Abstract
Several methods are available for obtaining the sorption isotherms of wood. Among these, the saturated salt and dynamic vapor sorption methods are the most frequently used ones. For the first time, the hygroscopic response of wood obtained using these two methods is compared. This is done by determining the 35 and 50 °C adsorption isotherms of juvenile and mature wood of Pinus pinea L. The hygroscopic behavior of the two types of wood is different, as the mature wood has a higher moisture content than the juvenile wood in the isotherms studied. Comparison of the static saturated salt method and dynamic vapor sorption shows few significant differences between the equilibrium moisture content obtained by each method during the adsorption process, both in a point by point comparison and in the comparison of quadratic polynomial forms of the Guggenheim Anderson-de Boer model. Moreover, in both methods the point of relative humidity from which multilayer sorption predominates over monolayer sorption is similar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Numerous studies have been conducted on the hygroscopic behavior of wood. The sorption isotherms of various species have been determined (Popper et al. 2009; Hill et al. 2010; Hoffmeyer et al. 2011; Zaihan et al. 2011; Fernandez et al. 2014), also differentiating between sapwood and heartwood (Ball et al. 2001; Telkki et al. 2013; Song et al. 2014) and between juvenile and mature wood (Lenth and Kamke 2001; Militz et al. 2003; Neimsuwan et al. 2008; Hill et al. 2011; Esteban et al. 2015; Simon et al. 2015). Other authors obtained the isotherms of wood that was modified thermally (Engelund et al. 2010; Jalaludin et al. 2010; Hill et al. 2012; Murata et al. 2013; Pearson et al. 2013) or chemically (Cao and Kamdem 2004; Dieste et al. 2010; Engelund et al. 2010; Xie et al. 2011; Popescu et al. 2014), and also of old wood (Esteban et al. 2006, 2008a, 2010; Bratasz et al. 2012; Popescu and Hill 2013). This variety of studies confirms the importance of knowing the hygroscopic behavior of wood and its repercussion on the physical and mechanical properties of this material (Skaar 1988; Siau 1995; Almeida and Hernandez 2006; Hill 2006; Kretschmann 2010; Rautkari et al. 2014).
The literature includes various methods for obtaining the equilibrium moisture content (EMC) in wood. Among these, the static gravimetric method of saturated salts has been widely used (Cao and Kamdem 2004; Almeida and Hernandez 2006; Esteban et al. 2008b; Majka and Olek 2008; Thygesen et al. 2010; Hoffmeyer et al. 2011; Murata et al. 2013; Olek et al. 2013; Fernandez et al. 2014). This method, described in COST Action E8, consists of keeping the samples in a container with saline solutions that generate known relative humidities (RH) until the wood reaches a state of equilibrium with the ambient RH. Samples are weighed at regular intervals until they reach a constant value corresponding to the equilibrium state.
However, new studies conducted increasingly use dynamic vapor sorption (DVS) (Engelund et al. 2010; Hill et al. 2010, 2012; Jalaludin et al. 2010; Xie et al. 2011; Zaihan et al. 2011; Popescu and Hill 2013; Popescu et al. 2014). This new technology generates the air relative humidity values by mixing dry nitrogen or air with saturated water vapor in automatically controlled proportions. The material is placed inside a small chamber where, as a result of a continuous flow of nitrogen and saturated water vapor over the sample, the equilibrium conditions of the material are reached in less time (Bingol et al. 2012). Changes in mass are constantly and automatically recorded.
The advantages of DVS over the saturated salt method are: (1) faster data acquisition (Engelund et al. 2011)—DVS technique requires a few days to determine a sorption isotherm whereas the salt method using desiccators takes months; (2) greater accuracy, as an automatic balance is used instead of a manual balance (Jalaludin et al. 2010); (3) continuous values for changes in mass are available as opposed to the discrete data of the static method (Zaihan et al. 2009), making it possible to monitor the process in real time and study changes over time; (4) the kinetic parameters of the sorption process that is related to the coefficients of diffusion, can be determined (Popescu et al. 2014) and (5) the possibility of obtaining all the values in the range of 0–96% RH (Engelund et al. 2011). The major disadvantage of DVS compared to the salt method is the high cost of the equipment (Arlabosse et al. 2003).
Very few authors have compared the adsorption behavior obtained using the saturated salt method and DVS. Levoguer and Williams (1999) and Surface Measurement Systems Ltd. (2012) determined the EMC in microcrystalline cellulose reference material (MCC RM 302) through DVS and compared the values with those obtained using the COST 90 procedure. The results showed similarities between the two methods for RH values above 33%. Below this value, the EMC values were higher when obtained by DVS than by the salt method. Arlabosse et al. (2003) determined this comparison in microcrystalline cellulose (MCC), pharmaceutical granules, PolyEtherBlocAmide (PEBA) membrane and sewage sludges. The EMC obtained showed small differences between the methods, which were more pronounced when the apparent diffusion coefficient in the material was higher than 10−9 m2/s. Paes et al. (2010) studied the two methods in samples of ball milled cellulose and concluded that they give similar results. Bingol et al. (2012) obtained the sorption behavior in two varieties of rice using both methods and established that they have similar behavior. None of these studies used wood as the material.
The objective of this study was to compare the saturated salt and dynamic vapor sorption methods for determining the hygroscopic behavior of wood, using the adsorption isotherms of juvenile and mature wood of Pinus pinea L. to obtain the degree of similarity of the results.
2 Materials and methods
The samples of Pinus pinea L. were collected in the municipalities of Coca and Navas de Oro, in the province of Segovia (Spain). All the trees were adults more than 70 years old. A radial slice was taken from the basal disc, obtained 50 cm from the ground. Samples were taken from the juvenile and mature wood zones, defining the juvenile zone as the fraction between rings 10–20 and the mature wood zone as the area with rings older than 50 years. All the samples chosen were from the heartwood, both earlywood and latewood.
2.1 Saturated salt method
The isotherms were obtained at 35 and 50 °C following the methodology described by COST Action E8 (Themelin et al. 1997). The dimensions of the test pieces for both juvenile and mature wood were 15 mm long, 10 mm wide and 1 mm thick, with no specific anatomical orientation. The samples used in the tests had not been through a drying cycle.
The isotherms were plotted using the 10 equilibrium points corresponding to 10 different salts (Table 1). The relative humidity values generated by each salt for each temperature (35 and 50 °C) were taken from Stokes and Robinson (1949), Robinson and Stokes (1959) and Wexler and Wildhack (1965). Three replicates were performed per study sample.
The test pieces were then placed in a desiccator with phosphorous pentoxide for 40 days to anhydrous state. Then the adsorption isotherms were plotted. The samples were considered to have reached equilibrium in each salt when the difference in mass was no more than 0.1% when they were weighed in a 24 h interval. This process took 1 month.
2.2 Dynamic vapor sorption
The sorption isotherms were determined using the DVS-Advantage instrument. The wood was ground in a Retsch SM 2000 mill and sifted to improve air circulation. The size of the particles used in the experiment was 0.5–1.0 mm. The isotherms were determined at 35 and 50 °C. The RH used was the same as in the static saturated salt method. Because of the high reproducibility of the instrument (Jalaludin 2012), only one sample was used per measurement. The initial mass of the sample was 20 mg. The sample was placed in the DVS instrument and dried to obtain the dry mass, then hydrated to reach the corresponding RH. The sample was considered to have reached equilibrium when the change in mass (dm/dt) was less than 0.001% min−1 for 10 min. The change in mass was recorded every minute.
In both methods the equilibrium moisture content was determined using the following equation:
Ww: wet mass (g), W 0: anhydrous mass (g).
The isotherms were fitted using the Guggenheim Anderson-de Boer (GAB) model, which has been widely used to model the sorption isotherms of wood (Kelsey 1957; Themelin et al. 1997; Hartley 2000; Esteban et al. 2008a, 2010; Lesar et al. 2009; Todoruk and Hartley 2011; Bratasz et al. 2012; Lam et al. 2012; Olek et al. 2013). It also allows a type II sorption curve to be drawn in the whole RH range (Hartley 2000; Esteban et al. 2008a, 2010):
X: EMC (%), X m : monolayer saturation moisture content (%), C g : Guggenheim constant (dimensionless), K: constant (dimensionless), RH: relative humidity (%)
The isotherm fits were considered valid when the correlation coefficient (R) was greater than 0.990 and the root mean square error (RMSE) was less than 4% (Viollaz and Rovedo 1999; Esteban et al. 2006, 2009).
2.3 Comparison of methods
The methods were compared in two ways. Firstly, the existence of significant differences between the DVS measurement and the interval of variation of the measurement with the static saturated salt method was analyzed, using a two-tailed test (Miller and Miller 2002) with a confidence level of 95%.
Secondly, the quadratic polynomial forms of the GAB equations of the methods were compared using the MATLAB program® Version 7.5.0 Curve Fitting Toolbox Version 1.2, which enables the confidence intervals of the fitted curves to be obtained.
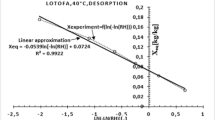
The GAB equation is expressed in quadratic polynomial form as follows:
This equation concurs with the polynomial form of the Hailwood–Horrobin model, which is also commonly used to model the isotherms of wood:
3 Results and discussion
Table 1 shows the EMC values of juvenile and mature wood for the static saturated salt method and the dynamic method (DVS). In all cases the isotherms describe a type II sigmoid (Fig. 1) characteristic of cellulosic materials (Avramidis 1997; Zaihan et al. 2011; Engelund et al. 2013). EMC values are higher in salts than in DVS for high RH and lower for the remaining RH, except in the juvenile and mature wood at 11.17% RH and 35 °C (Table 1). The most likely explanation for this is that the ambient conditions for high RH are obtained with greater accuracy with DVS than with saturated salts.
Table 1 shows the relation between the sorption behavior of juvenile and mature wood. In all cases the EMC values are higher in the mature wood than in the juvenile wood and therefore there is a relation between growth ring location and sorption behavior. Other authors obtained similar results (Zobel et al. 1968; Militz et al. 2003; Neimsuwan et al. 2008; Esteban et al. 2015; Simon et al. 2015). In contrast, Lenth and Kamke (2001), Majka and Olek (2008) and Hill et al. (2015) obtained higher EMC values in juvenile wood than in mature wood. The difference in this behaviour is mainly due to the chemical composition of each type of wood (Esteban et al. 2015; Simon et al. 2015).
The isotherm fits with the GAB model are valid, as they have R values greater than 0.990 and RMSE values less than 4% for all cases (Table 2). The saturation moisture content of the monolayer (X m ) is higher in the mature wood than in the juvenile wood at both temperatures. The RH values where multilayer sorption starts to predominate over monolayer sorption are at around 30% (Table 2), supporting observations in other species (Esteban et al. 2009, 2010, 2015; Fernandez et al. 2014). The similar value using the saturated salt method and DVS indicates the good correlation between the methods for obtaining the EMC.
Figure 2 shows the experimental EMC of DVS and the intervals of variation of the measurements with the saturated salt method.
A good correlation between salts and DVS was obtained, confirming the findings of other studies on different materials (Levoguer and Williams 1999; Arlabosse et al. 2003; Paes et al. 2010; Bingol et al. 2012; Surface Measurement Systems Ltd. 2012). There is only one RH value for which the methods differ. In the mature wood, at both 35 and 50 °C, this difference occurs for RH at around 40%, and in the juvenile wood it depends on the temperature, as it is 75% RH at 35 °C and 30% at 50 °C. Bingol et al. (2012) compared the EMC obtained with salts and with DVS in various forms of two varieties of rice. The differences between the two methods occurred in different RH ranges depending on the form of rice—in some cases for 0 and 98% RH and in others for RH around 40–80%. Therefore, it is not possible to establish a specific range of differences.
Table 3 shows the values of the quadratic polynomials of the GAB model using the saturated salt method and DVS. It was observed that the values of the two methods are similar (except in the mature wood at 50 °C and 30.54% RH), creating overlaps between their intervals of variation. This indicates that there are no significant differences between the methods.
No studies have compared the saturated salt method and DVS using the quadratic polynomials of the GAB model. However, this method can be considered valid, as the results obtained concur with those determined by comparing the EMC of DVS with the intervals of variation of the measurements using salts.
4 Conclusion
The isotherms of both juvenile and mature wood of Pinus pinea describe type II sigmoids and fit satisfactorily according to the GAB model. For any temperature and method, the juvenile wood has lower EMC than the mature wood.
The saturated salt and DVS methods show no significant differences when comparing the EMC points obtained with each one, both in the point-by-point comparison and using the quadratic polynomial form of the GAB model. This result is strengthened by the similar point of relative humidity from which multilayer sorption predominates over monolayer sorption in the two methods.
References
Almeida G, Hernandez RE (2006) Changes in physical properties of tropical and temperate hardwoods below and above the fiber saturation point. Wood Sci Technol 40(7):599–613
Arlabosse P, Rodier E, Ferrasse J, Chavez S, Lecomte D (2003) Comparison between static and dynamic methods for sorption isotherm measurements. Dry Technol 21(3):479–497
Avramidis S (1997) The basics of sorption. In: Hoffmeyer Preben (ed) Proceedings of International Conference on Wood-Water Relations. 16–17 June 1997, Copenhagen, Denmark. pp 1–16
Ball RD, Simpson IG, Pang S (2001) Measurement, modelling and prediction of equilibrium moisture content in Pinus radiata heartwood and sapwood. Holz Roh Werkst 59(6):457–462
Bingol G, Prakash B, Pan Z (2012) Dynamic vapor sorption isotherms of medium grain rice varieties. LWT-Food Sci Technol 48(2):156–163
Bratasz L, Kozlowska A, Kozlowski R (2012) Analysis of water adsorption by wood using the Guggenheim-Anderson-de Boer equation. Eur J Wood Prod 70(4):445–451
Cao JZ, Kamdem DP (2004) Moisture adsorption characteristics of copper-ethanolamine (Cu-EA) treated southern yellow pine (Pinus spp.). Holzforschung 58(1):32–38
Dieste A, Krause A, Mai C, Militz H (2010) The calculation of EMC for the analysis of wood/water relations in Fagus sylvatica L. modified with 1,3-dimethylol-4,5-dihydroxyethyleneurea. Wood Sci Technol 44(4):597–606
Engelund ET, Klamer M, Venas TM (2010) Acquisition of sorption isotherms for modified woods by the use of dynamic vapour sorption instrumentation. Principles and practice. IRG/WP 10-40518
Engelund ET, Klamer M, Venas TM (2011) Adsorption boundary curve influenced by step interval of relative humidity investigated by dynamic vapour sorption equipment. IRG/WP 11-40547
Engelund ET, Thygesen LG, Svensson S, Hill CAS (2013) A critical discussion of the physics of wood-water interactions. Wood Sci Technol 47(1):141–161
Esteban LG, Fernandez FG, Casasus AG, de Palacios P, Gril J (2006) Comparison of the hygroscopic behaviour of 205-year-old and recently cut juvenile wood from Pinus sylvestris L. Ann For Sci 63(3):309–317
Esteban LG, de Palacios P, Fernandez FG, Guindeo A, Conde M, Baonza V (2008a) Sorption and thermodynamic properties of juvenile Pinus sylvestris L. wood after 103 years of submersion. Holzforschung 62(6):745–751
Esteban LG, de Palacios P, Fernandez FG, Guindeo A, Cano NN (2008b) Sorption and thermodynamic properties of old and new Pinus sylvestris wood. Wood Fiber Sci 40(1):111–121
Esteban LG, de Palacios P, Fernandez FG, Martin JA, Genova M, Fernandez-Golfin JI (2009) Sorption and thermodynamic properties of buried juvenile Pinus sylvestris L. wood aged 1170 ± 40 BP. Wood Sci Technol 43(7–8):140–151
Esteban LG, de Palacios P, Fernandez FG, Garcia-Amorena I (2010) Effects of burial of Quercus spp. wood aged 5910 ± 250 BP on sorption and thermodynamic properties. Int Biodeter Biodegr 64(5):371–377
Esteban LG, Simon C, Fernandez FG, de Palacios P, Martin-Sampedro R, Eugenio ME, Hosseinpourpia R (2015) Juvenile and mature wood of Abies pinsapo Boissier: sorption and thermodynamic properties. Wood Sci Technol 49(4):725–738
Fernandez FG, Esteban LG, de Palacios P, Simon C, Garcia-Iruela A, de la Fuente J (2014) Sorption and thermodynamic properties of Terminalia superba Engl. and Diels. and Triplochiton scleroxylon K. Schum. through the 15, 35 and 50 °C sorption isotherms. Eur J Wood Prod 72(1):99–106
Hartley ID (2000) Application of the Guggenheim-Anderson-de Boer sorption isotherm model to klinki pine (Araucaria klinkii Lauterb.). Holzforschung 54(6):661–663
Hill C (2006) Wood modification. Chemical, thermal and other processes. Wiley, Chichester, UK
Hill C, Norton AJ, Newman G (2010) The water vapour sorption properties of sitka spruce determined using a dynamic vapour sorption apparatus. Wood Sci Technol 44(3):497–514
Hill C, Moore J, Jalaludin Z, Leveneu M, Mahrdt E (2011) Influence of earlywood/latewood and ring position upon water vapour sorption properties of sitka spruce. Int Wood Prod J 2(1):12–19
Hill C, Ramsay J, Keating B, Laine K, Rautkari L, Hughes M, Constant B (2012) The water vapour sorption properties of thermally modified and densified wood. J Mater Sci 47(7):3191–3197
Hill CAS, Ramsay J, Gardiner B (2015) Variability in water vapour sorption isotherm in Japanese larch (Larix kaempferi Lamb.)—earlywood and latewood influences. Int Wood Prod J 6(2):53–59
Hoffmeyer P, Engelund ET, Thygesen LG (2011) Equilibrium moisture content (EMC) in Norway spruce during the first and second desorptions. Holzforschung 65(6):875–882
Jalaludin Z (2012) The water vapour sorption behaviour of wood. Thesis, Edinburgh Napier University
Jalaludin Z, Hill CAS, Samsi HW, Husain H, Xie Y (2010) Analysis of water vapour sorption of oleo-thermal modified wood of Acacia mangium and Endospermum malaccense by a parallel exponential kinetics model and according to the Hailwood-Horrobin model. Holzforschung 64(6):763–770
Kelsey KE (1957) The sorption of water vapour by wood. Aust J Appl Sci 8:42–54
Kretschmann DE (2010) Mechanical properties of wood. In: Wood handbook, wood as an engineering material. General Technical Report FPL-GTR-190. US Department of Agriculture, Forest Service, Forest Products Laboratory, Madison. pp 1–46
Lam PS, Sokhansanj S, Bi XT, Lim CJ, Larsson SH (2012) Drying characteristics and equilibrium moisture content of steam-treated Douglas fir (Pseudotsuga menziesii L.). Bioresour Technol 116:396–402
Lenth CA, Kamke FA (2001) Equilibrium moisture content of wood in high-temperature pressurized environments. Wood Fiber Sci 33(1):104–118
Lesar B, Gorisek Z, Humar M (2009) Sorption properties of wood impregnated with boron compounds, sodium chloride and glucose. Dry Technol 27(1):94–102
Levoguer CL, Williams DR (1999) The characterisation of pharmaceutical materials by dynamic vapour sorption. Dyn Vapour Sorp Appl Note 101:1–5
Majka J, Olek W (2008) Sorption properties of mature and juvenile lime wood (Tilia sp.). Folia Forestalia Polonica B 65–75
Militz H, Busetto D, Hapla F (2003) Investigation on natural durability and sorption properties of Italian chestnut (Castanea sativa Mill.) from coppice stands. Holz Roh Werkst 61(2):133–141
Miller JN, Miller JC (2002) Estadística y quimiometría para química analítica. Prentice Hall, Madrid
Murata K, Watanabe Y, Nakano T (2013) Effect of thermal treatment on fracture properties and adsorption properties of spruce wood. Materials 6(9):4186–4197
Neimsuwan T, Wang S, Taylor AM, Rials TG (2008) Statics and kinetics of water vapor sorption of small loblolly pine samples. Wood Sci Technol 42(6):493–506
Olek W, Majka J, Czajkowski L (2013) Sorption isotherms of thermally modified wood. Holzforschung 67(2):183–191
Paes SS, Sun SM, MacNaughtan W, Ibbett R, Ganster J, Foster TJ, Mitchell JR (2010) The glass transition and crystallization of ball milled cellulose. Cellulose 17(4):693–709
Pearson H, Gabbitas B, Ormarsson S (2013) Equilibrium moisture content of radiata pine at elevated temperature and pressure reveals measurement challenges. J Mater Sci 48(1):332–341
Popescu CM, Hill CAS (2013) The water vapour adsorption-desorption behaviour of naturally aged Tilia cordata Mill. wood. Polym Degrad Stab 98(9):1804–1813
Popescu CM, Hill CAS, Curling S, Ormondroyd G, Xie YJ (2014) The water vapour sorption behaviour of acetylated birch wood: how acetylation affects the sorption isotherm and accessible hydroxyl content. J Mater Sci 49(5):2362–2371
Popper R, Niemz P, Croptier S (2009) Adsorption and desorption measurements on selected exotic wood species. Analysis with the Hailwood-Horrobin model to describe the sorption hysteresis. Wood Res 54(4):43–55
Rautkari L, Honkanen J, Hill CAS, Ridley-Ellis D, Hughes M (2014) Mechanical and physical properties of thermally modified Scots pine wood in high pressure reactor under saturated steam at 120, 150 and 180 °C. Eur J Wood Prod 72(1):33–41
Robinson RA, Stokes RH (1959) Electrolyte solutions. Academic Press, New York
Siau JF (1995) Wood: influence of moisture on physical properties. Department of Wood Science and Forest Products, Virginia Polytechnic Institute and State University, Blacksburg, VA
Simon C, Esteban LG, de Palacios P, Fernandez FG, Martin-Sampedro R, Eugenio ME (2015) Thermodynamic analysis of water vapour sorption behaviour of juvenile and mature wood of Abies alba Mill. J Mater Sci 50(22):7282–7292
Skaar C (1988) Wood-water relations. Springer-Verlag, Berlin
Song KL, Yin YF, Salmen L, Xiao FM, Jiang XM (2014) Changes in the properties of wood cell walls during the transformation from sapwood to heartwood. J Mater Sci 49(4):1734–1742
Stokes RH, Robinson RA (1949) Standard solutions for humidity control at 25 °C. Ind Eng Chem 41:2013
Surface Measurement Systems Ltd. (2012) Moisture sorption of EC standard reference material RM 302 on a DVS instrument. Dynamic Vapour Sorption, Application Note 02
Telkki VV, Yliniemi M, Jokisaari J (2013) Moisture in softwoods: fiber saturation point, hydroxyl site content, and the amount of micropores as determined from NMR relaxation time distributions. Holzforschung 67(3):291–300
Themelin A, Rebollo J, Thibaut A (1997) Method for defining the behaviour of lignocellulosic produces at sorption: application to tropical wood species. In: Hoffmeyer Preben (ed) Proceedings of International Conference on Wood-Water Relations. 16–17 June 1997, Copenhagen, Denmark. pp 17–32
Thygesen LG, Engelund ET, Hoffmeyer P (2010) Water sorption in wood and modified wood at high values of relative humidity. Part I: Results for untreated, acetylated, and furfurylated Norway spruce. Holzforschung 64(3):315–323
Todoruk TM, Hartley ID (2011) Sorption isotherm of lodgepole pine (Pinus contorta Dougl. Ex Loud.) pulp chips affected by the mountain pine beetle (Dendroctonus ponderosae). Holzforschung 65(1):109–112
Viollaz PE, Rovedo CO (1999) Equilibrium sorption isotherms and thermodynamic properties of starch and gluten. J Food Eng 40(4):287–292
Wexler A, Wildhack WA (1965) Humidity and moisture: measurement and control in science and industry, vol 3. Fundamentals and standards. Reinhold Publishing Corporation, New York
Xie Y, Hill CAS, Xiao Z, Mai C, Militz H (2011) Dynamic water vapour sorption properties of wood treated with glutaraldehyde. Wood Sci Technol 45(1):49–61
Zaihan J, Hill CAS, Curling S, Hashim WS, Hamdan H (2009) Moisture adsorption isotherms of Acacia mangium and Endospermum malaccense using dynamic vapour sorption. J Trop For Sci 21(3):277–285
Zaihan J, Hill CAS, Hashim WS, Dahlan JM, Sun DY (2011) Analysis of the water vapour sorption isotherms of oil palm trunk and rubberwood. J Trop For Sci 23(1):97–105
Zobel B, Matthias M, Roberds JH, Kellison RC (1968) Moisture content of southern pine trees. Tech Rep No. 37. School For Res NC State Univ Raleigh
Acknowledgements
This study is part of the AGL2009-12801 project of the 2008–2011 Spanish National Plan for Scientific Research, Development and Technological Innovation, funded by the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Simón, C., Fernández, F.G., Esteban, L.G. et al. Comparison of the saturated salt and dynamic vapor sorption methods in obtaining the sorption properties of Pinus pinea L.. Eur. J. Wood Prod. 75, 919–926 (2017). https://doi.org/10.1007/s00107-016-1155-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-016-1155-6