Abstract
We sought to determine the influence of single-nucleotide polymorphisms (SNPs) in RANKL, RANK, and OPG on volumetric bone mineral density (vBMD) and bone geometry at the radius in men. Pairwise tag SNPs (r 2 ≥ 0.8) for RANKL (n = 8), RANK (n = 44), and OPG (n = 22) and five SNPs near RANKL and OPG strongly associated with areal BMD in genomewide association studies were previously genotyped in men aged 40–79 years in the European Male Ageing Study (EMAS). Here, these SNPs were analyzed in a subsample of men (n = 589) who had peripheral quantitative computed tomography (pQCT) performed at the distal (4%) and mid-shaft (50%) radius. Estimated parameters were total and trabecular vBMD (mg/mm3) and cross-sectional area (mm2) at the 4% site and cortical vBMD (mg/mm3); total, cortical, and medullary area (mm2); cortical thickness (mm); and stress strain index (SSI) (mm3) at the 50% site. We identified 12 OPG SNPs associated with vBMD and/or geometric parameters, including rs10505348 associated with total vBMD (β [95% CI] = 9.35 [2.12–16.58], P = 0.011), cortical vBMD (β [95% CI] = 5.62 [2.10–9.14], P = 0.002), cortical thickness (β [95% CI] = 0.08 [0.03–0.13], P = 0.002), and medullary area (β [95% CI] = −2.90 [−4.94 to −0.86], P = 0.005) and rs2073618 associated with cortical vBMD (β [95% CI] = −4.30 [−7.78 to −0.82], P = 0.015) and cortical thickness (β [95% CI] = −0.08 [−0.13 to −0.03], P = 0.001). Three RANK SNPs were associated with vBMD, including rs12956925 associated with trabecular vBMD (β [95% CI] = −7.58 [−14.01 to −1.15], P = 0.021). There were five RANK SNPs associated with geometric parameters, including rs8083511 associated with distal radius cross-sectional area (β [95% CI] = 8.90 [0.92–16.88], P = 0.029). No significant association was observed between RANKL SNPs and pQCT parameters. Our findings suggest that genetic variation in OPG and RANK influences radius vBMD and geometry in men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Areal bone mineral density (BMDa) as determined by dual-energy X-ray absorptiometry (DXA) is an important determinant of future fracture risk [1, 2]. However, other factors, including bone size and shape, also influence bone strength and susceptibility to fracture [3, 4]. Lower bone width, cortical area, and thickness as measured by DXA at both axial and peripheral skeletal sites have been associated with higher risk of fragility fractures [5]. Quantitative computed tomography (QCT) provides additional information on bone compartments, including volumetric bone mineral density (vBMD) at both predominantly cortical and trabecular sites, and geometric parameters of bone, including area, cortical thickness, and strength [6]. QCT measurements can be made at both axial and appendicular sites; the forearm is the most common appendicular site. Previous studies have shown that radius vBMD and geometry-based parameters are associated with risk of fracture in both men [7] and women [8–11].
Genetic factors are known to influence both bone mass and structure. Data from family and twin studies suggest that genetic factors explain about 50% of variation in the total radius and trabecular vBMD and up to 40% of cortical vBMD [12, 13]. In addition, a large proportion of the variation in geometric parameters such as radius cross-sectional area (27%) and cortical thickness (51%) is attributable to genetic factors [13].
The RANKL/RANK/OPG signaling pathway has a critical role in bone remodeling. Higher OPG plasma concentration has been associated with higher femoral neck BMDa, width, and strength [14]. Strong evidence of association has previously been found between single-nucleotide polymorphisms (SNPs) within these genes and bone-related phenotypes in both candidate gene studies [15] and genomewide association studies (GWAS) [16–19]. Despite the critical importance of the RANKL/RANK/OPG signaling pathway, few studies have examined the association between polymorphisms within genes in this pathway and either vBMD or bone geometric parameters. Paternoster et al. [20] investigated the association between four SNPs near RANKL, RANK, and OPG strongly associated with lumbar spine or hip BMDa in GWAS and tibial peripheral QCT (pQCT) measures (both density and geometry) in young European individuals. They observed significant associations between OPG (rs4355801 and rs6993813) and RANK (rs3018362) SNPs and cortical vBMD. Recently, a GWAS of pQCT outcomes at the tibia has been published by the same group. In this study, a single SNP (rs1021188) in the vicinity of RANKL was associated with lower cortical vBMD at the genomewide significant level and less strongly with geometric parameters. It was also associated with higher plasma levels of soluble RANKL [21]. Yamada et al. [22] also examined the association between rs2073617 and rs3134069 in OPG and vBMD in the distal and proximal radius in 2,220 Japanese men and women and found significant associations with radial vBMD in women. However, Yerges et al. [23, 24] found no associations between tagging and potentially functional SNPs in RANKL, RANK, and OPG and vBMD at the femoral neck and lumbar spine in 862 Caucasian men in the Osteoporotic Fractures in Men (MrOS) study.
Here, we used data from the European Male Ageing Study (EMAS), a population-based study of aging, to determine whether SNPs in RANKL, RANK, and OPG are associated with vBMD and bone geometric parameters at the mid-shaft and distal radius in middle-aged and elderly European men.
Materials and Methods
Study Participants
Men aged 40–79 years were recruited from population registers in European centres, as described before [25]. Stratified sampling was used, with the target of recruiting equal numbers of men in each of four 10-year age bands (40–49, 50–59, 60–69, and 70–79 years). Subjects were invited to participate by letter, and those who agreed were asked to attend for a more comprehensive assessment including a blood sample for genetic analysis. pQCT was performed in a subsample of subjects in two centers (Manchester, UK, and Leuven, Belgium). Participants were subsequently excluded from the analysis if they reported that at least one of their parents or grandparents was born outside Europe or North America or if they reported use of antiosteoporotic medications or systemic glucocorticoids. Ethical approval in each center was obtained in accordance with local practice and requirements, and subjects gave informed consent.
pQCT
pQCT measurements were performed in the nondominant radius using an XCT-2000 scanner (Stratec, Pforzheim, Germany) in each center following the manufacturer’s standard quality-assurance procedures. Total and trabecular vBMD (mg/mm3) and bone cross-sectional area (mm2) were measured at the distal radius (4%, voxel size 0.4 mm). Cortical vBMD (mg/mm3); total, cortical, and medullary area (mm2); cortical thickness (mm); and stress strain index (SSI, mm3) were measured at the mid-shaft radius (50%, voxel size 0.6 mm). The detailed methodology for these measurements has been described previously [26].
The European Forearm Phantom (EFP) was measured for cross-calibration between the two centers; 10 repeat measurements were taken in slices 1–4. The differences were less than precision error for total, trabecular, and cortical vBMD and for cortical area; therefore, no cross-calibration was performed between the two centers.
The short-term precision values of two repeat measurements with repositioning were as follows: total vBMD 2.1 and 1.3%; trabecular vBMD 1.27 and 1.42%; cortical vBMD 0.77 and 0.71%; and cortical area 2.4 and 1.3% in Manchester (n = 22) and Leuven (n = 40), respectively.
Genotype Data
Genotype data for pairwise tagging SNPs in RANKL (receptor activator of NF-κB ligand, official gene symbol TNFSF11) (n = 8), RANK (receptor activator of NF-κB, official gene symbol TNFRSF11A) (n = 44), and OPG (osteoprotegerin, official gene symbol TNFRSF11B) (n = 22) and for additional OPG (n = 3) and RANKL (n = 2) SNPs associated with BMDa in GWAS were generated in EMAS as previously described. These SNPs were previously tested for association with markers of bone turnover, calcaneal quantitative ultrasound parameters, and BMDa at the lumbar spine and total hip as measured by DXA in this cohort [15].
Statistical Analysis
Linear regression under an additive genetic model was used to test for association between SNPs and the outcome variables, adjusting for center using PLINK (1.05) [27]. Significant results were further adjusted for age, weight, and height. The results are reported as the change in the mean of the outcome variable (β coefficient) for each copy of the minor allele with 95% confidence intervals (CIs). For significant associations, the interaction between SNP and center was tested to determine if there was any between-center heterogeneity using STATA (9.2; StataCorp LP, 4905 Lakeway Drive, College Station, TX).
Assuming a 0.05 type I error, minor allele frequencies (MAFs) of 0.05–0.45, and 588 individuals, this study had >80% power to detect differences of at least 0.4 SD (MAF = 0.05) and 0.2 SD (MAF = 0.45) for all outcomes under an additive genetic model. The power of the study was also calculated assuming a more conservative type I error of 0.0008 to take into account multiple testing, which was calculated by dividing 0.05 by the number of independent genotyped SNPs according to the method described by Li and Ji [28]. With a 0.0008 type I error, the study had >80% power to detect differences of at least 0.4 SD (MAF = 0.05) and 0.3 SD (MAF = 0.45) for all outcomes. Statistical power was calculated using Quanto v1.2.3 software [29].
Results
Subject Characteristics
In total, 589 men, mean (± SD) age 60 (± 11) years, were included in the analysis. Characteristics of the pQCT parameters are presented in Table 1.
Genetic Association Analysis
RANKL
We identified four RANKL tag SNPs (rs346588, rs9562414, rs633137, and rs346574) associated with greater medullary area at the mid-shaft radius. However, none of these associations remained significant after adjustment for age, weight, and height (Supplementary Table 1).
There was no association between the two SNPs near RANKL selected from GWAS (rs9594738 and rs9594759) and pQCT outcomes.
RANK
We identified three RANK SNPs associated with vBMD: rs9962159 associated with lower total vBMD, rs12956925 and rs4524035 associated with lower trabecular vBMD at the distal radius. Four RANK SNPs (rs9951012, rs8083511, rs6567276, and rs17665435) were associated with the distal radius cross-sectional area. A single SNP, rs12959396, was associated with both higher cortical thickness and smaller medullary area at the mid-shaft radius, but the association with medullary area did not remain significant after adjustment for age, weight, and height (Table 2). Only modest linkage disequilibrium (LD) exists between the associated SNPs, r 2 ≤ 0.36.
There was no evidence of heterogeneity between centers for SNP associations with pQCT parameters as tested by SNP center interaction analysis.
In order to account for the number of independent SNPs tested (n = 62), which was calculated by the method described by Li and Ji [28], SNP associations would need to reach a P value of <0.0008 to achieve statistical significance. None of the SNPs reached this level of significance.
OPG
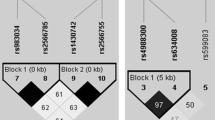
We identified seven tag SNPs in OPG associated with total and/or cortical vBMD: rs3134058 and rs3102724 associated with lower total vBMD at the distal radius; rs2073618, rs3134057, and rs1032129 associated with lower cortical vBMD at the mid-shaft radius; and rs10505348 and rs2073617 associated with both total and cortical vBMD (Table 3). There was a moderate to high LD (r 2 > 0.50) between most of these SNPs (Fig. 1). The SNPs rs2073618 and rs3134058 (r 2 = 0.75) were also associated with lower cortical thickness, and rs10505348 was associated with both higher cortical thickness and smaller medullary area at the mid-shaft radius. The tag SNP rs1032128 was only associated with lower cortical thickness at the mid-shaft radius. We also identified two tag SNPs in OPG, rs3102735 and rs4876868 (r 2 = 0.65), associated with higher SSI; but the association between rs3102735 and SSI did not remain significant after adjustment for age, weight, and height (Table 3).
All three SNPs near OPG selected from GWAS (rs4355801, rs6469804, and rs6993813) were associated with higher cortical vBMD at the mid-shaft radius, and rs4355801 was also associated with SSI (Table 3). No significant association was observed between these SNPs and the other pQCT outcomes.
There was no evidence of heterogeneity between centers for SNP associations with pQCT parameters as tested by SNP center interaction analysis.
None of the SNPs reached the level of significance required to correct for multiple testing (P < 0.0008).
Discussion
To our knowledge, this study is the first in which SNPs within RANKL, RANK, and OPG have been tested for association with bone vBMD and geometric parameters measured by pQCT with good gene coverage. These results are complementary to our previous analysis, which suggested that SNPs within RANKL, RANK, and OPG influence bone turnover and BMDa in this population of men [15].
We found multiple SNPs in RANK and OPG associated with radius geometry or vBMD (cortical, trabecular, and total), with some SNPs associating with both. Some of these SNPs were also associated with bone turnover and BMDa in our previous study [15].
Multiple SNPs in RANK in low LD were associated with total or trabecular vBMD or radius geometric parameters. Two SNPs, rs6567276 and rs17665435, which were associated with distal radius cross-sectional area, were also associated with serum levels of N-terminal propeptide of procollagen I (PINP) and C-terminal cross-linked telopeptide of collagen type I (CTX-I) bone turnover markers in this population, as reported previously [15]. The association between rs6567276 and PINP was still significant in the pQCT subpopulation. The effect on distal radius cross-sectional area was in the opposite direction to their effect on bone turnover markers [15] (Table 3).
Eight tag SNPs in OPG were associated with vBMD and/or cortical thickness, with some SNPs associating with both and in the same direction; e.g., rs10505348 was associated with higher vBMD (both total 4% site and cortical 50% site) and cortical thickness. Some of the OPG SNPs associated here with vBMD have previously been associated with lumbar spine BMDa (with the same direction of effect) in a large GWAS meta-analysis at the genomewide significant level (rs10505348, rs2073617, and rs2073618) [17] and in this population as reported previously [15] (Table 3). Furthermore, rs2073617 [30, 31], rs2073618 [31–38], and rs1032129 [39] have previously been reported in association with lower BMDa measured by DXA at different skeletal sites. As QCT measures of BMD are not confounded by bone size, this provides evidence that associations seen previously in OPG are truly related to density. The SNP rs2073617 has also been reported in association with lower vBMD measured by pQCT at the proximal radius in Japanese premenopausal women [22]. Most of the SNPs associated with vBMD and/or cortical thickness in our current study were also associated with bone turnover markers in this population, as previously reported [15]. A number of these associations remained significant when the analysis was limited to the subpopulation with pQCT. A lack of association with PINP or CTX-I in the subpopulation may be due to the reduced statistical power in the subpopulation; however, the effects of SNPs on bone turnover were in the opposite direction to their effects on vBMD and cortical thickness [15] (Table 3).
The association between SNPs within OPG and serum levels of OPG and soluble RANKL have been investigated in a few studies [34, 37, 40]. Jorgensen et al. [40] did not observe any significant association between rs3102735, which was associated with SSI in EMAS, and serum OPG levels. Kim et al. [37] also did not observe any significant association between rs3102735 and rs2073618 and between serum OPG and soluble RANKL. However, in the Zhao et al. study [34], the Asn-Asn genotype of rs2073618 was associated with lower serum OPG levels compared to the Lys-Lys genotype. This is consistent with our findings in which the C allele (Asn) was associated with higher bone turnover markers, lower BMD, and lower cortical thickness. OPG acts as a decoy receptor and blocks RANKL effects. RANKL increases the production, activity, and survival of osteoclasts [41]. Therefore, SNPs that decrease the activity of OPG would be expected to have a negative impact on bone health due to increased resorption.
All three SNPs near OPG selected from GWAS were associated with higher cortical vBMD at the mid-shaft radius. In a recent study of young (between 15 and 20 years) European individuals (1,492 males and 1,621 females from the Avon Longitudinal Study of Patents and Children [ALSPAC] and 935 males from the Gothenburg Osteoporosis and Obesity Determinants [GOOD] study), rs6993813 and rs4355801 were associated with higher cortical vBMD at the tibia [20].
Our findings contrast with those of Yerges et al. [23], who investigated SNPs in RANKL, RANK, and OPG and femoral neck (total, cortical, and trabecular) and lumbar spine (total) vBMD. They did not observe any significant association between RANKL, RANK, and OPG SNPs and vBMD (total, cortical, or trabecular). The apparent discrepant findings may relate to different sites of assessment. In addition, their gene coverage for SNPs with MAF > 5% ranged from 1 to 100% per gene (64% on average); therefore, they might not have genotyped the associating SNPs [23, 24]. Our gene coverage for SNPs with MAF > 5% was >98%.
EMAS is a multicenter, population-based study; and standard methods were used to minimize any variation between centers. However, it has some limitations which should be considered. As the response rate was about 39%, those who declined to participate may have differed from those who agreed to participate. Therefore, caution is needed in interpreting the results, such as absolute values of the phenotype data. However, considering that the main analysis was based on a within-cohort analysis, it is unlikely that the main findings would be influenced by any selection factors. False results might be produced due to population substructure. There was no evidence of any between-center heterogeneity, and the likelihood of population stratification was minimized by excluding subjects of non-European ancestry. However, we were unable to use methods such as genomic control or principal component analysis to determine if there was any population substructure as these methods need data on a large number of SNPs. False-positive associations might be detected due to multiple testing, whereas genuine associations might be missed due to power constraints. Based on the method described by Li and Ji [28], there were 62 independent SNPs in RANKL (n = 8), RANK (n = 36), and OPG (n = 18). Using Bonferroni correction to correct for multiple testing, the new cut-off for the P value will be 0.0008 (0.05/62 = 0.0008). None of the associations we found here reached this level of significance. Some of the SNPs (i.e., in OPG) associated with radius vBMD in the present study have, however, previously been associated with lumbar spine BMDa in large-scale GWASs. A technical limitation of pQCT measurement of cortical vBMD is the influence of the partial volume effect, which is due to the limited spatial resolution of pQCT. Therefore, some of the associations with cortical vBMD may be in part due to technical constraints: the thinner the cortices, the lower vBMD. We tried to minimize technical artifacts by using a higher threshold for analysis of cortical vBMD (960 mg/cm3) rather than the traditional 710 mg/cm3 [42], to try to reduce the partial volume effect.
To summarize, these findings add to our knowledge of the possible influence of genetic variation in the RANKL/RANK/OPG signaling pathway on bone and suggest that SNPs in OPG and RANK affect radius vBMD and geometry, especially cortical thickness, in men. The association of OPG is more convincing as the results are consistent with previous findings and SNPs are often associated with multiple bone-related phenotypes. However, these findings need to be validated in independent populations. If they are confirmed, fine mapping and functional studies will be needed to identify the causal variants.
References
Dargent-Molina P, Schott AM, Hans D, Favier F, Grandjean H, Baudoin C, Meunier PJ, Breart G (1999) Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int 9:188–192
Briot K, Roux C (2005) What is the role of DXA, QUS and bone markers in fracture prediction, treatment allocation and monitoring? Best Pract Res Clin Rheumatol 19:951–964
Bouxsein ML (2005) Determinants of skeletal fragility. Best Pract Res Clin Rheumatol 19:897–911
Seeman E, Delmas PD (2006) Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261
Szulc P, Munoz F, Duboeuf F, Marchand F, Delmas PD (2006) Low width of tubular bones is associated with increased risk of fragility fracture in elderly men—the MINOS study. Bone 38:595–602
Adams JE (2009) Quantitative computed tomography. Eur J Radiol 71:415–424
Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA (2011) Bone strength measured by peripheral quantitative computed tomography and the risk of non-vertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res 26:63–71
Vico L, Zouch M, Amirouche A, Frere D, Laroche N, Koller B, Laib A, Thomas T, Alexandre C (2008) High-resolution pQCT analysis at the distal radius and tibia discriminates patients with recent wrist and femoral neck fractures. J Bone Miner Res 23:1741–1750
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD (2007) Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433
Clowes JA, Eastell R, Peel NF (2005) The discriminative ability of peripheral and axial bone measurements to identify proximal femoral, vertebral, distal forearm and proximal humeral fractures: a case control study. Osteoporos Int 16:1794–1802
Schneider P, Reiners C, Cointry GR, Capozza RF, Ferretti JL (2001) Bone quality parameters of the distal radius as assessed by pQCT in normal and fractured women. Osteoporos Int 12:639–646
Wang X, Kammerer CM, Wheeler VW, Patrick AL, Bunker CH, Zmuda JM (2007) Genetic and environmental determinants of volumetric and areal BMD in multi-generational families of African ancestry: the Tobago Family Health Study. J Bone Miner Res 22:527–536
Havill LM, Mahaney MC, Binkley L, Specker BL (2007) Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res 22:737–746
Samelson EJ, Broe KE, Demissie S, Beck TJ, Karasik D, Kathiresan S, Kiel DP (2008) Increased plasma osteoprotegerin concentrations are associated with indices of bone strength of the hip. J Clin Endocrinol Metab 93:1789–1795
Roshandel D, Holliday K, Pye SR, Boonen S, Borghs H, Vanderschueren D, Huhtaniemi IT, Adams JE, Ward KA, Bartfai G, Casanueva F, Finn JD, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Silman AJ, Wu FC, Thomson W, O’Neill TW (2010) Genetic variation in the RANKL/RANK/OPG signaling pathway is associated with bone turnover and bone mineral density in men. J Bone Miner Res 25:1830–1838
Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371:1505–1512
Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41:1199–1206
Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K (2008) Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365
Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Snorradottir S, Center JR, Nguyen TV, Alexandersen P, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K (2009) New sequence variants associated with bone mineral density. Nat Genet 41:15–17
Paternoster L, Ohlsson C, Sayers A, Vandenput L, Lorentzon M, Evans DM, Tobias JH (2010) OPG and RANK polymorphisms are both associated with cortical bone mineral density: findings from a metaanalysis of the Avon Longitudinal Study of Parents and Children and Gothenburg Osteoporosis and Obesity Determinants cohorts. J Clin Endocrinol Metab 95:3940–3948
Paternoster L, Lorentzon M, Vandenput L, Karlsson MK, Ljunggren O, Kindmark A, Mellstrom D, Kemp JP, Jarett CE, Holly JM, Sayers A, St Pourcain B, Timpson NJ, Deloukas P, Davey SG, Ring SM, Evans DM, Tobias JH, Ohlsson C (2010) Genome-wide association meta-analysis of cortical bone mineral density unravels allelic heterogeneity at the RANKL locus and potential pleiotropic effects on bone. PLoS Genet 6:e1001217
Yamada Y, Ando F, Niino N, Shimokata H (2003) Association of polymorphisms of the osteoprotegerin gene with bone mineral density in Japanese women but not men. Mol Genet Metab 80:344–349
Yerges LM, Klei L, Cauley JA, Roeder K, Kammerer CM, Moffett SP, Ensrud KE, Nestlerode CS, Marshall LM, Hoffman AR, Lewis C, Lang TF, Barrett-Connor E, Ferrell RE, Orwoll ES, Zmuda JM (2009) A high-density association study of 383 candidate genes for volumetric bone density at the femoral neck and lumbar spine among older men. J Bone Miner Res 24:2039–2049
Yerges LM, Klei L, Cauley JA, Roeder K, Kammerer CM, Ensrud KE, Nestlerode CS, Lewis C, Lang TF, Barrett-Connor E, Moffett SP, Hoffman AR, Ferrell RE, Orwoll ES, Zmuda JM (2010) Candidate gene analysis of femoral neck trabecular and cortical volumetric bone mineral density in older men. J Bone Miner Res 25:330–338
Lee DM, O’Neill TW, Pye SR, Silman AJ, Finn JD, Pendleton N, Tajar A, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D, Wu FC (2009) The European Male Ageing Study (EMAS): design, methods and recruitment. Int J Androl 32:11–24
Ward KA, Roberts SA, Adams JE, Mughal MZ (2005) Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone 36:1012–1018
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Li J, Ji L (2005) Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95:221–227
Gauderman WJ, Morrison JM (2006) QUANTO 1.1: a computer program for power and sample size calculations for genetic-epidemiology studies. http://hydra.usc.edu/gxe
Ohmori H, Makita Y, Funamizu M, Hirooka K, Hosoi T, Orimo H, Suzuki T, Ikari K, Nakajima T, Inoue I, Hata A (2002) Linkage and association analyses of the osteoprotegerin gene locus with human osteoporosis. J Hum Genet 47:400–406
Vidal C, Brincat M, Xuereb AA (2006) TNFRSF11B gene variants and bone mineral density in postmenopausal women in Malta. Maturitas 53:386–395
Choi JY, Shin A, Park SK, Chung HW, Cho SI, Shin CS, Kim H, Lee KM, Lee KH, Kang C, Cho DY, Kang D (2005) Genetic polymorphisms of OPG, RANK, and ESR1 and bone mineral density in Korean postmenopausal women. Calcif Tissue Int 77:152–159
Arko B, Prezelj J, Kocijancic A, Komel R, Marc J (2005) Association of the osteoprotegerin gene polymorphisms with bone mineral density in postmenopausal women. Maturitas 51:270–279
Zhao HY, Liu JM, Ning G, Zhao YJ, Zhang LZ, Sun LH, Xu MY, Uitterlinden AG, Chen JL (2005) The influence of Lys3Asn polymorphism in the osteoprotegerin gene on bone mineral density in Chinese postmenopausal women. Osteoporos Int 16:1519–1524
Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2010) Associations between osteoprotegerin polymorphisms and bone mineral density: a meta-analysis. Mol Biol Rep 37:227–234
Moffett SP, Oakley JI, Cauley JA, Lui LY, Ensrud KE, Taylor BC, Hillier TA, Hochberg MC, Li J, Cayabyab S, Lee JM, Peltz G, Cummings SR, Zmuda JM (2008) Osteoprotegerin Lys3Asn polymorphism and the risk of fracture in older women. J Clin Endocrinol Metab 93:2002–2008
Kim JG, Kim JH, Kim JY, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM (2007) Association between osteoprotegerin (OPG), receptor activator of nuclear factor-kappaB (RANK), and RANK ligand (RANKL) gene polymorphisms and circulating OPG, soluble RANKL levels, and bone mineral density in Korean postmenopausal women. Menopause 14(5):913–918
Garcia-Unzueta MT, Riancho JA, Zarrabeitia MT, Sanudo C, Berja A, Valero C, Pesquera C, Paule B, Gonzalez-Macias J, Amado JA (2008) Association of the 163A/G and 1181G/C osteoprotegerin polymorphism with bone mineral density. Horm Metab Res 40:219–224
Jurado S, Nogues X, Agueda L, Garcia-Giralt N, Urreizti R, Yoskovitz G, Perez-Edo L, Salo G, Carreras R, Mellibovsky L, Balcells S, Grinberg D, ez-Perez A (2010) Polymorphisms and haplotypes across the osteoprotegerin gene associated with bone mineral density and osteoporotic fractures. Osteoporos Int 21:287–296
Jorgensen HL, Kusk P, Madsen B, Fenger M, Lauritzen JB (2004) Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratios. J Bone Miner Metab 22:132–138
Boyce BF, Xing L (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 9(Suppl 1):S1
Ward KA, Adams JE, Hangartner TN (2005) Recommendations for thresholds for cortical bone geometry and density measurement by peripheral quantitative computed tomography. Calcif Tissue Int 77:275–280
Acknowledgements
EMAS is funded by the Commission of the European Communities Fifth Framework Programme Quality of Life and Management of Living Resources grant QLK6-CT-2001-00258 and by Arthritis Research UK. For additional information regarding EMAS, contact Frederick Wu, MD; Department of Endocrinology, Manchester Royal Infirmary, Manchester, UK. The authors thank the men who participated in the eight countries and the research/nursing staff in the eight centers for data entry and project coordination: C. Pott, Manchester; E. Wouters, Leuven; M. Nilsson, Malmö; M. del Mar Fernandez, Santiago de Compostela; M. Jedrzejowska, Lodz; H.-M. Tabo, Tartu; A. Heredi, Szeged; and C. Moseley, Manchester. D. V. is a senior clinical investigator supported by the Clinical Research Fund of the University Hospitals Leuven, Belgium. S. B. is senior clinical investigator of the Fund for Scientific Research-Flanders, Belgium (F.W.O.-Vlaanderen) and holder of the Leuven University Chair in Metabolic Bone Diseases.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
T. W. O’Neill and W. Thomson equally contributed to this study.
F. C. W. Wu is the coordinator for the European Male Ageing Study (EMAS).
The EMAS Study Group: Florence (Gianni Forti, Luisa Petrone, Glovanni Corona); Leuven (Dirk Vanderschueren, Steven Boonen, Herman Borghs); Lodz (Krzysztof Kula, Jolanta Slowikowska-Hilczer, Renata Walczak-Jedrzejowska); London (Ilpo Huhtaniemi); Malmö (Aleksander Giwercman); Manchester (Frederick Wu, Alan Silman, Terence O’Neill, Joseph Finn, Philip Steer, Abdelouahid Tajar, David Lee, Stephen Pye); Santiago (Felipe Casanueva, Mary Lage); Szeged (Gyorgy Bartfai, Imre Földesi, Imre Fejes); Tartu (Margus Punab, Paul Korrovitz); Turku (Min Jiang).
The authors have stated that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Roshandel, D., Holliday, K.L., Pye, S.R. et al. Influence of Polymorphisms in the RANKL/RANK/OPG Signaling Pathway on Volumetric Bone Mineral Density and Bone Geometry at the Forearm in Men. Calcif Tissue Int 89, 446–455 (2011). https://doi.org/10.1007/s00223-011-9532-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9532-y