Abstract
The main objective of this study was to compare in the assessment of risk of fractures in postmenopausal women two algorithms for 10-year fracture risk evaluation, the WHO-endorsed FRAX® and the Italian FRAX-derived version (DeFRA), which considers BMD of different bone sites and allows the inclusion of other data. In a secondary analysis, we compared the performance of the tools in discriminating subjects who sustained previous major fractures from those who did not. The 10-year fracture risk score was evaluated in a sample of 989 climacteric women using FRAX and DeFRA tools. Bone mineral density was also included in the calculation of these algorithms. Comparing how the subjects were assigned to different risk classes by the two tools, we found that DeFRA attributed higher risk categories than FRAX, among women in the subgroups between 50 and 59 and, mostly, 60–69 years of age. ROC curve analysis showed that DeFRA had the same discriminative ability to identify previous major osteoporotic fractures compared to FRAX (AUC = 0.74 for both). If confirmed by prospective studies, our findings would suggest that DeFRA might be ascribed as at least equivalent to FRAX or perhaps slightly most appropriate in the categorization of the fracture risk, particularly in women aged 60–69 years, a period in which bone densitometry analysis is highly recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis (PO) is one of the most important diseases related to postmenopausal estrogen deprivation. Osteoporosis is characterized by reduced bone mass and impairment of bone architecture, leading to an increased risk of fragility fractures (FFs). According to a recent epidemiological study in 2010, 22 million women and 5.5 million men living in the European Union suffered from PO, with more than 3.5 million new FFs sustained [1, 2]. Therefore, it is mandatory upon the clinical practitioner, to identify patients at high risk of FFs in whom treatment will result in the greatest benefit while avoiding treatment of low-risk individuals where the expected benefit will be small [3, 4].
Osteoporosis is diagnosed when the bone mineral density (BMD) is lower than 2.5 standard deviations below the average BMD in healthy young Caucasian women (a T-score of −2.5 SD or less), however BMD fails to deliver an accurate estimate of fracture risk, since more than 50 % of FFs occur in osteopenic patients [5].
Fracture is the outcome of several risk factors, and this multiplicity should be taken into consideration in the assessment of fracture risk for an individual. Several risk prediction tools that integrate the weight of clinical risk factors (CRFs) for fracture, with or without information on BMD, have been developed in recent decades. Official guidelines [6] suggest the use of these tools to identify screening candidates among young postmenopausal women aged 50–64 years or to individualize candidates to antifracturative therapy in women older than 65 years of age.
The performance of fracture risk assessment tools has recently been critically reviewed. This systematic comparison revealed that the performance of the various algorithms is generally fair, with diagnostic accuracy between 0.6 and 0.8. It has also been estimated that, when not including BMD value in calculating the score for the estimation of the risk, the simplest methods are equivalent if not superior to the more complex ones [7, 8]. The Fracture Risk Assessment Tool (FRAX), developed by the WHO Collaborating Centre for Metabolic Bone Diseases, currently represents the most commonly used tool for calculating individual fracture risk based upon that individual’s risk factor profile [9].
Despite the validation of FRAX, several studies have raised important concerns regarding the algorithm’s ability to accurately quantify the fracture risk [10]. Among the limitations highlighted by these studies, the most critical are the underestimation of the number and sites of previous fractures and the lack of details on some risk factors (e.g., dose of glucocorticoids, units of alcohol per day or number of cigarettes smoked, etc.).
In Italy, the SIOMMMS (Italian Society for Osteoporosis, Mineral Metabolism, and Bone Diseases) developed an adapted version of FRAX, called DeFRA (FRAX-derived, ver 1.0), based on local epidemiological studies [11].
This is a novel, under validation, tool based on the data published for the development of FRAX and yielding results almost superimposable with those obtained using the “progenitor” [12]. The main goal of the developers of DeFRA was to better define the individual absolute risk of fracture by introducing into the algorithm graduated, not dichotomous variables (smoking, corticosteroid dose, alcohol units), number and sites of previous fragility fractures, other diseases potentially causing bone loss (e.g, connectivitis), BMD of either the spine or femur.
To the best of our knowledge, no previous studies have compared the performance of FRAX with that of DeFRA in women at climacteric age. The current study was undertaken to compare these two tools in estimating risk of fractures. In a secondary analysis, we compared the performance of FRAX and DeFRA in discriminating women who sustained previous major fractures from those who did not.
Methods
Design of the Study
The present study was a cross-sectional population-based study performed in the northeastern region of Italy. A group of women who were referred to the Menopause and Osteoporosis Center of the University of Ferrara for osteoporosis management from 2012 to 2013 were enrolled in this study. The investigation was undertaken within the framework of a protocol approved by the Medical School Ethics, participants in the cohorts included in this analysis provided informed consent at enrollment.
Collection of personal details, age, weight, height, menopausal status, concomitant main pathologies, and pharmacological treatments was performed by a trained nurse at the time of bone density examination. Individuals were included in the study if they were women of Caucasian origin aged between 50 and 90 years old. These age criteria were based on 50 years being the minimum age criterion for DeFRA and 90 years being the maximum age criterion for the FRAX algorithm. Subjects were excluded if they had been on osteoporosis treatment for more than 3 years at the time of evaluation. Of the 1654 consecutive attendants to the ambulatory clinic, 1023 women were eligible for the study; only 989 who had complete data records were finally included in the analysis.
Medical History and Physical Examination
The interview included gathering the clinical risk factors considered by the FRAX and DeFRA web tools to estimate the individual 10-year fracture risk: body mass index (BMI), parental and personal history of hip or vertebral fragility fractures, prior low-trauma fractures at other sites after 50 years of age, long-term use and dosage of glucocorticoids, smoking habits (number of cigarettes/day), alcohol intake (number of units/day), rheumatoid arthritis, and other connective tissue diseases (e.g., scleroderma, psoriatic arthritis, and systemic lupus), causes of secondary osteoporosis (diabetes mellitus, hypogonadism, osteogenesis imperfecta in adults, untreated chronic hyperthyroidism, early menopause, chronic malnutrition or malabsorption, chronic liver diseases, or others) as indicated in Table 1.
Secondary causes of osteoporosis are not considered in the estimation of both DeFRA and FRAX, because the possible weight of these factors in the final estimate of the risk has already been taken into account by BMD values imputed in the algorithm. Table 1 shows the CRFs that are considered when calculating the individual 10-year fracture risk using FRAX and DeFRA.
Fracture Risk Assessment
The individual 10-year fracture risk was assessed by the FRAX tool for Europe-Italy (available online at the website http://www.shef.ac.uk/FRAX) considering CRFs for osteoporosis collected in the case history and the T-score for femoral neck BMD. The output is the 10-year probability of a hip fracture and the 10-year probability of a major osteoporotic fracture (hip, clinical vertebral, humerus, or wrist); only the latter was considered in the present work.
DeFRA was also used to evaluate the individual 10-year fracture risk (website https://DeFRA-osteoporosi.it). It was possible to calculate DeFRA-risk only for the female population aged 50 years or more by inputting the lowest T-score between femoral and vertebral BMD (as recommended by the International Society for Clinical Densitometry [13]). The output is the 10-year probability of a major osteoporotic fracture (clinical vertebral, hip, forearm, or proximal humerus fractures).
In comparing the ability of FRAX and DeFRA to retrospectively identify subjects with OFs, we did not include responses regarding prior fracture while calculating the scores of the two tools.
BMD Measurement
The measurements were performed at both spine and femoral sites using an Hologic Discovery DXA scanner subject to weekly quality and precision controls. The total value for the spine had to originate from at least two normal vertebrae according to ISCD official position [13].
Statistical Analysis
Data were analyzed using SPSS 17.00 for Windows (Chicago, IL, USA). Unpaired Student’s t test (for normal variables) and the Mann–Whitney (for non-normal variables) analysis were used to identify significant differences between groups. The χ 2 test was used to compare differences in categorical variables. This type of variable was used to stratify the sample in subgroups according to scores of either of the two algorithms. The ability of the two algorithms under examination to discriminate those women who sustained previous fractures from those who did not was measured as the areas under the receiver operating characteristic (ROC) curves (AUC). A two-tailed probability value <0.05 was considered statistically significant.
Results
The main characteristics of the sample are presented in Table 2. The mean age of the population (n = 989) was 63.6 years (range 50–89), and taking into account the age class distribution, 36 % (n = 354) of the women were younger than 60 years and were in a relatively early postmenopausal status. Only 24 % (n = 239) of the sample was more than 70 years of age. The large majority of the sample subjects were women in spontaneous postmenopause (83 %), while only 5.4 % were in the menopausal transition. The average age at menopause was 49.5 years, in the subgroup of women who underwent a natural menopause, the mean age was 50 years.
Table 2 also shows the prevalence of the CRFs considered by FRAX and DeFRA. At least one CRF was found in 41 % of the subjects, but the presence of more than one was quite infrequent. The CRFs with the highest prevalence were smoking (12.9 %), a parental history of fractures (11.2 %) and secondary osteoporosis (10.3 %). Considering the latter, early menopause was the most common condition (9.8 %). Previous hip or spine fractures were found in 7.4 % of the subjects, and 2.4 % of the women had a previous non-spine/non-hip fragility fracture.
On the basis of the BMD found at the different sites examined by DXA scanning, the population was classified into different clusters using T-score, as indicated by the densitometric WHO criteria for postmenopausal women [5] (see the mentioned classification in Online Resource 1). Specifically, 49 % of the women had osteopenia, and 26 % were osteoporotic at the vertebral site; at the femoral neck, 17 % of the subjects were osteoporotic and 61 % were osteopenic. In women younger than 65 years, osteoporosis was present in 24 % of subjects at the vertebral site and 11.5 % at the femoral neck.
The FRAX mean score for major fractures was significantly lower (p < 0.001) than that calculated by DeFRA (FRAX 8.0 (1.9–53); DEFRA 9.6 (0.3–50.1) p < 0.001). In 51 % of cases, the DeFRA value was computed considering the spine T score.
Table 3 represents the cross-tabulation of the FRAX and DeFRA fracture risk categories assigned to study participants. From this analysis we found that the two sample stratifications were significantly (p < 0.001) different from each other. In particular, while the proportion of women at lower risk (score < 10 %) was comparable, only 70, 32, 25, and 75 % of subjects scoring 10–19, 20–29, 30–39, and >40 % for major fracture risk according to FRAX, respectively, belonged to the same DeFRA category.
The distribution of subjects according to risk categories by DeFRA and FRAX scores was also examined in three sample subgroups of different age (range 10 years) (Table 4). Interestingly, the classifications based on the FRAX and DeFRA scores were significantly different within each age subsample. The most relevant differences were observed in the 50–59 and 60–69 year subgroups, where women classified as having a risk exceeding 10 % by FRAX were much less frequent compared to those classified by DeFRA. In subjects older than 69 years, DeFRA identified 19 patients with a 10-year risk fracture of more than 40 %, while FRAX identified only 4.
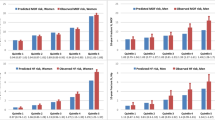
Figure 1 displayed the comparison between mean scores of DeFRA and FRAX according to the presence or absence of previous major fractures. As shown the figure, the 10-year absolute fracture risks as assessed by the 2 algorithms were similarly, and significantly, higher in women with fractures compared to the other women. Women with previous fractures were older and had a higher prevalence of parental fracture history, low BMI, and secondary causes of osteoporosis (e.g., premature menopause) compared to those in the other subgroup. The main characteristics, including CRFs distribution of the two sample subjects are shown in Table b (see supplementary data).
Finally, we checked the ability of the two algorithms (in which prior fractures were not inputted) to retrospectively predict previous fractures by the determination of AUC under the ROC curve. The accuracy for the FRAX model was very similar to that obtained for DeFRA, with both values reflecting a discrimination that was better than random chance (AUC = 0.5). More specifically the data obtained from ROC analysis were the following: FRAX: 0.736 (0.677–0.794); DeFRA: 0.742 (0.688–0.797).
Discussion
The evaluation of osteoporotic risk must be a relevant part of the overall assessment of postmenopausal woman [14]. Clinicians caring for women at climacteric age play a critical role in the prevention of PO and osteoporotic FFs by the identification and proper treatment of women at high risk for fracture. Moreover, since 2002, the progressive decrease in the use of menopausal hormone therapy (MHT) for the treatment of postmenopausal complaints [15, 16] has increased the risk of accelerated bone loss during the menopausal transition and the risk for fractures later in life. The use of algorithms, such as FRAX, to assess the absolute 10-year fracture risk is the currently recommended approach to assist physicians in identifying women who need anti-fracturative treatments. Some studies suggest that the FRAX tool may not be sufficiently sensitive for early postmenopausal women, and recent data confirm the low sensitivity of this tool in predicting initial and recurrent FFs [17–19]. In Italy, the DeFRA algorithm has been created to overcome some critical limitations of FRAX; in our study, we compare the two tools in assessing the 10-year absolute fracture risk in a selected population of postmenopausal women. Overall, our data show that the CRFs considered in the computation of the two algorithms were present in only 49 % of the sample subjects. The mean value of the estimated absolute fracture risk overall was low, but the DeFRA tool produced significantly higher values (9.6 and 8 % for DeFRA and FRAX, respectively). Taking into account the way which the subjects were assigned to different risk classes by the two tools, it became clear that DeFRA assigned higher risk categories than FRAX only among women in the subgroups aged between 50 and 59 and 60–69 years (whereas older women were similarly stratified by the two tools). This discrepancy was most evident in 60–69 years subgroup where 5 % of the women, instead of 0.5 %, were attributed a high fracture risk (i.e., ≥20 [5]). Considering the whole sample, the use of DeFRA resulted in an increase by 2 % of the absolute number of post-menopausal women estimated to be at high risk by FRAX.
The tendency of the WHO-endorsed tool to underestimate the risk for fracture, especially non-femoral fractures and mostly in the younger age groups, has been emphasized in a recent work [20]. Moreover, some Authors [21, 22] evaluated the effects of the, frequently highlighted, discordance between BMD T-score at lumbar spine (LS) and femoral neck (FN) in relation to fracture risk assessment. They found that a marked difference in BMD T-scores between the two sites can contribute to fracture risk, independently of FRAX probabilities that incorporate FN BMD alone.
Though not surprising, our data document that a higher grading of risk factors and the inclusion of spine BMD in the calculation of the score could improve the estimate of the risk of fracture, particularly between postmenopausal women, in the age group where BMD measurement begins to be recommended, although this has to be confirmed by prospective studies.
In our study, 9.5 % of subjects reported previous fragility fractures, which alone represent an indication for therapy according to the main international guidelines. Compared to the fracture-free subsample, these women were found to be significantly different with regard to age, CRFs, and FRAX and DeFRA mean scores (see the main characteristics of the two subsamples in Online Resource 1). Interestingly, the mean value of FRAX in women with a history of previous fractures was slightly above the intervention threshold (11.3 %) set by European guidelines (ESCEO) for Italian women of ages comparable to those of our sample [1]. In addition, similar results were obtained using the two tools in the identification of previous fractures. Overall, our population is characterized by a low prevalence of CRFs and estimated values of absolute fracture risk that mainly range from low to medium, irrespective of the presence of a high percentage of osteoporosis and osteopenia diagnosed before 65 years. These data underscore how the menopausal transition and early postmenopausal period represent an ideal window for preventive intervention aimed to counteract the deterioration of bone density and skeletal structure and to correct modifiable lifestyle risk factors to reduce the incidence of fractures later in life. The use of algorithms to evaluate the absolute fracture risk is recommended in a climacteric clinical setting; however, as recent data suggest, none of the current osteoporosis risk-based strategies is optimal [23]. At present, alternative strategies for properly identifying subjects at high risk of fracture are under examination in several countries. Some Authors have suggested that artificial neural networks (ANNs) analysis could represent a reliable approach because it is a novel statistical-mathematical model; beyond traditional statistics, which attempts to better understand the link between BMD values, CRFs, and fragility fractures [24]. In addition, the GLOW group study has recently proposed an empirically based 5-year composite fracture risk model for fracture sites that display similar risk profiles [25]. In this frame, a further interesting suggestion would be to integrate new predictive models with markers of bone turnover, as well as a number of other non-rheumatologic comorbidities associated with increased fracture risk (e.g., chronic obstructive pulmonary disease, Parkinson’s disease, multiple sclerosis, inflammatory bowel disease) [26].
Finally, some important limitations of the study must be acknowledged. First, our investigation, along with many others dealing with systematic comparison between FRAX and other tools [18, 19, 27], included a few subjects with relevant and graduated risk factors, such as different doses of corticosteroids, alcohol units, etc. This is an important issue, since a higher grading in the collection of CRFs represents one of the main differences between the 2 algorithms. To overcome this caveat, future studies should include a larger number of subjects under corticosteroid therapy or affected by other connective tissue diseases (besides RA). Second, a relevant limitation of our work lies in the fact that we only performed a retrospective analysis of ability of the two tools to predict fractures. We are aware that the results obtained by this type of approach give only a partial indication of the predictive power of DeFRA and FRAX, that, in turn, needs to be verified by longitudinal studies. It is fair to outline, however, that this is the first attempt to compare the assessment of the fracture risk by FRAX and DeFRA in a large population of postmenopausal women.
Conclusion
The presented data showed that, the use of DeFRA resulted in an increase by 2 % of the absolute number of post-menopausal women, mostly aged 60–69 years, estimated to be at high risk by FRAX. Thus, DeFRA might be ascribed as at least equivalent to FRAX or perhaps slightly more appropriate in the categorization of the fracture risk in those women in whom, usually, bone density examination is recommended and in subgroups of patients with specific risk factors.
References
Hernlund E, Svedbom A, Ivergård M, et al. (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136. doi: 10.1007/s11657-013-0136-1
Piscitelli P, Brandi M, Cawston H et al (2014) Epidemiological burden of postmenopausal osteoporosis in Italy from 2010 to 2020: estimations from a disease model. Calcif Tissue Int 95:419–427. doi:10.1007/s00223-014-9910-3
Rossini M, Di Munno O, Gatti D et al (2011) Optimising bisphosphonate treatment outcomes in postmenopausal osteoporosis: review and Italian experience. Clin Exp Rheumatol 29:728–735
Kanis JA, McCloskey E, Branco J et al (2014) Goal-directed treatment of osteoporosis in Europe. Osteoporos Int 25:2533–2543. doi:10.1007/s00198-014-2787-1
Cosman F, de Beur SJ, LeBoff MS et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. doi:10.1007/s00198-014-2794-2
Kanis JA, McCloskey EV, Johansson H et al (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57. doi:10.1007/s00198-012-2074-y
Rubin KH, Abrahamsen B, Friis-Holmberg T et al (2013) Comparison of different screening tools (FRAX®, OST, ORAI, OSIRIS, SCORE and age alone) to identify women with increased risk of fracture. A population-based prospective study. Bone 56:16–22. doi:10.1016/j.bone.2013.05.002
Sandhu SK, Nguyen ND, Center JR et al (2010) Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int 21:863–871. doi:10.1007/s00198-009-1026-7
Kanis JA, McCloskey EV, Johansson H et al (2008) Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int 19:1395–1408. doi:10.1007/s00198-008-0712-1
Kanis JA, Oden A, Johansson H, McCloskey E (2013) Pitfalls in the external validation of FRAX: response to Bolland, et al. Osteoporos Int 24:391–392. doi:10.1007/s00198-012-1985-y
Adami S, Bianchi G, Brandi ML et al (2010) Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: project rationale and description. Clin Exp Rheumatol 28:561–570
Adami S, Bertoldo F, Gatti D et al (2013) Treatment thresholds for osteoporosis and reimbursability criteria: perspectives associated with fracture risk-assessment tools. Calcif Tissue Int 93:195–200. doi:10.1007/s00223-013-9748-0
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16:455–66. doi: 10.1016/j.jocd.2013.08.004
Jane FM, Davis SR (2014) A practitioner’s toolkit for managing the menopause. Climacteric 17:564–579. doi:10.3109/13697137.2014.929651
Karim R, Dell RM, Greene DF et al (2011) Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. Menopause 18:1172–1177. doi:10.1097/gme.0b013e31821b01c7
Gambacciani M (2012) HRT misuse and the osteoporosis epidemic. Climacteric 15:10–11. doi:10.3109/13697137.2011.639527
Trémollieres FA, Pouillès J-M, Drewniak N et al (2010) Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J Bone Miner Res 25:1002–1009. doi:10.1002/jbmr.12
Roux S, Cabana F, Carrier N et al (2014) The World Health Organization Fracture Risk Assessment Tool (FRAX) underestimates incident and recurrent fractures in consecutive patients with fragility fractures. J Clin Endocrinol Metab 99:2400–2408. doi:10.1210/jc.2013-4507
Crandall CJ, Larson J, Gourlay ML et al (2014) Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US Preventive Services Task Force strategy and two traditional strategies in the Women’s Health Initiative. J Bone Miner Res 29:1661–1666. doi:10.1002/jbmr.2174
Baró F, Cano A, Sánchez Borrego R et al (2012) Frequency of FRAX risk factors in osteopenic postmenopausal women with and without history of fragility fracture. Menopause 19:1193–1199. doi:10.1097/gme.0b013e31825d65c5
Leslie WD, Lix LM, Johansson H et al (2011) Spine-hip discordance and fracture risk assessment: a physician-friendly FRAX enhancement. Osteoporos Int 22:839–847. doi:10.1007/s00198-010-1461-5
Johansson H, Kanis JA, Odén A et al (2014) Impact of femoral neck and lumbar spine BMD discordances on FRAX probabilities in women: a meta-analysis of international cohorts. Calcif Tissue Int 95:428–435. doi:10.1007/s00223-014-9911-2
Rubin KH, Friis-Holmberg T, Hermann AP et al (2013) Risk assessment tools to identify women with increased risk of osteoporotic fracture: complexity or simplicity? A systematic review. J Bone Miner Res 28:1701–1717. doi:10.1002/jbmr.1956
Eller-Vainicher C, Chiodini I, Santi I et al (2011) Recognition of morphometric vertebral fractures by artificial neural networks: analysis from GISMO Lombardia Database. PLoS ONE 6:e27277. doi:10.1371/journal.pone.0027277
FitzGerald G, Compston JE, Chapurlat RD et al (2014) Empirically based composite fracture prediction model from the Global Longitudinal Study of Osteoporosis in Postmenopausal Women (GLOW). J Clin Endocrinol Metab 99:817–826. doi:10.1210/jc.2013-3468
Gregson CL, Dennison EM, Compston JE et al (2014) Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int 25:85–95. doi:10.1007/s00198-013-2438-y
Rubin KH, Holmberg T, Rothmann MJ et al (2015) The Risk-Stratified Osteoporosis Strategy Evaluation study (ROSE): a Randomized Prospective Population-Based Study. Design and Baseline Characteristics. Calcif Tissue Int 96:167–179. doi:10.1007/s00223-014-9950-8
Acknowledgments
The Authors want to thank M. Cristina Castaldini, Stefania Ferrazzini, and Valentina Lea Poetto for their meaningful contribution in data collection and processing.
Conflict of interest
Gloria Bonaccorsi, Enrica Fila, Carlo Cervellati, Arianna Romani, Melchiore Giganti, Maurizio Rossini, Pantaleo Greco, and Leo Massari report no conflicts of interests.
Funding
This work was not supported by external funds.
Human and Animal Rights and Informed Consent
The present population-based study was conducted in accordance to the Declaration of Helsinki (World Medical Association, http://www.wma.net). An informed consent was signed by all the study subjects.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonaccorsi, G., Fila, E., Cervellati, C. et al. Assessment of Fracture Risk in A Population of Postmenopausal Italian Women: A Comparison of Two Different Tools. Calcif Tissue Int 97, 50–57 (2015). https://doi.org/10.1007/s00223-015-0009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0009-2