Abstract
Fractures of the radial head are common; however, it remains to be determined whether the radial head has to be considered as a typical location for fractures associated with osteoporosis. To investigate whether the human radial head shows structural changes during aging, we analyzed 30 left and 30 right human radial heads taken from 30 individuals. The specimens taken from the left side were analyzed by peripheral quantitative computed tomography (pQCT) and micro-CT. The specimens taken from the right elbow joint were analyzed by radiography and histomorphometry. In these specimens pQCT revealed a significant decrease of total and cortical bone mineral density (BMDto BMDco) with aging, regardless of sex. Histomorphometry revealed a significant reduction of cortical thickness (Ct.Th), bone volume per tissue volume (BV/TV), and trabecular thickness (Tb.Th) in male and female specimens. In this context, mean BV/TV and mean trabecular number (Tb.N) values were significantly lower and, accordingly, mean trabecular separation (Tb.Sp) was significantly higher in female samples. The presented study demonstrates that the radial head is a skeletal site where different age- and sex-related changes of the bone structure become manifest. These microarchitectural changes might contribute to the pathogenesis of radial head fractures, especially in aged female patients where trabecular parameters (BMDtr and Tb.Sp) change significantly for the worse compared to male patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fractures of the radial head or neck account for about 1.5–4% of all fractures and for 26% of all elbow fractures [1, 2]. Radial head fractures are classified according to Mason [3], who introduced three clinical types: type I fractures are fissure fractures or marginal sector fractures without displacement, type II fractures are dislocated marginal sector fractures, and type III fractures are comminuted fractures of the whole radial head. Undisplaced fractures usually result in minimal residual deficits independent of treatment, whereas displaced fractures have been described as having the risk of an unfavorable outcome [4].
Based on biomechanical and clinical evidence, radial head fractures are caused by a fall on the extended forearm or a direct trauma to the elbow [5]. While it is recognized that the trauma energy and the position of the elbow at the time of injury are important factors in the pathogenesis and severity of radial head fractures, the possible role of age- and sex-related changes in the radial head remains largely undefined.
In a retrospective analysis of 426 patients (235 females, 191 males) who were treated in our department with a fracture of the radial head, we observed a significant (P < 0.001) difference of the average age at trauma between female (mean 55.8 ± 21.4 years, 48% of injured patients older than 60 years) and male (mean 39.2 ± 15.6 years, 9.4% of injured patients older than 60 years) patients [6].
Based on this epidemiologic observation, it is fair to assume that age- and sex-related bone loss occurs within the radial head and thereby might constitute a structural risk factor for fracture independent of the trauma mechanism. However, age- and sex-related structural changes within the radial head have never been studied in detail.
In addition, osteoporosis is the most frequent bone disease and constitutes a major health concern in the aging population in the Western Hemisphere [7]. It is characterized by a low bone mass caused by an imbalance between osteoblastic bone formation and osteoclastic bone resorption, a physiologically balanced process called “remodeling” that normally maintains bone mass almost constant [8].
If, however, the radial head is a skeletal region that is prone to display the characteristic changes of osteoporosis in its microarchitecture, then one has to consider that radial head fractures can occur as osteoporotic fractures as it is the case for fractures of the distal radius, the proximal femur, the proximal humerus, and the spine. Furthermore, as due to the demographic changes in developed countries osteoporotic fractures will double within the next 25 years, this means that fractures of the radial head will gain even more clinical relevance in aged patients.
Therefore, the aim of this study was to determine whether the bone structure of the radial head shows age- and sex-related changes. The question was addressed by analysis of 30 left and 30 right human radial heads taken from 30 individuals at autopsy. The specimens were subjected to morphologic, radiographic, and histologic analyses or micro-computed tomographic (μCT) and peripheral quantitative CT (pQCT) analyses.
Materials and Methods
Specimens
Radial heads, 30 left and 30 right, were harvested from 30 patients at autopsy (15 females, 15 males, five females and five males per age group; age groups 20–40 [32.5 ± 5.37], 41–60 [51.3 ± 5.03], 61–80 [69.2 ± 5.58] years). This study was carried out according to the existing rules and regulations of Hamburg University School of Medicine. All patients had died in accidents or of acute disease without known prolonged periods of immobilization. Iliac crest biopsies were obtained from all autopsy cases to exclude any metabolic diseases known to affect the skeleton, i.e., chronic kidney disease, hyperparathyroidism, Cushing disease, malignancy, or chronic liver disease. Also, donors on drugs known to affect calcium metabolism were excluded.
pQCT
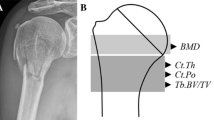
The 30 left radial heads were measured by pQCT, using a micro-computer-controlled, translation-rotation tomographic device with an X-ray source [9] (voxel size 0.2 mm, XCT-2000; Stratec, Pforzheim, Germany). Following generation of a scout view, pQCT sectional images of every analyzed specimen were performed 5 mm below a tangent adjacent to the lowest point of the radial joint cavity (Fig. 1). On the basis of the measurement scan of the radial head, total (BMCto), trabecular (BMCtr), and cortical (BMCco) bone mineral content values were analyzed. Furthermore, total (BMDto), trabecular (BMDtr), and cortical (BMDco) bone mineral density values were calculated. Therefore, the cortical bone area (CBA) was defined through a threshold algorithm detecting bone with high density in a defined region of interest (ROI). All voxels with a lower attenuation coefficient than the given threshold were eliminated. The threshold levels were kept constant for all patients (Fig. 2; trabecular bone 200–600 mg/cm3, cortical bone >600 mg/cm3).
von Kossa–stained grinding of a radial head specimen. For histomorphometric analysis of the radial head, a 5-mm-thin section was cut out of the center of the radial head in the frontal plane, ground to a thickness of 1 mm, and stained using a modification of the von Kossa method. On the macroscopic view of the radial head, the ROI for histomorphometric analysis were defined. The subchondral lamella adjacent to the proximal humeroradial facet is marked by a thick line. The second ROI encircled by a thin line shows the area underneath the subchondral lamella passing into the radial column. The distal end of this region is represented by the transition from the radial head to its neck. For pQCT analysis the measurement scan was performed in the axial plane 5 mm distal from the proximal humeroradial facet
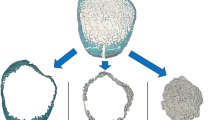
Representative axial pQCT scans of female radial heads from the three age groups (upper panels). Notice the loss of mineralized bone with age. Corresponding structural analysis of the three radial heads by μCT, which demonstrates the loss of cortical and trabecular bone volume with age (lower panels). Scale bar = 1 cm
μCT Analysis
To visualize the trabecular microarchitecture of the 30 left radial heads, μCT was performed, using a μCT40 scanner (Scanco-Medical, Bassersdorf, Switzerland). The samples were placed in alcohol and scanned at 55 keV and 145 μA, a field of vision of 36.8 mm, and a matrix of 1,024 × 1,024 × 808 at a nominal resolution of 37 μm with an isotropic voxel size. The 3-D reconstruction was visualized using Scanco Image Processing Software (Scanco-Medical) (Fig. 2).
Sample Preparation and Contact Radiography
From the 30 samples taken from the right elbow of the individuals a 5-mm-thin section was cut out of the center of the radial head in the frontal plane with a diamond saw. Bone marrow was rinsed out thoroughly, and the specimens were macerated in hydrogen peroxide solution for 72 hours, followed by the generation of contact radiographs from each section using a Faxitron X-ray (Wheeling, IL) cabinet. After removing adhering fat tissue, the specimens were dehydrated, embedded in plastic, ground to a thickness of 1 mm, polished, and attached to slides. The surface of this preparation of the radial head was then stained using a modification of the von Kossa method [10]. The high contrast yielded by this silver staining enabled subsequent morphologic evaluation by an automatic computer-assisted analyzing system. Histomorphometric evaluation of the trabecular architecture was performed for every right radial head as follows.
Qualitative and Quantitative Histomorphometry
Two-dimensional analysis was carried out by dark and light field microscopy using a stereomicroscope (Zeiss, Göttingen, Germany). Standard histomorphometry was performed using the Osteoquant workstation (Bioquant Image Analysis, Nashville, TN) on an axioscope II microscope (Zeiss). Histomorphometric analysis was carried out according to the standards of the ASBMR histomorphometric standardization committee [11] as previously described [12]. Analysis was carried out for each radial head from the right elbow of the individuals and included the following parameters: bone volume per tissue volume (BV/TV, %), trabecular number (Tb.N, mm−1), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), subchondral lamellar bone volume per tissue volume (Subch.Lam BV/TV, %), and mean cortical thickness 15 mm below the lowest point of the radial head joint facet (Ct.Th, μm). Histomorphometric analysis was performed for two different ROI as depicted in Fig. 1.
Statistical Methods
Statistical evaluation was performed using SPSS software for Windows (SPSS, Inc., Chicago, IL). Results are presented as means ± standard deviations of the measurements. Changes of morphometric parameters correlated with age or gender were assessed by ANCOVA. P < 0.05 was considered significant.
Results
pQCT of the 30 left radial head specimens revealed a significant decrease of BMDto with age for men and women (Figs. 2, 3 and Table 1) (P = 0.039). This was mainly caused by a loss of cortical bone mass (BMDco) during aging (P = 0.024). Furthermore, the mean values of BMCto were significantly lower in female compared to male individuals (P = 0.0004). Regarding the analysis of trabecular bone parameters, pQCT revealed a significant age-dependent decrease of BMDtr in women (P = 0.011), which could not be confirmed for male samples. A significant loss of cortical bone mass with aging was monitored by pQCT as BMDco (P = 0.024) and BMCco (P = 0.03) likewise decreased with age regardless of gender. In summary, changes of BMCco and BMDco are age-dependent and changes of BMDtr are age- and sex-dependent.
Plotting of the age- and sex-related changes of the pQCT data. BMDto and BMDco were significantly reduced in aging regardless of sex (BMDto, P = 0.039; BMDco, P = 0.024). The mean values of BMCto were significantly lower in female compared to male patients (P = 0.0004) but showed only a slight but not significant reduction with age. Whereas BMCco was significantly reduced in aging regardless of sex (P = 0.03). Interestingly, mean values for BMDtr in female specimens were significantly lower (P = 0.0014) and significantly decreased with age (P = 0.011) compared to males. BMCtr was significantly lower in female samples than in male samples (P = 0.0004), and there was a slight but significant increase with age regardless of sex (P = 0.04)
The loss of trabecular bone volume with aging in women was qualitatively confirmed by 3-D reconstruction after micro-CT of the left radial head specimens (Fig. 2). These age-related structural changes in the proximal radius of the left side were also observed by contact X-ray analysis and on surface-stained grindings (Fig. 4) of the radial head specimens taken from the contralateral right elbow joint. Qualitatively, the highest radiodensity was found in the subchondral and cortical–subcortical regions in female and male specimens under the age of 60 years, whereas the central and cortical–subcortical regions in female specimens over 60 years appeared as radiolucent zones. The changes in radiolucency with aging monitored by contact X-ray were quantitatively confirmed by histomorphometric analysis (Fig. 5 and Table 2). In line with pQCT, histomorphometric analysis detected significant age-related changes of several trabecular bone parameters. In male and female specimens BV/TV (P = 0.011) and Tb.Th (P = 0.0006) significantly decreased with age. Tb.Sp significantly increased with age within the radial head from female individuals, corresponding to the decrease of BMDtr (P = 0.037). Interestingly, Tb.N was significantly higher in males compared to females regardless of age (P = 0.002), and there was only a slight but not significant age-related decrease (P = 0.21).
Contact radiographs (upper panels) and corresponding surface-stained grindings (lower panels) (thickness 1 mm, von Kossa staining) of three radial head preparations. In contrast to the low sensitivity of conventional X-ray analysis, contact X-ray demonstrates an increase in radiolucency with aging; this bone loss is confirmed in the block-grindings at the structural level. Scale bar = 1 cm
Plotting of the age- and sex-related changes of the histomorphometric data. BV/TV and Tb.Th significantly decreased with age (BV/TV, P = 0.011; Tb.Th, P = 0.0006), but there was only a slight and insignificant reduction of Tb.N for male and female samples. Mean values for Tb.Sp were significantly higher in female specimens (Tb.Sp, P = 0.0009) and significantly increased with age compared to males (Tb.Sp, P = 0.037). Subch.Lam BV/TV and Ct.Th (15 mm) significantly decreased with age regardless of sex (Subch.Lam BV/TV, P = 0.009; Ct.Th, P = 0.0009)
A significant age-related decrease in Subch.Lam BV/TV was observed for female as well as male specimens (P = 0.009). Interestingly, throughout all ages Subch.Lam BV/TV was at least fourfold higher than the corresponding BV/TV of the entire preparation. Analogous to pQCT, histomorphometry of the radial head 15 mm below the lowest point of the radial head joint facet confirmed a significant reduction of cortical bone mass (Ct.Th) with aging, regardless of sex (P = 0.0009) (Fig. 5 and Table 2).
Discussion
Since Mason’s fundamental work on fractures of the head of the radius published in the British Journal of Surgery in 1954, there is consensus that this injury is mainly caused by an indirect trauma through the long axis of the radius [1–3]. Amis and Miller [13] tested the influence of different angles of force impact on fracture patterns of 40 human elbow specimens. In their series fractures were created by an average indirect impact of 2,900 N along the axis of the radius and in all cases the radial head fracture took a longitudinal path.
However, it is widely accepted that skeletal mass is the one of the three determinants of structural bone strength—besides geometric properties and material quality—which can be measured best and can estimate future fracture risk well [14–17]. For the distal radius, a skeletal site where bone mass changes during aging are of dramatic clinical relevance, the correlations between bone mass and bone strength have been repeatedly reported [18, 19].
Regarding the microarchitecture of the radial head, already Mason observed a “columnar type of dense cancellous bone running in a more or less longitudinal pattern.” Mason concluded that “in consequence, fractures of the radial head tend to be oblique or longitudinal in direction” [3]. This longitudinal orientation of trabeculae in the radial head results from modeling and remodeling in the proximal radius. Modeling contributes to skeletal strength homeostasis by adjusting bone mass and geometric properties to withstand loading conditions [14].
In this context, it is somehow surprising that even though Mason’s microstructural observation was made half a century ago, up to now a comprehensive morphometric analysis of the microstructure of the radial head is missing. Hence, to characterize the michroarchitecture of the radial head and to answer the question of whether the bone structure of the radial head shows age- and sex-related changes, which might explain the clinically apparent high number of female patients of advanced age sustaining a radial head fracture (48% of injured female patients older than 60 years), we performed the present study. A total of 60 specimens were subjected either to radiography and histology or to μCT and pQCT analyses as described previously [10, 12, 20, 21].
pQCT revealed a significant decrease of BMDto and BMDco with aging, regardless of sex. Histomorphometry revealed a significant reduction of cortical and trabecular parameters (Ct.Th, BV/TV, and Tb.Th) in male and female specimens, although the deterioration of bone mass monitored by changes of BV/TV, Tb.N, and Tb.Sp was significantly more pronounced in female than in male samples.
As it is widely accepted that BMD, bone geometry, and bone microarchitecture are all components that determine bone strength by the bone’s ability to withstand loading [22], our findings strongly suggest that the presented structural changes within the radial head might contribute to the pathogenesis of radial head fractures, especially in aged female patients where trabecular parameters (BMDtr and Tb.Sp) change significantly for the worse compared to male patients.
Looking through the recently published literature on the structure and bone mass of the radial head, only a few publications were found. In 2003 Gordon et al [23] presented a study on 13 radial heads (mean age 70.5 ± 9.5 years; seven male, six female) to determine the mechanical properties of subchondral cancellous bone. To detect regional variations in bone stiffness and strength in the radial head, they performed indentation tests on 3-mm-thick slices of the radial heads in four different quadrants (AL, AM, PL, PM). Unfortunately, in that study multiple linear regression tests could not detect a clear influence of the patients’ sex and age on bone stiffness and strength of the radial head. Probably this was due to the advanced mean age of 70.5 years of the tested specimens. A young control group was not included in the study.
With regard to the humeroradial joint, Eckstein et al [24] were able to identify a direct influence of loading conditions and geometric configurations of the joint to the pattern of subchondral bone distribution, which again reflects the potential of a mechanobiological adaptation of the bone. Because of these mechanobiological principles of skeletal remodeling, Mason′s microstructural observation fitted exactly with the fracture paths resulting from the biomechanical experiments performed by Amis and Miller [13] 40 years later.
In another study on the subchondral bone density in 36 human elbow specimens by CT osteoabsorptiometry, Eckstein et al [25] described a typical pattern of bone mineralization within the radial head. They found a central density maximum in the fovea, with mineralization falling off concentrically toward the margins of the radial head. The authors concluded that the presence of this central density maximum in the fovea of the radial head indicates a predominantly central pressure transmission in the humeroradial joint. Our findings, that Subch.Lam BV/TV was at least fourfold higher than the corresponding BV/TV of the entire preparation throughout all different groups, are in line with the observations of Eckstein et al [25]. In addition, the significant age-related decrease of Subch.Lam BV/TV in female as well as in male specimens monitored by the present investigation leads to an impaired function of this region of the radial head to withstand transition forces from the articular cartilage to the radial head, resulting in a higher susceptibility to fractures of this skeletal site.
As in Gordon et al [23], in the study by Eckstein et al [25] the average age of the specimens was advanced (76.8 years). Therefore, again the age-related morphological changes in the radial head could not be differentiated.
In a study by Koslowsky et al [26] the subchondral bone density of the radial head was measured with subtraction densitometry. In line with the findings of Eckstein et al [25], an eccentric distribution of the subchondral bone density reflecting an eccentric force transmission through the radiohumeral joint was again detected. Even though a number of 37 specimens with a range of 36–90 years of age were examined, Koslowsky et al did not analyze the influence of age on regional BMD. They found an asymmetric bone density pattern of the radial head with maxima at the ulnar-dorsal and ulnar-ventral areas of the radial head, giving an explanation for the higher incidence of fractures in the lateral area of the radial head.
Concerning the methodology of the present work, one has to evaluate the data resulting from pQCT measurements with caution as several articles have discussed a poor short-term precision of pQCT measurements at the appendicular skeleton, leading to less sensitivity of this technique in discriminating osteoporotic bone mass changes if compared to other techniques (i.e., DXA, SPA) [27, 28]. Furthermore, it has to be considered that pQCT and 2-D histomorphometry are susceptible to sampling errors as the amount of analyzed bone volume per sample is limited. In this context Ashe et al. [29] examined the accuracy of pQCT for evaluating the bone strength of 10 pairs of aged fresh-frozen radial specimens and found that pQCT scans varied systematically as they are affected by analysis mode, resolution, and thresholding. A reason for the imprecise bone mass measurements by pQCT as well as by histomorphometry might be that only thin slices of the whole bone specimen are determined, making them susceptible to sampling errors.
Another limitation concerns the study design. As this is a cross-sectional study, the monitored structural changes of the bone might be rather cohort effects than real age effects, e.g., due to changes in the nutritional status of the oldest subjects born in the late 1930s.
In conclusion, our findings provide evidence that the radial head shows distinct age-related changes in its microarchitecture that affect both the cortical and the trabecular compartments and that are known to reduce the biomechanical stability of the bone. This deterioration of the microarchitecture of the radial head in aging might contribute to the pathogenesis of radial head fractures, especially in aged female patients. This implies that fractures of the radial head are at least in part osteoporotic fractures, which may lead to an increase of the number of radial head fractures in the elderly due to the demographic changes in the Western world.
References
Herbertsson P, Josefsson PO, Hasserius R, Besjakov J, Nyqvist F, Karlsson MK (2004) Fractures of the radial head and neck treated with radial head excision. J Bone Joint Surg Am 86:1925–1930
Herbertsson P, Josefsson PO, Hasserius R, Karlsson C, Besjakov J, Karlsson M (2004) Uncomplicated Mason type-II and III fractures of the radial head and neck in adults. A long-term follow-up study. J Bone Joint Surg Am 86:569–574
Mason ML (1954) Some observations on fractures of the head of the radius with review of one hundred cases. Br J Surg 42:123–132
Ikeda M, Sugiyama K, Kang C, Takagaki T, Oka Y (2005) Comminuted fractures of the radial head. Comparison of resection and internal fixation. J Bone Joint Surg Am 87:76–84
Radin EL, Riseborough EJ (1966) Fractures of the radial head. A review of eighty-eight cases and analysis of the indications for excisison of the radial head and operative treatment. J Bone Joint Surg Am 48:1055–1064
Gebauer M, Rücker AH, Barvencik F, Rueger JM (2005) Therapy for radial head fractures. Unfallchirurg 108:657–667
Brown SA, Rosen CJ (2003) Osteoporosis. Med Clin North Am 87:1039–1063
Rodan GA, Martin TJ (1981) Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int 33:349–351
Schneider P, Reiners C, Cointry GR, Capozza RF, Ferretti JL (2001) Bone quality parameters of the distal radius as assessed by pQCT in normal and fractured women. Osteoporos Int 12:639–646
Amling M, Herden S, Pösl M, Hahn M, Ritzel H, Delling G (1996) Heterogeneity of the skeleton: comparison of the trabecular microarchitecture of the spine, the iliac crest, the femur, and the calcaneus. J Bone Mineral Res 11:36–45
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2:595–610
Amling M, Hahn M, Wening VJ, Grote HJ, Delling G (1994) The microarchitecture of the axis as the predisposing factor for fracture of the base of the odontoid process. A histomorphometric analysis of twenty-two autopsy specimens. J Bone Joint Surg Am 76:1840–1846
Amis AA, Miller JH (1995) The mechanisms of elbow fractures: an investigation using impact tests in vitro. Injury 26:163–168
Kimmel DB (1993) A paradigm for skeletal strength homeostasis. J Bone Miner Res 8:515–522
Ross PD, Wasnich RD, Davis JW (1990) Fracture prediction models for osteoporosis prevention. Bone 11:327–331
Currey JD (2003) The many adaptations of bone. J Biomech 36:1487–1495
Keaveny TM, Hayes WC (1993) A 20-year perspective on the mechanical properties of trabecular bone. J Biomech Eng 115:534–542
Eckstein F, Kuhn V, Lochmüller EM (2004) Strength prediction of the distal radius by bone densitometry—evaluation using biomechanical tests. Ann Biomed Eng 32:487–503
Lochmüller EM, Kristin J, Matsuura M, Kuhn V, Hudelmaier M, Link TM, Eckstein F (2008) Measurement of trabecular bone microstructure does not improve prediction of mechanical failure loads at the distal radius compared with bone mass alone. Calcif Tissue Int 83:293–299
Gebauer M, Lohse C, Barvencik F, Pogoda P, Rueger JM, Püschel K, Amling M (2006) Subdental synchondrosis and anatomy of the axis in aging. A histomorphometric study on 30 autopsy cases. Eur Spine J 15:292–298
Rupprecht M, Pogoda P, Mumme M, Rueger JM, Püschel K, Amling M (2006) Bone microarchitecture of the calcaneus and its changes in aging: a histomorphometric analysis of 60 human specimens. J Orthop Res 24:664–674
Müller R (2003) Bone microarchitecture assessment: current and future trends. Osteoporos Int 5:89–95
Gordon KD, Duck TR, King GJ, Johnson JA (2003) Mechanical properties of subchondral cancellous bone of the radial head. J Orthop Trauma 17:285–289
Eckstein F, Jacobs CR, Merz BR (1997) Mechanobiological adaptation of subchondral bone as a function of joint incongruity and loading. Med Eng Phys 19:720–728
Eckstein F, Muller-Gerbl M, Steinlechner M, Kierse R, Putz R (1995) Subchondral bone density in the human elbow assessed by computed tomography osteoabsorptiometry: a reflection of the loading history of the joint surfaces. J Orthop Res 13:268–278
Koslowsky TC, Mader K, Brandenburg A, Hellmich M, Koebke J (2008) Subchondral bone density of the radial head measured with subtraction densitometry. Surg Radiol Anat 30:113–118
Formica CA, Nieves JW, Cosman F, Garrett P, Lindsay R (1998) Comparative assessment of bone mineral measurements using dual X-ray absorptiometry and peripheral quantitative computed tomography. Osteoporos Int 8:460–467
Grampp S, Lang P, Jergas M, Glüer CC, Mathur A, Engelke K (1995) Assessment of the skeletal status by peripheral quantitative computed tomography of the forearm: short-term precision in vivo and comparison to dual X-ray absorptiometry. J Bone Miner Res 10:1566–1576
Ashe MC, Khan KM, Kontulainen SA, Guy P, Liu D, Beck TJ, McKay HA (2006) Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int 17:1241–1251
Acknowledgements
The authors acknowledge the support of Claudia Mueldner for excellent technical assistance in preparing the samples for qualitative and quantitative histomorphometry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Matthias Gebauer, Florian Barvencik and Marcus Mumme contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gebauer, M., Barvencik, F., Mumme, M. et al. Microarchitecture of the Radial Head and Its Changes in Aging. Calcif Tissue Int 86, 14–22 (2010). https://doi.org/10.1007/s00223-009-9304-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9304-0