Abstract
Peripheral quantitative computed tomography (pQCT) is widely used for clinical and research purposes. For accurate determination of bone geometry (bone cross-sectional area, cortical thickness, and cortical area), volumetric bone mineral density (vBMD) and cortical bone mineral content (BMC), it is important to select the appropriate thresholds. A Stratec XCT-2000 scanner was used to compare current standard practice with new optimized thresholds. Currently, a single threshold of 710 mg/mL for the measurement of cortical vBMD and geometry is used. We hypothesised that this threshold may not be optimal and used the European Forearm Phantom (EFP) and patient data to test more appropriate thresholds. A single slice (1.2 mm width, 0.4 mm pixel size) was made at section 4 of the EFP (representing the diaphyseal portion of a long bone). The EFP has a known cortical thickness of 2.5 mm and, therefore, the correct threshold for geometry would be that which measures cortical thickness as 2.5 mm. Thresholds were altered at approximately the 50% value between soft tissue (60 mg/mL) and peak density (879 mg/mL), and cortical thickness versus threshold was plotted; the correct threshold for geometry was 460 mg/mL. By expressing this threshold as a percentage of the range of density values in the EFP ([460–60]/[879–60] = 49%) and then applying this percentage to in vivo data, the optimum threshold for geometry can be determined: ([1240−79] × 0.49) + 79 = 648 mg/mL. For cortical vBMD of in vivo bone measurements at the midshaft site of the radius, thresholds were varied around the peak value (1240 mg/mL), and the threshold was set to that which gave a cortical density of 1240 mg/mL; the threshold for cortical density was, therefore, 1200 mg/mL. A subset of radius scans from a population of young healthy females was analyzed using the new thresholds (648 mg/mL for bone geometry, 1200 mg/mL for cortical vBMD) versus the current threshold (710 mg/mL). For bone geometry, the mean difference between the analysis based on the new threshold and that based on the manufacturer-recommended threshold ranged between 2.1% and 14% (total area = 2.1%, cortical thickness = 14%, cortical area = 3.7%). Although there was a 10% difference between the analysis based on the new threshold and that based on the manufacturer-recommended threshold, this difference was not systematic. Thresholds will significantly affect results obtained from pQCT. The current threshold of 710 mg/mL is inadequate for accurate determination of bone geometry and cortical vBMD. New thresholds of 648 mg/mL for geometry and 1,200 mg/mL for cortical vBMD should be used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Peripheral quantitative computed tomography (pQCT) is increasingly being used in both research and clinical practice. The technique has the advantage over dual energy X-ray absorptiometry (DXA) in that it measures volumetric bone mineral density (vBMD) and is, therefore, size independent; it can also provide bone geometry, bone strength, and muscle cross-sectional area [1]. The separate analysis of cortical and trabecular bone is also advantageous when studying the response to a therapeutic intervention or the etiology of a disease that may differentially affect the two types of bone (cortical/trabecular), for example, hyperparathyroidism, which predominantly affects cortical bone [2].
The accurate measurement of bone by pQCT, or any QCT (axial or micro) method, is limited by the finite geometric resolution of the technique. In this paper, we discuss the analysis of cortical bone. Figure 1 shows a typical density profile though the midshaft radius. The analysis of a computed tomography (CT) image is achieved by taking density profiles through the bone. When considering the measured density from the periosteum through to the medullary cavity: it gradually increases to its maximal cortical value and decreases as it crosses the bone boundary. This increase and decrease across the boundaries is caused by the partial volume effect in which voxels contain both bone and soft tissue, and, therefore, the measured density is a combination of the two tissues. When cortical bone is analyzed, there are two main outcome variables to consider, bone geometry and cortical vBMD; thresholds must be set in order to analyze the image. The selection of the threshold will significantly affect the assessment of bone geometry and cortical vBMD. Although most applications of pQCT are to assess bone geometry at cortical sites, in some circumstances, a measurement of cortical density may be necessary, for example, to evaluate osteomalacia, hyperparathyroidism, or the response to therapeutic intervention.
The optimum threshold for an accurate determination of cortical wall thickness is based upon a given percentage of the difference between the densities of cortical bone and immediately adjacent tissue (soft tissue, marrow) [3]. The density of cortical bone is the peak of the density profile across the cortex only if the cortical width is sufficiently large. Otherwise, the peak is artificially lowered owing to the partial-volume effect. Therefore, the cortical value is a universal quantity to be established for a given scanner and imaging protocol. With the use of this method of threshold selection, cortical wall thicknesses of approximately 1 mm may be evaluated with accuracy [3]; the threshold is approximately midway between the density of the cortex and adjacent tissue (Fig. 2).
For cortical density, the cortex must be sufficiently thick for one voxel to lie completely within the cortical bone [3]. To ensure that the measured density is not subject to the partial volume effect, that is, only including voxels composed entirely of bone, the threshold that should be selected is at the top of the density profile of the bone, thereby eliminating any voxels subject to the partial volume effect (Fig. 2). Cortical density is accurately determined when the cortical width is approximately 2 mm or greater [3–5].
The purpose of this study was to investigate whether our current, manufacturer-recommended threshold of 710 mg/mL used for analyzing scans of the midshaft radius was optimal. We hypothesized that the current, single threshold is not adequate for either cortical geometry or cortical vBMD, or both, and that new, optimized thresholds should be determined.
Methods
In vitro Measurements
The European Forearm Phantom (EFP) [6] was used to determine the optimum threshold for cortical thickness determination in vivo. The EFP consists of four sections that correspond to different portions of the forearm. Section 4 mimics the radial midshaft, representing a circular bone of 10 mm outer diameter and a cortical thickness of 2.5 mm; the cortical density is 800 mg/mL.
For this study, a histogram profile of the EFP was obtained from the image. Thresholds were then altered sequentially, starting in the midregion of the density between the bone-mimicking material and “soft tissue” (water), and the cortical thickness measured. Each threshold was plotted against cortical thickness; the correct threshold was that which resulted in a cortical thickness of 2.5 mm (Fig. 3).
Expressing this threshold as a percentage of the range of density values within the EFP gave the correct percentage to apply to in vivo data to determine the bone geometry threshold that would most accurately measure cortical thickness. Therefore, the geometry threshold fraction f g is
where f g : threshold resulting in EFP cortical thickness of 2.5 mm
t 1 : cortical density
t 2 : soft-tissue density
Using the values of the EFP, we obtain
To calculate the actual threshold for in vivo bone data, the equation needs to be solved for t g :
In Vivo
Scans of a healthy adult were used to determine the in vivo thresholds for cortical density and bone geometry. A histogram profile of the bone was obtained, and the densities for cortical bone and soft tissue were determined from the peaks of the histogram. Cortical bone had a density of 1240 mg/mL, soft tissue had a density of 79 mg/mL. Therefore, the threshold t g for bone geometry was calculated to be
To obtain the appropriate threshold td for the cortical density, thresholds were sequentially altered from the maximum value in steps of 50 mg/mL; near the correct threshold, steps of 5 mg/mL were used to improve accuracy. The correct threshold was that which gave a cortical density of 1240 mg/mL; the correct threshold was found to be 1200 mg/mL.
The measurement of bone mineral content (BMC) in the cortical midshaft is also subject to partial volume effect. To obtain accurate measurements of cortical BMC the parameter should be calculated from cortical area derived from t g and density from t d :
where BMC: cortical bone mineral content
CtvBMD: cortical volumetric bone mineral density based on threshold t d
a: cortical bone area based on threshold t g .
pQCT Protocol
pQCT measurements were made using a Stratec XCT-2000 scanner (Stratec, Pforzheim, Germany), software version 5.50d. In vitro measurements consisted of a single 1.2-mm slice made at section 4 of the EFP. All measurements in vivo were taken from the nondominant side. Measurements were taken at 50% of the forearm length proximal to the distal radial joint surface. At the 50% midshaft radius, total bone area (mm2), cortical area (mm2), cortical thickness (mm), cortical vBMD (mg/cm3) and cortical BMC (mg/mm), were evaluated.
In Vivo Evaluation of Thresholds
To test the results obtained from the thresholds determined in-house in comparison to those currently used, we used a dataset drawn from a study of Ethnic Peak Bone Mass in females. The North West Multi Centre and University of Manchester Research Ethics Committee approved the study. A subset of the scans was taken (n = 130), comprising caucasians, Urdus, and Gujaratis, aged between 17 and 35 years. Scans were analyzed using the current threshold and then analyzed again with the new thresholds.
Statistical Analysis
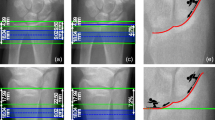
Data were entered into an SPSS database (SPSS 11.5 for Windows, Chicago, IL, USA). Absolute and percentage mean differences between old and new thresholds were calculated. Bland and Altman plots (Fig. 4) were used to test whether the differences were systematic and significant [7].
Scatter and Bland & Altman plots to illustrate differences between the new and old thresholds for (a) bone geometry (648 vs. 710 mg/mL), (b) cortical bone mineral content (combination of density threshold of 1200 mg/mL and cortical area threshold of 648 mg/mL vs. 710 mg/mL), and (c) cortical density (1,200 vs. 710 mg/mL). The upper panels show scatterplots of absolute values with each threshold; the lower panels show the difference between the two techniques as well as the mean ± 2 standard deviations (SDs).
Results
From our in vitro and in vivo analyses of pQCT data, we determined that two separate thresholds should be used for the analysis of cortical bone. For bone geometry, a threshold of t g = 648 mg/mL was used, for cortical vBMD, a threshold of t d = 1200 mg/mL, and cortical BMC was calculated using t g and t d . Table 1 shows the results of using these thresholds in comparison to that used in current practice (710 mg/mL). Figure 4a–c illustrates the results of cortical thickness, cortical BMC, and cortical vBMD, respectively, using the new thresholds. The use of new thresholds altered the results obtained from pQCT to a varying extent, whereas differences in total bone and cortical area were moderate (2.1% and 3.7%, respectively), those in cortical thickness (14%), cortical BMC (13.5%) and cortical vBMD (10.4%) were considerably greater.
Discussion
From the data presented in our paper, we have demonstrated how threshold selection significantly alters the results obtained from a pQCT scanner. The implications of threshold selection for the monitoring of changes and accurate assessment of bone geometry and density in adults and children should be considered. We have found that the average cortical thickness increases by 8% per year in children (Manchester normative data study, unpublished data). In adults, the age-related decline after 50 years of age is approximately 14% over a decade (Dayton normative database, unpublished data). Therefore, a difference of cortical thickness of 14% by changing thresholds from 710 to 648 mg/mL has a large impact on the accuracy of results obtained. In addition, BMC values alter significantly when calculated using the new cortical density and area (13.5%). The differences in cortical area, cortical BMC, and thickness are systematic and will, therefore, have little impact upon calculation of change over the period of a longitudinal study. Cortical density in children increases with age by an average of 9 mg/mL (0.9%) per year (Dayton normative database, unpublished data); in adults, a decline of 2.4% per decade is observed [3]. Therefore, the 10% nonsystematic difference in cortical density assessment using our new threshold would definitively alter the assessment of cortical bone density in both children and adults, even in assessing changes in longitudinal studies.
Cortical thickness significantly affects the assessment of cortical vBMD, owing to partial volume averaging [3, 4]. Therefore, in a growing child, an apparent increase is seen until the thickness is greater than approximately 2 mm; however, in reality, cortical vBMD is changing at a lower rate [3]. With use of the higher threshold determined in our study, some of this problem may be overcome, as long as the bone width is more than approximately 2 mm, making accurate assessment of density in younger children possible.
The higher threshold selection will assess only a few voxels of bone in the young child and should give a more accurate reflection of the cortical vBMD of the individual. An alternative to this technique has been proposed in which a small region of interest is outlined in the central cortex; this also overcame the partial volume effect [8].
An example of a condition in which cortical density may be affected and require accurate assessment is osteomalacia in which the mineral-to-collagen ratio is affected and mineral is reduced, thereby reducing cortical vBMD. Use of a lower threshold for analysis may not detect the condition, as vBMD is underestimated by an average of 10% when using the lower threshold. Another clinical condition, hyperparathyroidism, results in cortical resorption on the surfaces of the bones, which often have a characteristic “pitted” appearance, in addition to an increase of the cortical remodeling space. Use of a low threshold would include the boundary regions in the analysis, and the density would appear to be even lower owing to the loss of periosteal bone. However, if just the inner cortex were studied, cortical vBMD may not be affected as much.
In conclusion, we have presented an approach to optimize threshold selection for analysis of pQCT scans. Although the differences between the new thresholds and current ones are systematic, the use of the new thresholds is expected to increase accuracy of the measurements obtained. To reduce partial volume averaging, cortical BMC should be calculated from cortical area and cortical vBMD. For cortical area we recommend a threshold of 648 mg/mL; for cortical vBMD, the threshold should be approximately 1200 mg/mL. Implementation of new thresholds should be made with caution; for example, in longitudinal or multicenter studies, all data should be analyzed with appropriate and consistent thresholds.
References
Schneider P, Reiners C (1998) Peripheral quantitative computed tomography. In: Genant H, Guglielmi G, Jergas M (eds). Bone densitometry and osteoporosis. Berlin, Heidelberg: Springer-Verlag, pp 349–363
Wishart J, Horowitz M, Need A, Nordin BE (1990) Relationship between forearm and vertebral mineral density in postmenopausal women with primary hyperparathyroidism. Arch Intern Med 150:1329–1331
Hangartner T, Gilsanz V (1996) Evaluation of cortical bone by computed tomography. J Bone Miner Res 11:1518–1525
Prevhal S, Engelke K, Kalender W (1999) Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Physics Med Biol 44:751–764
Binkley T, Specker B (2000) pQCT measurement of bone parameters in young children—validation of technique. J Clin Densitom 3:9–14
Ruegsegger P, Kalender W (1993) A phantom for standardization and quality control in peripheral bone measurements by pQCT and DXA. Phys Med Biol 38:1963–1970
Bland J, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Vol: 307–310
Kibe L, Zemel BS, Leonard MB (2004) Assessment of cortical bone mineral density by peripheral quantitative computed tomography in children: impact of partial volume effects. J Bone Miner Res 19 (Suppl 1): S230
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ward, K.A., Adams, J.E. & Hangartner, T.N. Recommendations for Thresholds for Cortical Bone Geometry and Density Measurement by Peripheral Quantitative Computed Tomography. Calcif Tissue Int 77, 275–280 (2005). https://doi.org/10.1007/s00223-005-0031-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-005-0031-x