Abstract
The human skeleton is a miracle of engineering, combining both strength and light weight to provide mechanical support to withstand the force of gravity and to transfer muscle forces during movement. The brain is well established as a master regulator of homeostasis in peripheral tissues. The discovery of bone regulation by central nervous system represents a growing area of study that is identifying novel regulatory axes between the nervous system and bone homeostasis, and revealing a far more complex, and interdependent bone biology than previously envisioned. This chapter examines the current understanding of the central regulation of bone homeostasis. Herein, we will discuss the contribution of central peptides, and other peptides such as leptin, and semaphorins and involvement of the brain in regulation of bone metabolism.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Central nervous system (CNS)
- Sympathetic nervous system (SNS)
- Hypothalamus
- Adrenergic
- Leptin
- Neuropeptide Y (NPY)
- Cannabinoid
- Semaphorins
- Melanocortin
- ACTH
- CART
- Energy
1 Introduction
Bone architecture adapts to changes in the mechanical strain generated within it to ensure that it maintains sufficient strength to withstand the loads to which it is subjected. As part of this adaptation, bone is remodeled throughout life by means of basic multicellular units (BMUs), consisting of osteoclasts and osteoblasts acting in a coordinated fashion to resorb existing bone and form new bone, respectively (Burger et al. 1995; Burger and Klein-Nulend 1999). The prevalent and widely accepted view has been that bone remodeling is controlled in a predominantly endocrine manner while simultaneously responding to local mechanical stimuli. Of late, the discovery of bone regulation by central nervous system represents an emerging area of study that is identifying novel regulatory axes between the nervous system and bone homeostasis.
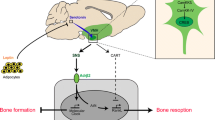
The brain is a powerful regulator of homeostatic processes in many peripheral tissues. There is now clear evidence for cross talk between the brain and bone via two distinct routes. The first comprises well-defined hormonal signals arising from neuroendocrine neurons of the hypothalamus and subsequently processed within the pituitary. The second, more recently appreciated pathway, consists of efferent neuronal discharges originating from the hypothalamus and processed through the brainstem. The hypothalamus, with its semipermeable blood brain barrier, is thus one of the most powerful regulatory regions within the body, integrating signals not only from peripheral tissues via the circulation but also from afferent neural return. A direct role of the nervous system in bone cells was strongly supported by immunocytochemistry studies which revealed the presence of innervation and neuropeptides receptors in bone cells (Elefteriou 2005). Furthermore, retrograde transsynaptic tracing has identified neural tracts from the femoral bone marrow linked direct to the central nervous system (Denes et al. 2005). These findings suggest the existence of neuronal connection between the brain and bone. These direct, neuronal pathways represent an emergent area of study identifying novel regulatory axes between the brain and the cells of bone. Moreover, this work is also providing insights into regulatory connections involving skeletal tissues that are proving unexpected, thereby outlining a level of interconnectedness that has been previously unappreciated. This chapter examines the expanded understanding of the central, neural outputs to bone metabolism and remodeling.
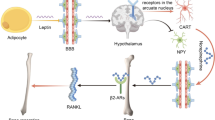
2 Leptin
Leptin is a small polypeptide hormone secreted into the peripheral circulation by adipocytes, and study of a mouse with nonfunctional leptin was the first indicator of the connection between the brain and bone. The leptin receptor in the hypothalamus senses the circulating levels of leptin which are positively correlated to total body adiposity and play a pivotal part in whole-body energy homeostasis. Mice with mutations in the leptin gene (ob/ob) or its receptor (db/db) display marked obesity accompanied by hyperglycemia, hyperinsulinemia, hypercorticism, and hypogonadism (Tartaglia et al. 1995; Halaas et al. 1995; Friedman and Halaas 1998). Interestingly, these mice also display marked bone phenotypes, which have been pivotal to understanding central control of bone but also link between energy and bone homeostasis. The hypogonadism and hypercorticism of ob/ob and db/db mice would be expected to result in reduced cancellous bone mass due to elevated bone loss, but, unexpectedly, it was found that cancellous bone volume was markedly increased in both models (Ducy et al. 2000). This increased bone mass was associated with a marked increase in bone formation, which surpassed the elevation of bone resorption. A key role for hypothalamic leptin activity in regulating bone mass was indicated following infusion of leptin into the cerebral ventricles, with no detectable change in serum leptin, which produced a decrease in cancellous bone volume in both ob/ob and wild-type mice (Ducy et al. 2000). The importance of central leptin signaling was further demonstrated by central infusion of leptin to one mouse of a pair of parabiosed ob/ob mice. With effective cross-circulation between the pair, correction of the ob/ob skeletal phenotype occurred only in the centrally infused recipient and not the contralateral mouse (Takeda et al. 2002). These findings provided strong evidence that, similar to the central regulation of obesity, the inhibition of osteoblast activity on cancellous bone surfaces is mediated by leptin within the hypothalamus .
The control of bone remodeling by leptin deficiency provides exciting new insights into neuronal mechanisms in skeletal physiology, but some reports in the literature suggest additional levels of complexity. Leptin action in cortical bone may be distinct from its effects in the cancellous compartment, as femoral length and total body bone mineral content (BMC ) are decreased in ob/ob mice and peripheral leptin treatment of immature (4-week old) ob/ob mice partially restored both parameters (Steppan et al. 2000). In addition, similar to the db/db mouse, leptin-resistant Zucker (fa/fa) rats carry an inactivating Gln269Pro mutation in the extracellular domain of the Ob-Rb leptin receptor gene (Takaya et al. 1996) and exhibit reduced femoral bone mineral density (BMD ) and calcium content (Mathey et al. 2002). Leptin can stimulate growth plate chondrocyte proliferation and differentiation in vitro (Nakajima et al. 2003); thus it is possible that the ob/ob-associated reductions in bone length and BMC may relate to growth plate rather than osteoblastic effects. Further complexity exists in ob/ob mice, between axial and appendicular skeletons. A subsequent study reported increased vertebral length and lumbar BMC in addition to reduced femur length mid-shaft cortical area and thickness and BMC (Hamrick et al. 2004). Whether these differences between effects of leptin deficiency on cortical and trabecular compartments or between appendicular and axial skeleton may relate to effects on muscle mass, marrow adipogenesis (Hamrick et al. 2004) or growth plate remains to be resolved; however, studies outlined below suggest a role for neuropeptide Y pathways in cortical bone control by leptin, district from the pathway to cancellous bone.
3 Leptin and Bone in Humans
Genetic studies in mice have convincingly demonstrated the association between leptin deficiency and altered skeletal homeostasis, but the limited genetic evidence in humans is mixed. Study of four individuals in a family carrying a missense mutation in the leptin gene showed only one exhibited low bone mass despite morbid obesity in all four; however, a high degree of consanguinity and multiple endocrine defects cloud interpretation of the observation (Ozata et al. 1999). Administration of leptin for a year to a young girl with a leptin mutation was associated with loss of body fat and an increase in whole-body bone mineral density (BMD ), as would be expected from mouse studies (Farooqi et al. 1999). Indirect clinical evidence suggests that if there is an association between leptin deficiency and bone mass in humans, BMD is reduced after self-imposed calorie restriction in anorexia nervosa, which can lead to extremely low levels of the nutritionally dependent hormones leptin and IGF-1 and amenorrhea, consistent with the low bone mass, reduced fertility, and suppressed activity of the somatotropic axis in ob/ob mice (Miller et al. 2004; Soyka et al. 1999). Reinforcing the potential importance of IGF-1 in this context, leptin treatment in patients with hypothalamic amenorrhea due to strenuous exercise or low body weight resulted in increased levels of both IGF-1 and bone formation markers (Welt et al. 2004). Such studies involving reduced nutritional intake, excessive exercise, and disrupted menses are not, however, amenable to simple interpretation in terms of direct leptin effects on bone vs indirect effects acting through altered reproductive and other neuroendocrine function.
Before the discovery of leptin, initial correlations between bone mass and body weight or fat mass (Reid 2002) were attributed either to changes in mechanical load or estrogen production related to fat tissue mass. But after the discovery of leptin, this additional factor was also investigated. Serum leptin levels and BMD are directly correlated in pre- and postmenopausal females by some investigators (Thomas et al. 2001) but not others (Rauch et al. 1998). Furthermore, a positive association between leptin levels and BMC was observed in a group of healthy nonobese women (Pasco et al. 2001), and a weak correlation was observed in postmenopausal osteoporotic women but not the controls (Odabasi et al. 2000). This uncertain relationship between leptin and bone may be gender-specific, as studies in males have reported either no association (Thomas et al. 2001) or a negative association (Sato et al. 2001) between leptin and BMD. In this context, it is interesting that one study which detected a positive association between leptin and BMD found no association between leptin and biochemical markers of bone resorption or formation and concluded that leptin is not likely to play a significant direct role in regulating bone cell activity (Goulding and Taylor 1998). However, this interpretation is challenged by direct examinations of bone cell responses to leptin in vitro.
4 Bone Cell Responses to Leptin in Vitro
Evidence for presence of the signal-transducing Ob-Rb leptin receptor or leptin-binding sites has been detected on ossifying fetal cartilage (Hoggard et al. 1997), immortalized marrow stromal cells (Thomas et al. 1999), chondrocytes (Steppan et al. 2000; Nakajima et al. 2003; Maor et al. 2002; Cornish et al. 2002), primary osteoblasts (Steppan et al. 2000; Cornish et al. 2002; Enjuanes et al. 2002; Reseland et al. 2001; Lee et al. 2002), and some but not all osteosarcoma cell lines (Reseland et al. 2001). Adding further to the possibility of peripheral actions of leptin is evidence of leptin production by primary human and rat osteoblast cultures (Morroni et al. 2004; Gordeladze et al. 2002).
A number of in vitro functional studies support direct leptin effects on chondrocytes and osteoblasts. Leptin has been observed to stimulate proliferation and differentiation of cultured growth plate chondrocytes (Nakajima et al. 2003) and to induce osteoblast differentiation and suppress adipogenesis in a human stromal cell line (Thomas et al. 1999). The hormone also has direct stimulatory effects on cell proliferation, differentiation, and function in human and rat osteoblastic cultures (Cornish et al. 2002; Gordeladze et al. 2002). In addition, leptin can inhibit osteoclastogenesis in vitro (Holloway et al. 2002; Burguera et al. 2001). One notable exception, the study which identified the central leptin pathway to bone, to these studies detected no osteoblastic expression of leptin or the leptin receptor long form and no effect of leptin treatment on formation of mineralized nodules in primary mouse osteoblastic cultures (Ducy et al. 2000). The majority of the in vitro evidence, however, supports peripheral actions of leptin in bone biology. It therefore remains possible that, particularly at high leptin concentrations direct, peripheral actions are prominent, and at low levels, central anti-actions may predominate.
5 Sympathetic Nervous System Regulation of Bone Mass
Although leptin deficiency is characterized by numerous endocrine changes, a humoral pathway was ruled out by parabiosis experiments in ob/ob mice (Takeda et al. 2002). This indicated that leptin signaling in the brain (supplied by brain-only infusion, intracerebroventricular icv) was responsible for the skeletal changes, without the necessity of a humoral signal. The ventromedial hypothalamus (VMH ) was identified as the source of the leptin signaling and origin of the signal to cancellous bone. Thus implicated sympathetic activity, as sympathetic tone, is known to be altered in leptin deficiency and was likely the important downstream mediator of central leptin signaling. Takeda and colleagues investigated whether the central actions of leptin might be mediated via the sympathetic nervous system (SNS ) and found that dopamine β-hydroxylase deficient mice, which are unable to produce epinephrine and norepinephrine, the catecholamine ligands for adrenergic receptors, also have high bone mass (Takeda et al. 2002). Importantly, icv infusion of leptin failed to reduce the bone mass of dopamine β-hydroxylase mice, indicating a requirement for a functional autonomic nervous system for leptin actions and identifying the sympathetic system as a neuronal mediator of leptin effects on bone (Takeda et al. 2002). This conclusion is supported by studies using β-adrenergic receptor (β-AR) agonists and antagonists. Administration of the β-agonist isoproterenol restored sympathetic activity in ob/ob mice and reduced cancellous bone mass in both wild-type and ob/ob mice without affecting body weight (Takeda et al. 2002). Conversely, administration of the nonselective β-adrenergic receptor antagonist propranolol increased bone mass in wild-type mice, demonstrating that modulation of SNS activity affects cancellous bone remodeling in a manner indicated by ob/ob mice (Takeda et al. 2002). Consistent with a role for SNS signaling in bone, mice deficient in ß2AR showed increased cancellous bone volume (Elefteriou et al. 2005), while deletion of a downstream mediator of ß2AR signaling, adenylyl cyclase 5, protects against age-related bone loss (Yan et al. 2007). Disruption of dopamine ß-hydroxylase, an enzyme generating adrenaline and noradrenaline, produced greater cancellous bone mass in wild-type mice. Importantly, reducing adrenergic signaling protected against cancellous bone loss following icv leptin, through loss of dopamine ß-hydroxylase in wild-type mice and propranolol treatment in ob/ob (Takeda et al. 2002). Unfortunately the effect of these agents on cortical bone was not systematically investigated. However, the novel role of ß-adrenergic signaling via SNS completed the central leptin signaling pathway to bone.
Consistent with the in vivo evidence for sympathetic control of bone formation, β-ARs have been identified on osteoblasts and in osteoblast-like cell lines. β1- and β2-adrenergic receptors are expressed at different levels in various human osteoblast-like cell lines (Kellenberger et al. 1998), and β2-adrenergic receptors have been identified on rat osteoblast-like cells, and in mouse primary osteoblast cultures (Takeda et al. 2002; Moore et al. 1993), suggesting that sympathetic activity might control bone formation through direct modulation of osteoblast function. Supporting this, administration of the specific β2-AR agonist, formoterol, induced cAMP and expression of the immediate-early gene c-fos in the SaOS-2 human osteosarcoma cell line (Kellenberger et al. 1998). Likewise, c-fos expression was inhibited by administration of a specific β2-AR antagonist, demonstrating that β2-ARs in osteoblasts are indeed functionally coupled to intracellular signaling pathways. Furthermore, in vitro studies have confirmed the ability of adrenergic signaling to alter bone remodeling. Administration of norepinephrine stimulated bone resorption in neonatal mouse calvariae in organ culture (Moore et al. 1993), and treatment of the MC3T3-E1 pre-osteoblastic mouse cell line with epinephrine stimulated expression of osteoclast differentiation factor (Takeuchi et al. 2000), suggesting that β-adrenergic stimulation of resorption may occur via an indirect osteoblast-mediated pathway.
6 Clinical Relevance of β-Adrenergic Control of Bone
Beta-blocker usage to treat cardiovascular diseases is prevalent, but effects on skeletal mass and strength had not been widely investigated prior to the evidence from mouse studies cited above. However, recent studies have assessed the effects of β-adrenergic antagonists on bone turnover, BMD , and fracture risk in human population-based studies. β-Blocker use was associated with a reduction in fracture risk and increased BMD at the hip and forearm in women over 50 years of age (Pasco et al. 2004), and reduced fracture risk in women and men between 30 and 79 years of age (Schlienger et al. 2004), consistent with the rodent model data. However, a third observational study contradicted these findings, with β-blocker usage associated with a threefold increase in fracture risk and reduced serum osteocalcin, an osteoblastic marker of bone formation, in premenopausal women (Rejnmark et al. 2004). The difference in this latter finding may relate to variations in study design. Thus, there is some epidemiological evidence to support the hypothesis that β-adrenergic signaling is involved in bone metabolism; however, placebo-controlled randomized clinical trials are necessary to more effectively assess potential therapeutic benefit from bone-specific β-adrenergic blockade in hypogonadal and age-related osteoporosis and other conditions that could benefit from stimulation of bone formation.
7 Neuropeptide Y and the Y Receptors
7.1 NPY System
The role of leptin in the hypothalamus was followed by the identification of a number of central pathways to bone. One neuronal system of particular importance to bone is the neuropeptide Y (NPY) system . The NPY system consists of three ligands: NPY, peptide YY (PYY ), and pancreatic polypeptide (PP) mediating its effects via G protein-coupled receptors, of which five have been identified to date, Y1, Y2, Y4, Y5, and y6 (Blomqvist and Herzog 1997; Lin et al. 2005). NPY, a 36-amino acid peptide, is widely expressed in the central and peripheral nervous systems, and is present in both sympathetic and parasympathetic nerve fibers , often co-released with noradrenaline during nerve stimulation. It also circulates in the blood from neuronal and adrenal sources. NPY is a well-characterized vasoconstrictor in these neurons, in addition to enhancing the action of other pressor agents (Pernow et al. 1987; Parker and Herzog 1999; Morris 1994). NPY-ergic neurons are abundant in the brain, with high levels in the hypothalamic arcuate nucleus (ARC) and VMH (Hokfelt et al. 1998). Central NPY action is associated with the regulation of food intake, cardiac and respiratory activity, and the release of pituitary hormones (Hokfelt et al. 1998; Wettstein et al. 1995). PYY is produced primarily from the endocrine L cells of the gastrointestinal tract with some expression in the pancreatic islets, and their function is related to satiety control and gastrointestinal regulation (Lundberg et al. 1984). Moreover, PYY expression has also been reported in brainstem neurons, although the functional significance of this localization is unknown (Ekblad and Sundler 2002). PP is produced by endocrine islet cells of the pancreas and regulates endocrine functions of the pancreas as well as satiety centrally (Ueno et al. 1999).
7.2 NPY–Leptin Interaction
In keeping with NPY’s role as a powerful modulator of energy homeostasis, both NPY and leptin have a close association within the hypothalamus . Indeed, a significant proportion of NPY-ergic neurons co-express the leptin receptor in the ARC (Mercer et al. 1996), and NPY expression is upregulated following the reduction in leptin due to starvation (Schwartz et al. 1995; Spanswick et al. 1997; Spiegelman and Flier 1996) and in leptin-deficient ob/ob mice (Wilding et al. 1993). Thus NPY is a critical downstream mediator of leptin-deficient starvation signaling in the hypothalamus and indices powerful increases in appetite and conservation of energy. Leptin receptors are also present in other nuclei in the hypothalamus, including the ventromedial hypothalamus, from which the leptin-mediated pathway to cancellous bone was shown to originate (Takeda et al. 2002). Thus the degree to which they function separately or can coordinate pathways is not known.
The first NPY model evaluated for skeletal activity was Y2 receptor null, Y2 −/− mice, due to the known co-expression of Y2 and leptin receptors on neurons within the arcuate nucleus (Baskin et al. 1999; Broberger et al. 1997). Initial analysis of germline Y2 −/− mice was similar to those following conditional deletion of Y2 receptors in the hypothalamus , demonstrating a role for central Y2 receptors in this pathway and the first specific gene deletion in the hypothalamus to alter bone homeostasis. Both models revealed a greater cortical and cancellous bone volume associated with a greater bone formation rate (Baldock et al. 2002, 2006). Bone resorption parameters were unchanged except for a modest increase in osteoclast number. Importantly, the skeletal changes observed in germline Y2 −/− mice and hypothalamic Y2 −/− mice occurred in the absence of any measurable changes in bone active endocrine factors. Thus these findings suggested that the bone anabolic effects after Y2 receptor deletion are mediated by a neuronal mechanism and not by endocrine effectors of bone turnover. The importance of the ARC to NPY–bone signaling was confirmed by viral-dependent overexpression of NPY restricted to the ARC resulting in marked bone loss, despite extreme weight gain (Baldock et al. 2005), consistent with stimulation of starvation pathways.
Several reports suggested that leptin- and NPY-mediated pathways to bone were similar. This was supported by findings in NPY, Y2 receptor, and leptin double mutant mice (Y2 −/− ;ob/ob) in which no additive effect on cancellous bone volume or formation was evident (Baldock et al. 2006). Additionally, male Y2 −/− Y4 −/− double NPY receptor knockout mice revealed a synergistic increase in cancellous bone volume compared with Y2 −/− mice (Sainsbury et al. 2003). This gender-specific effect was coincident with a marked reduction in plasma leptin in male, but not in female Y2 −/− Y4 −/− mice (Sainsbury et al. 2003), suggesting an additive effect of the Y2 receptor and leptin on bone.
However, when NPY was continuously administered into wild-type mice, mimicking the increase in ob/ob, the treatment reduced cancellous bone volume, suggesting that NPY and leptin might use different pathways to control bone mass (Ducy et al. 2000). Consistent with this notion, NPY −/− mice display a different bone phenotype to ob/ob, while both display anabolic effects in cancellous bone, and no additive effect as outlined above; however, they do differ in cortical bone. Leptin-deficient mice have markedly reduced cortical bone production, with shorter and smaller bones, particularly when corrected for the greater body weight of these very obese mice (Baldock et al. 2006), leading to their reduced whole-body BMC (Steppan et al. 2000). In contrast, NPY −/− mice display a generalized bone anabolic effect, with greater whole-body BMC and larger cortical bones, with greater bone formation similar to Y2 −/− mice (Lee et al. 2011a). The nature of the relationship with leptin was examined in NPY −/− ;ob/ob double mutant mice (Wong et al. 2013). NPY levels are markedly elevated in ob/ob mice, as a direct effect of reduced activation of the leptin receptor in NPY-ergic neurons (Erickson et al. 1996). Loss of NPY in ob/ob mice had very specific effects upon bone metabolism, with a correction of the cortical deficit through a correction of bone formation; NPY −/− ;ob/ob cortical bone was returned to wild-type levels, indicating that the reduction in bone mass in ob/ob is driven by the increase in ARC NPY signaling following leptin withdrawal. However, there was no difference in cancellous bone between ob/ob and NPY −/− ;ob/ob, indicating that the VMH /SNS /β2 pathway is not altered by NPY. Thus this model identified two anatomically distinct pathways from two hypothalamic nuclei to bone, which respond to deficiency in the critical marker of energy homeostasis, leptin. In response to leptin deficiency, a condition associated with reduced energy storage (as fat), and thus starvation, NPY from the arcuate nucleus inhibits bone formation and bone accrual, thereby conserving energy. Simultaneously, the VMH alters SNS activity to maintain cancellous bone volume, perhaps to maintain some of the calcium and trace elements stored in bone tissue.
7.3 NPY in Bone Tissue
It is interesting to note that with leptin, the receptors and ligands present in the hypothalamus were also present in the cells of bone. There is also evidence that NPY-positive autonomic nerves are present in healthy bone tissue, particularly in association with blood vessel walls, suggestive of a role in modulation of vascular tone rather than direct effects on bone cells (Bjurholm et al. 1988; Ahmed et al. 1993; Lindblad et al. 1994; Sisask et al. 1996). In addition, sympathetic denervation has been observed to reduce NPY immunoreactive nerves in the periosteum (Hill and Elde 1991). Very early studies demonstrated that NPY treatment in osteoblastic cell lines inhibited the cAMP response to parathyroid hormone and norepinephrine (Bjurholm 1991; Bjurholm et al. 1992), suggesting the presence of functional Y receptors on bone cells and a possible regulatory role for NPY in bone.
Similar to leptin, direct NPY-mediated effects in bone were confirmed in mutant mouse models. Two NPY receptors, Y1 and Y2, have been connected with bone homeostasis. These receptors are abundant in the hypothalamus as well as in peripheral nerves (Kishi and Elmquist 2005; Kopp et al. 2002; Naveilhan et al. 1998). Similar to the phenotype resulting from Y2 receptor deletion, germline Y1 receptor deficiency resulted in a generalized anabolic phenotype with greater cortical bone and cancellous accrual, although with an additional increase in bone resorption (Baldock et al. 2007). However, unlike the anabolic effects in hypothalamus-specific Y2 deletion (Parker and Herzog 1999), loss of hypothalamic Y1 receptors had no effect on bone homeostasis, indicating a noncentral mechanism for Y1 action in bone. The existence of a direct Y1-mediated effect on bone anabolism was further suggested following identification of Y1 expression in osteoblastic cells in vivo (Baldock et al. 2007). NPY treatment of calvarial osteoblast cultures markedly decreased cell numbers, an effect absent in cultures from Y1 receptor knockout mice, indicating functional osteoblastic Y1 receptors. The direct regulation of osteoblasts by NPY was confirmed in osteoblast-specific Y1 receptor knockout mice, which displayed an increase in bone formation similar to germline Y1-null mice (Lee et al. 2011b). Loss of Y1 receptor has also been demonstrated to regulate mesenchymal stem cell activity and mineralization of osteoblastic cultures in vitro (Lee et al. 2010). In addition, NPY is produced by osteoblasts and elevation of NPY production in osteoblast-specific NPY transgenic mice shown an opposing phenotype to Y1 receptor deletion, with reduced bone volume and bone formation (Matic et al. 2012). The integration of NPY within the osteoblast lineage was confirmed by a study demonstrating the NPY and Y1 receptor expression is controlled in a differentiation-dependent manner and responds to loading, a fundamental aspect of bone physiology (Igwe et al. 2009). Adding a new dimension to neuronal/bone interactions, a recent study, which produced an osteoblast-specific deletion of p38a-Mapk14 demonstrated alterations in bone homeostasis, but also increased energy expenditure and reduced adiposity (Rodriguez-Carballo et al. 2015). Interestingly, these metabolic changes were associated with reduced NPY production by osteoblasts and blocked by i.p. NPY administration. Thus, taken together these models confirmed an NPY-mediated pathway from the arcuate nucleus of the hypothalamus to the osteoblast, as well as an active NPY loop within the osteoblast lineage. Moreover, they indicate that osteoblastic NPY can modulate whole-body energy homeostasis, as does central NPY in the hypothalamus, illustrating extremely novel interorgan communication from bone cells.
7.4 Cannabinoid Receptors
Endocannabinoid signaling has been identified as one of the central pathways to bone which mediates its actions via two cannabinoid receptors, CB1 and CB2 (Howlett et al. 2002). CB1 is primarily found within the CNS and accounts for most of the CNS actions of cannabinoid drugs and endocannabinoids (Mackie 2008), while CB2 is predominantly expressed in peripheral tissues (Tam et al. 2008). Endocannabinoids are generated as needed, whereas other neurotransmitters are released from vesicles. The main endogenous ligands of CB1 and CB2 receptors are 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA or anandamide) (Devane et al. 1992; Mechoulam et al. 1995).
It has been recently reported that endocannabinoids regulate bone homeostasis by modulating adrenergic signaling. The activation of CB1 receptor signaling on presynaptic nerve terminals in bone inhibits norepinephrine release by sympathetic neurons to balance the tonic sympathetic restrain of bone formation (Ishac et al. 1996; Niederhoffer et al. 2003). However, CB1 receptor inactivation not only increased bone mineral but also provided protection against ovariectomy-induced bone loss (Idris et al. 2005). Moreover, CB1 receptor inactivation inhibited osteoclastogenesis and bone resorption, while CB1 receptor-deficient mice were resistant to these effects suggesting cannabinoid signaling acts via the CB1 receptor to regulate osteoclasts (Idris et al. 2005).
CB2 receptor is abundantly expressed in osteoblasts, osteocytes, and osteoclasts. CB2 knockout mice have accelerated age-related cancellous bone loss and cortical expansion due to increased bone turnover (Ofek et al. 2006). These results corroborate with human genetic association studies linking CNR2 gene (encoding CB2) and reduced bone mass in women (Karsak et al. 2005; Yamada et al. 2007) and in vitro pharmacological studies demonstrating a direct activation of CB2 in osteoclasts. These in vitro studies indicate that CB2 signaling contributes to the maintenance of bone mass by stimulating stromal cells/osteoblasts directly and by inhibiting monocytes/osteoclasts. Also CB2 agonists, which are not psychoactive, attenuated ovariectomy-induced bone loss in mice suggesting that these compounds might be used for the treatment of low bone mass diseases (Ofek et al. 2006). Taken together, these data suggest that the cannabinoid system plays an important role in the regulation and maintenance of bone mass through the signaling of both the central and skeletal cannabinoid receptors. This system, along with the NPY system, highlights the importance of centrally expressed receptors but also the locally expressed receptors in bone.
7.5 Semaphorins
Semaphorins are a family of both secreted and membrane-associated proteins that can regulate cell–cell interactions as well as cell differentiation, morphology, and function. The best-characterized biological role of semaphorins is their ability to provide attractant or repellent cues for migrating cells and growing neurites, i.e., axons and dendrites (Derijck et al. 2010; Tran et al. 2007). Originally characterized as axon guidance molecules, recent studies have demonstrated that the semaphorin–plexin system has an important role in the crosstalk between osteoblasts and osteoclasts (Negishi-Koga and Takayanagi 2012; Kang and Kumanogoh 2013). Semaphorins are divided into eight subclasses, of which classes III–VII are vertebral semaphorins . Most of the effects of semaphorins are mediated by plexin and neuropilin receptors. Recent studies have shown that semaphorin 3A (SEMA3A) and semaphorin 4D (SEMA4D) are involved in bone homeostasis.
In mice, SEMA3A is produced by osteoblasts and inhibits osteoclast formation from precursor cells (Hayashi et al. 2012). SEMA3A-null mice have reduced bone density, and systemic administration of SEMA3A prevents bone loss in a mouse model of menopause, indicating that SEMA3A could be a potential therapeutic agent to reduce bone loss (Hayashi et al. 2012). SEMA3A not only has an inhibitory effect on osteoclast differentiation but it also repels osteoclast precursors. SEMA3A from autocrine or paracrine sources also promotes osteoblast formation and differentiation. Recent studies have shown that SEMA3A was shown to have a crucial role in the development of proper sensory innervation of bone tissue, and neuronal but not osteoblastic SEMA3A was found to be responsible for bone loss in SEMA3A-null mice (Fukuda et al. 2013). These data indicate that SEMA3A, through its modulation of sensory innervation during development, regulates bone metabolism.
SEMA4D is highly and selectively expressed by osteoclasts and it inhibits osteoblast formation and differentiation. Consistent with its role in inhibition of osteoblast formation, mice lacking SEMA4D or its receptor plexin-B1 have elevated bone mass (Negishi-Koga et al. 2011; Dacquin et al. 2011). Moreover, it has been reported that SEMA4D-specific antibody was able to reduce bone loss in ovariectomized mice by increasing bone formation (Negishi-Koga et al. 2011). In vitro studies have shown that plexin-B1-mediated, ERBB2-dependent RhoA activation is responsible for the inhibition of osteoblast differentiation. These data are supported by in vivo studies showing that the osteoblast-specific expression of RhoA mimics global SEMA4D and plexin-B1 knockout mouse phenotypes (Negishi-Koga et al. 2011).
7.6 POMC and Melanocortin System
The melanocortin system is involved in diverse physiological functions from coat color to body weight homoeostasis. Pro-opiomelanocortin (POMC)-derived peptides such as melanocortins, adrenocorticotropic hormone (ACTH), and β-endorphin exert their pleiotropic effects via binding to melanocortin receptors (MCRs) and opioid receptors. Of the five MCRs identified as G-protein-coupled receptors, MC1–MC5 (Beltramo et al. 2003; Nijenhuis et al. 2001), melanocortin 4 receptor (MC4R) is abundantly expressed in hypothalamic neurons. MC4R is a major regulator of bone homeostasis with patients lacking in MC4R exhibiting a high bone mineral density resulting from a decrease in bone resorption (Farooqi et al. 2000). Importantly, the greater BMD is still evident after correction of the obesity that is characteristic of MC4R deficiency (Farooqi et al. 2000). Most studies on POMC peptides and their receptors in bone have concentrated on effects of melanocortin peptides rather than on expression of POMC, POMC-derived peptides, and effects of endogenous opioids. In a mouse model, daily subcutaneous administration of 0.2 μg/kg ACTH (amino acids 1–24) protected against glucocorticoid-induced osteonecrosis of the femoral head (Isales et al. 2010). Moreover, ACTH stimulated vascular endothelial growth factor (VEGF) production, which supported the maturation and survival of osteoblasts. It has been observed that induction of VEGF expression and secretion from osteoblasts was mediated by MC2R. ACTH appeared to modulate osteogenic differentiation as well. In the human osteoblast-like Saos2 cells of an osteosarcoma origin, ACTH had a biphasic effect on transcripts of collagen type I, the major collagen in bone, for which expression is strongly elevated during osteoblast differentiation. ACTH at 10 nm increased collagen I mRNA and thus differentiation, whereas at lower concentrations, it opposed osteoblast differentiation (Isales et al. 2010). Interestingly, ACTH immunoreactivity and ACTH secretion was described in rat osteoclastic cells (Sun et al. 2006), but the significance of this finding remains to be shown. Contrary to ACTH, in vitro administration of 10−8 m α-MSH increased proliferation of fetal rat osteoblasts without affecting their differentiation. In cultures of mouse bone marrow, α-MSH also stimulated osteoclast formation from their precursors but has no effect on mature osteoclasts.
7.7 CART in Bone Homeostasis
Studies in mice have implicated the involvement of hypothalamic neuropeptide, CART in bone homeostasis. CART, a neuropeptide precursor protein involved in the regulation of food intake and energy expenditure, is broadly expressed in the hypothalamus and the peripheral organs such as the pancreas and adrenal glands (Elefteriou et al. 2005). Interestingly, the phenotype of leptin-deficient (ob/ob) mice suggested that leptin could be affecting bone resorption via central effects on CART. ob/ob mice, unlike the ß2-adrenergic receptor null mice, showed decrease in hypothalamic CART expression and increased bone resorption, thereby implicating CART as a potential regulator of bone resorption. Moreover, intraperitoneal treatment with leptin in ob/ob mice was able to restore the decreased CART expression (Kristensen et al. 1998). Consistent with this hypothesis, CART knockout mice are osteopenic due to an increase in bone resorption (Elefteriou et al. 2005). Interestingly, CART-deficient mice express higher levels of RANKL in bone than wild-type mice, with in vitro osteoclast differentiation experiments indicating that the effect of CART bone is not cell autonomous, suggesting a local mechanism for the central CART changes (Elefteriou et al. 2005). Moreover, hypothalamic CART expression is elevated in MC4R −/− mice, which display a high bone mass phenotype due to decreased osteoclast formation and activity(Elefteriou et al. 2005; Ahn et al. 2006), as evident in human studies. Further, MC4R mutant mice lacking one or two copies of CART exhibited a significantly lower bone mass (Elefteriou et al. 2005; Ahn et al. 2006), demonstrating increased CART signaling plays an important role in the low bone resorption/high bone mass phenotype observed in MC4R-null mice.
8 Conclusion
It has become clear in the last decade or so that direct neural connections between the brain and the cells of bone represent a major regulatory axis controlling bone homeostasis. However, signals from the major neuronal populations arising from hypothalamus are critical for sensing the current status of energy stores on a body-wide scale, and it is these nerves which appear to play a pivotal role in regulating bone mass. In this manner, integration of energy balance and nutritional state can be achieved not only through modulation of energy intake but also expenditure, through control of activity at a tissue level, in this case bone metabolism. Bone is a large and protein/ nutrient dense tissue and as such represents a significant energy store. In addition, osteoblasts are very active protein synthetic cells, requiring a substantial amount of energy to renew lost bone. Thus bone, being an energy store and requiring energy to function, is a vital element of the regulatory processes coordinated with the brain. This connection between energy and bone homeostasis renders many of these interactions context-dependent, with responses particularly evident in starvation-type context , such as low leptin, CART, or POMC or greater NPY. While this relationship may help shed light upon skeletal responses to altered nutrient balance, such as obesity or anorexia, it also means that care must be taken when comparing mouse results to population-based studies in humans. The parallels between bone regulatory pathways in the hypothalamus and within the cells of bone themselves present potential therapeutic opportunities to effect bone directly, without the complexity of central hypothalamic responses. Given the power of some of these pathways to control bone cell activity, such agents may offer exciting avenues for therapeutic development in the years to come.
References
Ahmed M, et al. Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine. 1993;18(2):268–73.
Ahn JD, et al. Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology. 2006;147(7):3196–202.
Baldock PA, et al. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109(7):915–21.
Baldock PA, et al. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res. 2005;20(10):1851–7.
Baldock PA, et al. Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res. 2006;21(10):1600–7.
Baldock PA, et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J Biol Chem. 2007;282(26):19092–102.
Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48(4):828–33.
Beltramo M, et al. Gene expression profiling of melanocortin system in neuropathic rats supports a role in nociception. Brain Res Mol Brain Res. 2003;118(1–2):111–8.
Bjurholm A. Neuroendocrine peptides in bone. Int Orthop. 1991;15(4):325–9.
Bjurholm A, et al. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25(2–3):119–25.
Bjurholm A, et al. Neuroendocrine regulation of cyclic AMP formation in osteoblastic cell lines (UMR-106-01, ROS 17/2.8, MC3T3-E1, and Saos-2) and primary bone cells. J Bone Miner Res. 1992;7(9):1011–9.
Blomqvist AG, Herzog H. Y-receptor subtypes – how many more? Trends Neurosci. 1997;20(7):294–8.
Broberger C, et al. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66(6):393–408.
Burger EH, Klein-Nulend J. Mechanotransduction in bone – role of the lacuno-canalicular network. FASEB J. 1999;13(12):S101–12.
Burger EH, et al. Function of osteocytes in bone – their role in mechanotransduction. J Nutr. 1995;125(7 Suppl):2020S–3S.
Burguera B, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142(8):3546–53.
Cornish J, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15.
Dacquin R, et al. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS One. 2011;6(10):26.
Denes A, et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005;134(3):947–63.
Derijck AA, Van Erp S, Pasterkamp RJ. Semaphorin signaling: molecular switches at the midline. Trends Cell Biol. 2010;20(9):568–76.
Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9.
Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207.
Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23(2):251–61.
Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005;62(19–20):2339–49.
Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20.
Enjuanes A, et al. Leptin receptor (OB-R) gene expression in human primary osteoblasts: confirmation. J Bone Miner Res. 2002;17(6):1135.
Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274(5293):1704–7.
Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. New Engl J Med. 1999;341(12):879–84.
Farooqi IS, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9.
Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70.
Fukuda T, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497(7450):490–3.
Gordeladze JO, et al. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85(4):825–36.
Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63(6):456–8.
Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–6.
Hamrick MW, et al. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34(3):376–83.
Hayashi M, et al. Osteoprotection by semaphorin 3A. Nature. 2012;485(7396):69–74.
Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264(3):469–80.
Hoggard N, et al. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci U S A. 1997;94(20):11073–8.
Hokfelt T, et al. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26(2–3):154–66.
Holloway WR, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17(2):200–9.
Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202.
Idris AI, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11(7):774–9.
Igwe JC, et al. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J Cell Biochem. 2009;108(3):621–30.
Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann N Y Acad Sci. 2010;1192:110–6.
Ishac EJ, et al. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–8.
Kang S, Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin Cell Dev Biol. 2013;24(3):163–71.
Karsak M, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005;14(22):3389–96.
Kellenberger S, et al. Formoterol and isoproterenol induce c-fos gene expression in osteoblast-like cells by activating beta2-adrenergic receptors. Bone. 1998;22(5):471–8.
Kishi T, Elmquist JK. Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry. 2005;10(2):132–46.
Kopp J, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111(3):443–532.
Kristensen P, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–6.
Lee YJ, et al. Leptin receptor isoform expression in rat osteoblasts and their functional analysis. FEBS Lett. 2002;528(1–3):43–7.
Lee NJ, et al. Critical role for Y1 receptors in mesenchymal progenitor cell differentiation and osteoblast activity. J Bone Miner Res. 2010;25(8):1736–47.
Lee NJ, et al. Y2 and Y4 receptor signalling attenuates the skeletal response of central NPY. J Mol Neurosci. 2011a;43(2):123–31.
Lee NJ, et al. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011b;48(3):461–7.
Lin S, et al. Compensatory changes in [125I]-PYY binding in Y receptor knockout mice suggest the potential existence of further Y receptor(s). Neuropeptides. 2005;39(1):21–8.
Lindblad BE, et al. Vasoconstrictive action of neuropeptide Y in bone. The porcine tibia perfused in vivo. Acta Orthop Scand. 1994;65(6):629–34.
Lundberg JM, et al. Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral neurons. J Neurosci. 1984;4(9):2376–86.
Mackie K. Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol. 2008;286:S60–5.
Maor G, et al. Leptin acts as a growth factor on the chondrocytes of skeletal growth centers. J Bone Miner Res. 2002;17(6):1034–43.
Mathey J, et al. Bone mass in obese diabetic Zucker rats: influence of treadmill running. Calcif Tissue Int. 2002;70(4):305–11.
Matic I, et al. Bone-specific overexpression of NPY modulates osteogenesis. J Musculoskelet Neuronal Interact. 2012;12(4):209–18.
Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90.
Mercer JG, et al. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol. 1996;8(10):733–5.
Miller KK, et al. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab. 2004;89(9):4434–8.
Moore RE, et al. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23(3):301–15.
Morris JL. Selective constriction of small cutaneous arteries by NPY matches distribution of NPY in sympathetic axons. Regul Pept. 1994;49(3):225–36.
Morroni M, et al. In vivo leptin expression in cartilage and bone cells of growing rats and adult humans. J Anat. 2004;205(4):291–6.
Nakajima R, et al. Effects of leptin to cultured growth plate chondrocytes. Horm Res. 2003;60(2):91–8.
Naveilhan P, et al. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87(1):289–302.
Negishi-Koga T, Takayanagi H. Bone cell communication factors and Semaphorins. Bonekey Rep. 2012;1(183):183.
Negishi-Koga T, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17(11):1473–80.
Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedeberg’s Arch Pharmacol. 2003;367(5):434–43.
Nijenhuis WA, Oosterom J, Adan RA. AgRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15(1):164–71.
Odabasi E, et al. Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol. 2000;142(2):170–3.
Ofek O, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A. 2006;103(3):696–701.
Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95.
Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11(4):1431–48.
Pasco JA, et al. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86(5):1884–7.
Pasco JA, et al. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19(1):19–24.
Pernow J, et al. Neuropeptide Y: presence in perivascular noradrenergic neurons and vasoconstrictor effects on skeletal muscle blood vessels in experimental animals and man. Regul Pept. 1987;19(5–6):313–24.
Rauch F, et al. Does leptin have an effect on bone in adult women? Calcif Tissue Int. 1998;63(6):453–5.
Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31(5):547–55.
Rejnmark L, et al. Fracture risk in perimenopausal women treated with beta-blockers. Calcif Tissue Int. 2004;75(5):365–72.
Reseland JE, et al. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res. 2001;16(8):1426–33.
Rodriguez-Carballo E, et al. p38alpha function in osteoblasts influences adipose tissue homeostasis. In: FASEB J Faseb. United States; 2015. p. 1414–25.
Sainsbury A, et al. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol Cell Biol. 2003;23(15):5225–33.
Sato M, et al. Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab. 2001;86(11):5273–6.
Schlienger RG, et al. Use of beta-blockers and risk of fractures. JAMA. 2004;292(11):1326–32.
Schwartz MW, Dallman MF, Woods SC. Hypothalamic response to starvation: implications for the study of wasting disorders. Am J Phys. 1995;269(5 Pt 2):R949–57.
Sisask G, et al. The development of autonomic innervation in bone and joints of the rat. J Auton Nerv Syst. 1996;59(1–2):27–33.
Soyka LA, et al. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84(12):4489–96.
Spanswick D, et al. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390(6659):521–5.
Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87(3):377–89.
Steppan CM, et al. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92(1–3):73–8.
Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–60.
Takaya K, et al. Molecular cloning of rat leptin receptor isoform complementary DNAs – identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun. 1996;225(1):75–83.
Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17.
Takeuchi T, et al. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol. 2000;61:5.
Tam J, et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008;22(1):285–94.
Tartaglia LA, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–71.
Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1360–8.
Thomas T, et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29(2):114–20.
Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–92.
Ueno N, et al. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117(6):1427–32.
Welt CK, et al. Recombinant human leptin in women with hypothalamic amenorrhea. New Engl J Med. 2004;351(10):987–97.
Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65(3):397–414.
Wilding JP, et al. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology. 1993;132(5):1939–44.
Wong IP, et al. Neuropeptide Y is a critical modulator of leptin’s regulation of cortical bone. J Bone Miner Res. 2013;28(4):886–98.
Yamada Y, Ando F, Shimokata H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int J Mol Med. 2007;19(5):791–801.
Yan L, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130(2):247–58.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kulkarni, R.N., Baldock, P.A. (2017). Bone and the Central Nervous System. In: Smith, S., Varela, A., Samadfam, R. (eds) Bone Toxicology. Molecular and Integrative Toxicology. Springer, Cham. https://doi.org/10.1007/978-3-319-56192-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-56192-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56190-5
Online ISBN: 978-3-319-56192-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)