treatment at 140 and 160 °C. For the third series of samples, S7–S9, the post-treatment at 180 °C probably induces more degradation in the wooden components structure, since the mass loss increases and, as was observed from set recovery tests, the samples did not adsorb water. This can suggest an increase in the amount of low molecular compounds, which are volatilised at low temperatures.
The main decomposition step corresponding to wood components takes place between 180 and 410 °C (Table 2)—varying for each series. For the first series, the post-treated samples show almost the same temperatures for the shoulder and for the maximum decomposition rate with the sample S0, and slightly higher temperatures for the end of the stage, increasing with increasing time of post-treatment. The mass losses decrease for the samples S1–S4 compared to S0, while the residual mass at 700 °C increased.
The second series (S4–S6) (Table 2) show lower onset temperatures compared to S0. The temperatures for the shoulder are almost the same for the samples S0, S4 and S5 and smaller for S6. The temperatures corresponding to the maximum decomposition rate and to the end of the stage are the same for S0 and S4–S6, with a small exception; the temperature of the end of the stage for sample S6 is lower than all others. The values of the mass losses are smaller for the samples with post-treatment at 160 °C for different times compared to S0 (no post-treatment), while the values for the residual mass at 700 °C are higher for post-treated samples than for S0. From Fig. 5b, a decrease in the shoulder with increasing time of the post-treatment can be observed. Because this shoulder is associated with hemicelluloses and amorphous cellulose, a decrease indicates an increase in hemicelluloses modification/degradation during wood treatment.
For the samples S7–S9 (the third series) (Table 2), the onset temperatures are similar to the S0 sample. The temperatures for the shoulder and for the maximum decomposition rate increase in the series but are not higher than that of the sample S0. Interestingly, even though the samples are different, they show the same temperature for the maximum decomposition rate and for the shoulder. The values of the mass loss of this step increase in the series but are also not higher than that of the S0 sample. The values of the residual mass at 700 °C are comparatively higher for the post-treated samples, especially for the S7 sample, when compared to the S0 sample. As for the other series, the shoulder decreases (see Fig. 5c) with the increase in post-treatment time, suggesting the occurrence of amorphous carbohydrates degradation/modification during this treatment.
In order to follow the kinetics of the second step (which involves overlapped processes such as decomposition, crosslinking between carbohydrates and/or between lignin and carbohydrate or recombination of the thermal degradation products during heating and then degradation), the activation energy and reaction order were evaluated by three integral methods: Coats–Redfern (CR) (1964), Flynn–Wall (FW) (1966) and Urbanovici–Segal (US) (1984).
Comparing the values of the overall activation energies for untreated and THM-densified wood without post-treatment, from Table 3 it can be observed that the values for the modified lime wood sample (S0) are higher than the values for untreated wood, indicating a higher stability of the obtained structure. Also, the values of the overall activation energies, corresponding to the first series (S1–S3) of samples, increase with increasing time of post-treatment, while for the second and third series (S4–S6 and S7–S9, respectively) of samples, they decrease with increasing time of post-treatment (Table 3). Additionally, except the S4 sample, all other values of the activation energies are lower than the value obtained for S0 sample, and except S6, S8 and S9 samples, all have higher values of the activation energies than untreated lime wood. Even though these samples show the lowest set recovery effect, they also exhibit lower thermal stability than the untreated lime wood.
From Table 3, it can be observed that the reaction order is 1.1 for untreated lime wood sample and increases to 1.9 for the S0 sample (THM-densified without post-treatment). For the first series (S1–S3) with a post-treatment at 140 °C, the reaction orders increase with the time of post-treatment from 1.9 to 2.1, while for the other two series with a post-treatment at 160 and 180 °C, increasing the time of post-treatment induces a decrease in the reaction orders, from 2.0 to 1.7 and 1.5. Again the samples S6, S8 and S9 exhibit the lowest reaction order values.
The authors are still not sure what make these samples to have improved properties for set recovery but lower thermal stability.
Thermogravimetric analysis indicates that changes in wood structure and composition have occurred as a consequence of the THM densification process and post-treating, and thus, it could be used as a complementary characterisation technique for these types of materials.
Conclusion
The thermogravimetric analysis shows that THM densification process of the lime wood in a closed system caused significant changes in its structure.
The set recovery tests evidenced that the post-treatment at 160 °C for 80 min or at 180 °C for 10, 15 and 20 min improves the dimensional stability of the samples suggesting a permanent fixation of transversal compression set, but also reduced their thermal stability, as observed from the thermogravimetric data. Additionally, these samples have lower values for the mass loss at the end of the decomposition stage and for the residual mass at 700 °C compared to the S0 sample.
The other samples (S1–S5) show high set recovery values and also exhibit higher values for the temperatures corresponding to the maximum decomposition rate and lower values for the mass loss at the end of the stage. Moreover, these samples have higher values for the residual mass at 700 °C and higher activation energies and reaction orders.
References
Assor C, Placet V, Chabbert B, Habrant A, Lapierre C, Pollet B, Perre P (2009) Concomitant changes in viscoelastic properties and amorphous polymers during the hydrothermal treatment of hardwood and softwood. J Agric Food Chem 57:6830–6837
Beall FC (1986) Thermal degradation of wood. In: Bever MB (ed) Encyclopedia of materials science and engineering. Pergamon Press, Oxford
Chang TC, Chang HT, Wu CL, Lin HY, Chang ST (2010) Stabilizing effect of extractives on the photo-oxidation of Acacia confusa wood. Polym Degrad Stab 95:1518–1522
Coats AW, Redfern JT (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Fang CH, Mariotti N, Cloutier A, Koubba A, Blanchet P (2012) Densification of wood veneers by compression combined with heat and steam. Eur J Wood Prod 70:155–163
Flynn JH, Wall LA (1966) A quick direct method for the determination of Activation Energy from Thermogravimetric data. J Polym Sci B Polym Let 4:323–328
Grønli MG, Varhegyi G, Di Blasi C (2002) Thermogravimetric analysis and devolatilization kinetics of wood. Ind Eng Chem Res 41:4201–4208
Heger F, Girardet F, Navi P (2003) In: Van Acker J, Hill C (eds) Proceedings of first European conference on wood modification. European Thematic Network for Wood Modification, Ghent, Belgium, p 33
Helsen L, Van den Bulck EJ (2000) Kinetics of the low-temperature pyrolysis of chromated copper arsenate-treated wood. J Anal Appl Pyrol 53:51–79
Higashihara T, Morooka T, Norimoto M (2000) Permanent fixation of transversely compressed wood by steaming and its mechanism. J Jpn Wood Res Soc 46:291–297
Hill CAS (2006) Wood modification: chemical, thermal and other processes. Wiley, Chichester
Inoue M, Sekino M, Morroka T, Rowell RM, Norimoto M (2008) Fixation of compressive deformation in wood by pre-steaming. J Trop For Sci 20:273–281
Korosec RC, Lavric B, Rep G, Pholeven F, Bukovec P (2009) Thermogravimetry as a possible tool for determining modification degree of thermally treated Norway spruce wood. J Therm Anal Calorim 98:189–195
Kutnar A, Kamke FA (2012) Influence of temperature and steam environment on set recovery of compressive deformation of wood. Wood Sci Technol 46:953–964
Kutnar A, Sernek M (2007) Densification of wood. Zbornikgozdarstva in Lesarstva 82:53–62
Navi P, Heger F (2004) Combined densification and thermo-hydro-mechanical processing of wood. MRS Bull 29:332–336
Navi P, Heger F (2005) Comportement thermo-hydromechanique du bois (Behaviour of Thermo-Hydro-Mechanical processes of wood). PPUR, Lausanne
Orfao JJM, Antunes FJA, Figueiredo JL (1999) Pyrolysis kinetics of lignocellulosic materials—3 independent reactions model. Fuel 78:349–358
Popescu MC, Popescu CM, Lisa G, Sakata Y (2011) Evaluation of morphological and chemical aspects of different wood species by spectroscopy and thermal methods. J Mol Struct 988:65–72
Shebani AN, Van Reenen AJ, Meincken M (2008) The effect of wood extractives on the thermal stability of different wood species. Thermochim Acta 471:43–50
Teng H, Wei YC (1998) Thermogravimetric studies on the kinetics of rice hull pyrolysis and the influence of water treatment. Ind Eng Chem Res 37:3806–3811
Tjeedsma BF, Militz H (2005) Chemical changes in hydrothermal treated wood: FTIR analysis of combined hydrothermal and dry heat-treated wood. Holz Roh Werkst 63:102–111
Urbanovici E, Segal E (1984) A new integral method of non-isothermal kinetic data evaluation. Thermochim Acta 80:389–393
Windeisen E, Strobel C, Wegener G (2007) Chemical changes during the production of thermo-treated beech wood. Wood Sci Technol 41:523–536
Acknowledgments
This work was performed under the framework of the COST Action FP0904. M. C. Popescu acknowledges the financial support of CNCSIS-UEFISCSU, project number PN II-RU PD 460/2010. C.-M. Popescu acknowledges the financial support of PERFORM-ERA “Postdoctoral Performance for Integration in the European Research Area” (ID-57649), financed by the European Social Fund and the Romanian Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popescu, MC., Lisa, G., Froidevaux, J. et al. Evaluation of the thermal stability and set recovery of thermo-hydro-mechanically treated lime (Tilia cordata) wood. Wood Sci Technol 48, 85–97 (2014). https://doi.org/10.1007/s00226-013-0588-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-013-0588-x