Abstract
Locomotor exercise may induce corticospinal excitability and/or cortical inhibition change in the knee extensors. This study investigated whether the mode of muscle contraction involved during a locomotor exercise modulates corticospinal and intracortical responsiveness. Eleven subjects performed two 45-min treadmill walking exercises in an uphill (+ 15%) or a downhill (− 15%) condition matched for speed. Maximal voluntary isometric torque (MVIC), voluntary activation level (VAL), doublet (Dt) twitch torque, and M-wave area of the knee extensors were assessed before and after exercise. At the same time-points, motor-evoked potential (MEP), cortical silent period (CSP), and short-interval cortical inhibition (SICI) were recorded in the vastus lateralis (VL) and rectus femoris (RF) muscles. After exercise, uphill and downhill conditions induced a similar loss in MVIC torque (− 9%; p < 0.001), reduction in VAL (− 7%; p < 0.001), and in M-wave area in the VL muscle (− 8%; p < 0.001). Dt twitch torque decreased only after the downhill exercise (− 11%; p < 0.001). MEP area of the VL muscle increased after the downhill condition (p = 0.007), with no change after the uphill condition. MEP area of the RF muscle remained stable after exercises. CSP and SICI did not change in the two conditions for both muscles. Downhill walking induces an increase in MEP area of the VL muscle, with no change of the CSP duration or SICI ratio. The eccentric mode of muscle contraction during a locomotor exercise can modulate specifically corticospinal excitability in the knee extensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aerobic exercise represents a potent strategy to induce neuroplastic changes in a non-exercised muscle (e.g., McDonnell et al. 2013; Singh et al. 2014b; Garnier et al. 2017). As a surrogate of neuroplasticity, amplitude of motor-evoked potential (MEP) evoked by single-pulse transcranial magnetic stimulation (TMS) tested the excitability of the corticospinal pathway (Davranche et al. 2015). Locomotor exercise of moderate intensity (60–70% maximal heart rate) induced neuroplastic changes that are not restricted to exercised muscles, and can increase corticospinal excitability of non-exercised muscle (McDonnell et al. 2013; Garnier et al. 2017). These increases in non-exercised muscles could be due in part to an increase in blood neurotrophins or hormone concentration during exercise that may favour synaptic plasticity (Rojas Vega et al. 2006). Exercise could also change cortico-cortical functional connectivity between the primary motor area and the supplementary motor cortex area or premotor areas reducing, thus, cortical inhibition (Byblow et al. 2007). In addition, locomotor exercise can modulate the responsiveness to non-invasive brain stimulation protocol applied after exercise. For instance, locomotor exercise can either facilitate the response of a stimulation protocol aiming to increase corticospinal excitability (Singh et al. 2014b; Garnier et al. 2017), or reverse the effect of a protocol aiming to depress corticospinal excitability (McDonnell et al. 2013) of a non-exercised hand muscle. Interestingly, exercise-induced corticospinal changes may depend upon exercise intensity (McDonnell et al. 2013) and exercise modality (e.g., uphill versus downhill treadmill exercise) (Garnier et al. 2017).
The effect of a single bout of aerobic exercise on a subsequent stimulation protocol is believed to depend upon the principle of metaplasticity whereby the excitability of a synaptic network conditioned the responsiveness to a subsequent protocol (Abraham and Bear 1996). Downhill treadmill exercise differed from uphill one by the different knee extensors modes of muscle contraction involved during exercise. Indeed, while knee extensors would predominantly act under an eccentric mode during downhill, uphill locomotion would mainly involve these muscles under a concentric mode (Abe et al. 2011). From a neural point of view, downhill exercise is characterized by greater cortical activities from the sensorimotor and supplementary motor areas compared to uphill exercise (Mazerie et al. 2012). This increased activity may result from the greater control required for eccentric contraction (Fang et al. 2004), or the processing of a greater amount of sensory afferent feedback from muscles conveyed to the brain during eccentric contraction (Fang et al. 2001). The paired-pulse TMS technique called short-interval cortical inhibition (SICI), providing insights about intracortical inhibition and more precisely the GABAA-ergic inhibition (McDonnell et al. 2006), is of interest to examine whether intracortical mechanisms could be modulated following locomotor exercise. For instance, using this technique, Singh and colleagues showed that cycling exercise modulated the balance between cortical facilitation and inhibition in a non-exercised hand muscle, in favour of a weaker inhibition (Singh et al. 2014a).
Corticospinal excitability change induced by locomotor exercise has been conducted also on exercised muscles. Particularly, changes occurring in the knee extensors have been investigated following cycling (Goodall et al. 2012; Fernandez-del-Olmo et al. 2013; Girard et al. 2013; Jubeau et al. 2014; O’Leary et al. 2015) and running protocols (Temesi et al. 2014). After exercise, corticospinal excitability of the knee extensors was increased (Fernandez-del-Olmo et al. 2013; Jubeau et al. 2014; Temesi et al. 2014) or unchanged (Goodall et al. 2012; Girard et al. 2013; O’Leary et al. 2015) when tested in active muscle. Consistently, severe-intensity exercise changed intracortical inhibition in the knee extensors, reducing the cortical silent period while increasing SICI (O’Leary et al. 2015), while moderate-intensity exercises induced no changes (Goodall et al. 2012; Fernandez-del-Olmo et al. 2013; Girard et al. 2013; Jubeau et al. 2014; Temesi et al. 2014; O’Leary et al. 2015). However, if the mode of muscle contraction modulates corticospinal excitability change in a non-exercised muscle following a locomotor exercise (Garnier et al. 2017), not is known on this effect for exercised muscles.
To date, it is well known that downhill walking (Mazerie et al. 2012) and isolated eccentric contractions (Fang et al. 2004) induce specific cortical activations; however, the effect of the mode of muscle contraction on corticospinal excitability changes following a locomotor exercise remains unknown. Within this context, this study aimed to determine whether changes in corticospinal excitability and cortical inhibition depend upon the mode of muscle contraction primarily involved in a locomotor exercise. We hypothesized that the higher cortical activity reported during downhill walking would increase corticospinal excitability, and reduced SICI in the knee extensors due to greater modulation of intracortical excitability.
Materials and methods
Participants
Eleven healthy volunteers (all male; age: 26.3 ± 6.8 years; mass: 73.5 ± 3.4 kg; height: 180.7 ± 5.0 cm; VO2max 57.9 ± 5.6 ml min−1 kg−1) with no history of neurological disorders or injuries were recruited for this study. Subjects were instructed not to perform any vigorous physical exercise or consume caffeine or nicotine the day before each session. All participants provided signed informed consent prior to the experimentation and were asked to declare if they were taking any medication or had any acute illness or injury. The study was approved by the French ethics committee (ClinicalTrials.gov Identifier: NCT03334526) and was in compliance with the standards set by the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (2008).
Experimental protocol
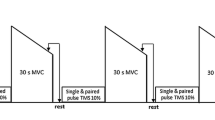
An overview of the experimental protocol is presented in Fig. 1. Participants attended the laboratory on 3 separate days divided into a familiarization session for the first visit and experimental sessions for the two subsequent ones. A time delay ranging from 96 h to 2 week separated sessions. The first session was devoted to determining the participants’ maximal oxygen uptake during an incremental treadmill test (see Garnier et al. 2018 for more details on the incremental treadmill test). Following completion of the incremental test, participants familiarized themselves with maximal voluntary isometric contractions as well as with transcranial magnetic stimulation and peripheral nerve stimulation protocols. During the subsequent two visits, participants performed a 45-min walking exercise in an uphill or downhill condition matched for speed (see “Experimental treadmill exercise” for more details). Corticospinal excitability, intracortical inhibition and neuromuscular function of the knee extensors were investigated before and after exercise (see “Peripheral nerve stimulation” and “Transcranial magnetic stimulation” for more details).
Overview of the experimental protocol. Each exercise condition followed the same experimental timeline involving neuromuscular assessment before (PRE), immediately after (POST) and 30 min after (POST30) completion of the exercise. The uphill and downhill conditions were performed at the same speed. CSP cortical silent period, Dt100 Hz electrical doublet at 100 Hz, MEP motor-evoked potential, MVIC maximal voluntary isometric contraction of the knee extensor muscles, PNS peripheral nerve stimulation, Pt single-pulse electrical stimulation, SICI short-interval cortical inhibition, TMS transcranial magnetic stimulation
Torque and electromyographic recordings
Corticospinal and neuromuscular measurements were ascertained on an isokinetic dynamometer (System pro 4, Biodex Medical System, New York). Participants sat with a 90° hip angle, the right leg positioned at 70° of flexion (0° = fully extended), and the knee joint axis aligned with the dynamometer axis. The lever arm was attached 2 cm above the malleolus with a non-compliant strap. To avoid upper body movement, participants were attached on the trunk with non-compliant strap. Torque signal was recorded at a sampling rate of 1000 Hz using the Biopac MP150 system (Biopac System, Santa Barbara, CA). To ensure reliability of the measurements across sessions, the ergometer’s participant settings were kept constant between sessions.
Electromyographic (EMG) activity of the vastus lateralis (VL) and the rectus femoris (RF) muscle were recorded with pairs of pre-gelled Ag/AgCl surface electrodes (recording diameter of 10 mm, centre-to-centre distance 20 mm; Mini KR, Controle Graphique S.A., Brie-Comte-Robert, France) placed over the muscle belly. The location of electrodes was marked on the skin with the indelible ink used to ensure the same stimulation position across the session. The reference electrode was set on the contralateral patella. EMG activity was recorded with a Biopac MP150 system at a sampling rate of 2000 Hz, bandpass filtered (10 Hz to 500 Hz), and stored for analysis with commercially available software (AcqKnowledge 4.2 for MP systems, Biopac System, Santa Barbara, CA).
Maximal voluntary isometric contraction and activation level
Assessment of knee extensor neuromuscular function was performed at the onset of the experimental sessions. The maximal voluntary isometric contraction (MVIC) peak torque of the knee extensors was measured during a 5-s trial performed before (PRE), 1.5 min after (POST), and 30 min after (POST30) completion of the exercise. The time-delay at POST was required to install the subject and ensure reliable recordings on the dynamometer after exercise, and was kept constant between participants and sessions. Before MVIC at PRE, subjects performed a specific warm-up including isokinetic knee extensor contractions at different angular velocities (from + 120° to − 60° s−1) and intensities (60–100% of the participant’s estimated maximal intensity). Finally, two 5-s near-maximal (~ 90% of the participant’s estimated maximal intensity) isometric contractions were performed. MVIC at PRE was defined as the best performance obtained on two consecutive 5-s trials with 1 min rest in between. An additional trial was performed as long as MVIC increased over 5% from the precedent best trial. Only one trial was performed to determine MVIC at POST and POST30. During MVIC, participants were verbally encouraged and a visual feedback of the current torque produced was displayed in front of the subjects.
The maximal voluntary activation level of the knee extensors (VAL) was calculated before and after exercise by comparing the mechanical response evoked by a 100 Hz doublet superimposed on the MVIC (Dt100 Hzsup) by the response evoked by a potentiated 100 Hz doublet applied after MVIC (Dt100 Hz) (see Fig. 1) using the following equation:
The correction proposed by Strojnik and Komi (1998) including the value of the torque at the moment of the stimulation was used if the superimposed doublet was applied slightly before or after the maximal torque.
Peripheral nerve stimulation
Knee extensors’ neuromuscular function was assessed with transcutaneous stimulations over the femoral nerve (pulse width 200 µs) using a high-voltage constant-current stimulator (model DS7AH, Digitimer Ltd., Hertfordshire, UK). The same experienced experimenter pressed a monopolar cathode-ball electrode (0.5 cm diameter) into the femoral triangle. The anode (10 × 5 cm rectangular electrode) was placed on the gluteus maximus opposite to the cathode. To determine stimulation intensity, pulse amplitude was increased until the peak twitch force and the M-wave area of the muscles under investigation plateaued. Intensity was further increased by 20% for measurements and kept constant within the same session. Single and doublet (100 Hz) pulses were applied at rest in a randomized order across sessions, with a 5-s interval between stimulations. Peak twitch torque (Pt) of the single (Pt1) and doublets (Pt100) was measured off-line. M-wave area was used for further normalizations.
Transcranial magnetic stimulation
Corticospinal excitability of the knee extensors was investigated using single and paired TMS pulses applied on the contralateral motor cortex area. For all the TMS procedures, the VL muscle was targeted to define stimulation site and stimulation intensity. Pulses were delivered through a double-coned coil orientated to induce a posterior-to-anterior current flow using two magnetic stimulators Magstim 2002 connected to a BiStim module (Magstim, Whitland, Dyfed, UK). The coil was held by the same experimenter throughout the session. Ten single and ten paired pulses were recorded at PRE, POST, and POST30 during a brief (~ 3-s) weak knee extensor contraction corresponding to 20% MVIC performed at PRE, with a 5-s interval between stimulations. At the onset of each experimental session, the optimal stimulation site was identified and marked on the scalp. The active motor threshold (AMT) was assessed and defined as the lowest stimulator intensity producing a distinguishable MEP observable from the background EMG, followed by an EMG cancellation phase in three consecutive trials. Then, stimulus intensity was increased by 5% increments of the maximal stimulator output until MEP amplitude plateaued.
The stimulus intensity of single-pulse TMS was defined as the minimal intensity that evoked an MEP amplitude corresponding to 50% of the maximal amplitude determined previously. This intensity was adjusted at PRE and kept constant for POST and POST30 measurements within the same session. For analysis, MEP area was normalized to the M-wave area recorded at the same time-point. The RMS-EMG activity preceding the stimulation was calculated over a 100-ms window for each muscle and normalized to the M-wave RMS of the corresponding time-point (RMS-EMGTMS). Cortical silent period (CSP) was determined manually as the duration between the stimulus artifact and the reappearance of a continuous EMG after the MEP. To ensure an accurate assessment of CSP, subjects were asked to maintain the desired force level after the TMS pulse.
Short-interval cortical inhibition (SICI) was measured in the knee extensors at PRE, POST, and POST30 using a paired-pulse TMS protocol. A sub-threshold conditioning stimulus (CS; 90% AMT) followed by a supra-threshold testing (TS) stimulus were applied with a 3-ms inter-stimulus interval (O’Leary et al. 2015). At PRE, the experimenter adjusted the intensity of the TS so that obtained a conditioned MEP amplitude equal to 50% of the amplitude of the single-pulse MEP. This SICI protocol has previously been showed to provide and excellent reliability (Temesi et al. 2017). The intensities of the CS and the TS were then kept constant at POST and POST30 within the same session. SICI was determined as the mean MEP area from the ten conditioned pulses and expressed relative to the mean area of the ten non-conditioned MEP. A ratio inferior at 100% denotes an inhibition of the conditioned MEP as function of the non-conditioned MEP, with an increase of the ratio mirroring a weaker inhibition.
Experimental treadmill exercise
To investigate the effect of the mode of muscle contraction, the two different conditions were matched for walking speed, duration, and absolute slope. These conditions would ensure a similar amount of total work performed at the end of each exercise (Alexander et al. 2017), and avoid a confounding effect of different magnitude neuromuscular fatigue between conditions (Garnier et al. 2019). Considering the greater energy cost of uphill locomotion compared to downhill (Minetti et al. 2002), the speed of the uphill condition was always considered as the reference speed. Consequently, all participants performed first the uphill condition and then the downhill condition. The speed of the uphill condition was adjusted throughout the exercise to maintain 75% of the heart rate reserve (HRR), calculated in accordance with Karvonen’s formula (Karvonen and Vuorimaa 1988). Heart rate during exercise was recorded every second using a heart rate monitor (Polar RS300; Polar Electro Oy, Kempele, Finland). The speed recorded every 2 min during the uphill condition was then replicated during the downhill exercise. Consequently, the experimenter adjusted the treadmill speed throughout each exercise to maintain either the target heart rate or the speed:
The constant “a” was 0.5 for the warm-up and 0.75 for the 45-min exercise.
Before the walking exercise, subjects performed a 10-min warm-up with 6 min of cycling at 50% HRR followed by 4 min of treadmill walking. During the 4 min of the warm-up, treadmill speed was adjusted to fit with either targeted heart rate (for uphill) or speed (for downhill). The slope during the warm-up was also adjusted by increasing (uphill) or decreasing (downhill) the slope by step of 5% at 1.5 min, 2.5 min, and 3.5 min (from 0 to ± 15%). Then, subjects performed a 45-min treadmill walking exercise with either + 15% (uphill), or − 15% slope (downhill) (see Fig. 1). These slopes were shown to increase specifically the concentric or eccentric part of the stretch–shortening cycle of the knee extensors during uphill or downhill locomotion, respectively (Minetti et al. 2002).
Statistical analysis
All data are presented as mean ± standard deviation (SD) in text and tables, and as mean ± 95% confidence interval (CI) in figures. The nature of the distribution was assessed for all variables using the Shapiro–Wilk test. A logarithmic transformation was applied to the data that violated the assumption of normality to ensure the relevant use of parametric testing. Sphericity was checked as appropriate and a Greenhouse–Geisser correction to the degree of freedom was applied when sphericity was violated. A Student t test for paired samples was used to determine significant differences between average heart rate during exercise. A two-way repeated-measures ANOVA tested the effect of condition (uphill vs downhill) and time (PRE, POST, and POST30) on corticospinal (MEP area, CSP duration, and SICI) and neuromuscular (MVIC, VAL, RMS-EMGTMS, M-wave area, Pt1, and Pt100) parameters. When significant, main effects and condition × time interaction were followed up with Tukey’s HSD tests. When interaction was significant, relevant simple main effects are reported. Effects size are reported as partial eta squared (\(\eta_{\text{p}}^{2}\)) and Cohen’s dz. Statistical analyses were performed with Statistica (StatSoft France, version 7.1, STATISTICA) and G*Power 3.1. The significance level was set at 0.05 (two-tailed) for all the analyses.
Results
Treadmill exercise
The walking exercises were performed at the same speed (4.3 ± 0.4 km h−1). The t test showed a significant difference of the average heart rate recorded during exercise (p <0.001; t = 14.86). Heart rate recorded during the uphill condition (159 ± 8 bpm) was, on average, greater than the downhill condition (99 ± 16 bpm; p <0.001; dz=4.480).
Neuromuscular function
Maximal voluntary isometric contraction and voluntary activation level
ANOVA revealed a significant time effect for the MVIC of the knee extensors (p <0.001; \(\eta_{\text{p}}^{2}\) = 0.605), with neither condition (p =0.171; \(\eta_{\text{p}}^{2}\) = 0.179), nor condition × time interaction (p =0.847; \(\eta_{\text{p}}^{2}\) = 0.016). MVIC decreased significantly from PRE (279.9 ± 51.6 N m) to POST (255.4 ± 46.2 N m) and POST30 (263.6 ± 49.7 N m) (all p < 0.015; all dz>1.140). No difference occurred between POST and POST30 (p = 0.061; dz = 0.659). A main effect of time was observed for VAL (p < 0.001; \(\eta_{\text{p}}^{2}\) = 0.518), with a significant decrease from PRE (85.5 ± 8.7%) to POST (80.4 ± 8.5%; p < 0.001; dz=0.554), but no difference between PRE and POST30 (83.7 ± 6.9%; p = 0.259; dz=0.176). VAL significantly increased between POST and POST30 (p = 0.002; dz=0.886). Neither main effect of condition (p = 0.240; \(\eta_{\text{p}}^{2}\) = 0.135), nor condition × time interaction (p = 0.483; \(\eta_{\text{p}}^{2}\) = 0.070) was observed for VAL.
Peripheral nerve stimulation
ANOVA showed a significant effect of time (p < 0.001; \(\eta_{\text{p}}^{2}\) = 0.519) for the M-wave area of the VL muscle, but no main effect of condition (p = 0.453; \(\eta_{\text{p}}^{2}\) = 0.057) or condition × time interaction (p = 0.407; \(\eta_{\text{p}}^{2}\) = 0.086). The area decreased significantly from PRE (0.092 ± 0.048 mV s) to POST (0.081 ± 0.044 mV s) (p < 0.001; dz=0.189) and POST30 (0.082 ± 0.043 mV s) (p < 0.001; dz=0.176). No difference was observed between POST and POST30 (p = 0.501; dz = 0.091). ANOVA reported a main effect of condition for the M-wave area of the RF muscle (p = 0.011; \(\eta_{\text{p}}^{2}\) = 0.490). M-wave area was lower in the uphill (0.019 ± 0.007 mV s) compared to the downhill condition (0.028 ± 0.009 mV s) (p = 0.011; dz = 0.696).
A follow-up test on the condition × time interaction of Pt1 and Pt100 (all p < 0.020; all \(\eta_{\text{p}}^{2}\) > 0.323) revealed that the downhill condition reduced peak twitches from PRE (Pt1 = 57.1 ± 8.7 N m; Pt100 = 93.9 ± 11.8 N m) to POST (Pt1 = 47.7 ± 8.9 N m; Pt100 = 84.2 ± 12.7 N m; all p < 0.001; all dz>1.396), and POST30 (Pt1 = 55.2 ± 9.5 N m; Pt100 = 83.6 ± 11.4 N m; all p < 0.001; all dz>1.513). No change of Pt1 and Pt100 occurred at POST and POST30 in the uphill condition (all p >0.093; all dz<0.817).
Transcranial magnetic stimulation
Change in muscular activity level
Normalized muscle activity (RMS-EMGTMS) of the knee extensors was measured during the TMS recordings performed at 20% MVIC. For the VL muscle, ANOVA showed a significant condition × time interaction for the RMS-EMGTMS (p = 0.029; \(\eta_{\text{p}}^{2}\) = 0.297). The normalized muscle activity was lower at POST in the uphill compared to downhill condition (p = 0.001; dz = 0.755). Normalized muscle activity did not change for the VL muscle from PRE to POST or POST30. ANOVA showed neither significant main effect of time or condition (all p >0.123; all \(\eta_{\text{p}}^{2}\) < 0.221), nor condition × time interaction (p = 0.166; \(\eta_{\text{p}}^{2}\) = 0.164) for the normalized muscle activity of the RF muscle.
Corticospinal excitability
Figure 4 shows EMG traces recorded for the RF and VL muscles following TMS for a representative subject. ANOVA showed a significant condition × time interaction (p = 0.038; \(\eta_{\text{p}}^{2}\) = 0.278) for the normalized MEP area of the VL muscle (see Fig. 2a). In the downhill condition, normalized MEP area increased from PRE to POST and POST30 (all p < 0.008; all dz>0.885) with no difference between POST and POST30 (p = 0.936; dz = 0.263). No difference occurred for the uphill condition (all p >0.759; all dz<0.314). ANOVA showed neither significant main effect of time or condition (all p >0.192; all \(\eta_{\text{p}}^{2}\) < 0.163), nor condition × time interaction (p = 0.058; \(\eta_{\text{p}}^{2}\) = 0.294) for the normalized MEP area of the RF muscle (see Fig. 2b).
Normalized MEP area and cortical silent period of the vastus lateralis (a, c), and rectus femoris muscles (b, d) recorded before (PRE), immediately (POST) and 30 min after (POST30) the completion of the exercise (N = 11; mean ± 95% CI). *A significant difference from the PRE value within the same condition. **p < 0.01; ***p < 0.001. aA significant condition effect (p < 0.05)
Cortical silent period
ANOVA showed neither significant main effect of time or condition (all p >0.155; all \(\eta_{\text{p}}^{2}\) < 0.191), nor condition × time interaction (p = 0.185; \(\eta_{\text{p}}^{2}\) = 0.165) for the CSP duration of the VL muscle (see Fig. 2c). For the RF muscle, the ANOVA revealed a significant main effect of the condition (p = 0.038; \(\eta_{\text{p}}^{2}\) = 0.383), with a greater CSP duration for the uphill compared to the downhill condition (p = 0.319; dz = 0.751; see Fig. 2d). Neither main effect of time (p = 0.289; \(\eta_{\text{p}}^{2}\) = 0.116), nor a condition × time interaction (p = 0.886; all \(\eta_{\text{p}}^{2}\) = 0.003) was observed for the CSP duration of the RF muscle.
Short-interval cortical inhibition
For both VL and RF muscles, ANOVA showed neither main effect of time (p >0.353; \(\eta_{\text{p}}^{2}\) < 0.098), condition (p >0.104; \(\eta_{\text{p}}^{2}\) < 0.242), nor condition × time interaction (p >0.094; \(\eta_{\text{p}}^{2}\) < 0.215) on SICI change (see Fig. 3).
Discussion
This study investigated whether corticospinal changes induced by treadmill exercise depended upon the mode of muscle contraction of the knee extensors. To this purpose, corticospinal excitability and intracortical inhibition were examined in the VL and RF muscles following uphill versus downhill walking exercise performed at the same speed for the same duration. The results showed that MEP area of the VL muscle increased after downhill walking but did not change after the uphill walking. MEP area of the RF muscle was not affected by exercise. Both CSP duration and SICI remained unchanged following walking exercises.
Neuromuscular fatigue
A similar reduction in the MVIC torque occurred after exercises, performed at the same speed but different slopes, at POST (~ − 9%) and POST30 (~ − 6%) evidencing a similar level of neuromuscular fatigue in the two conditions. This MVIC reduction was accompanied at POST by a reduction of the VAL (~ − 5%), recovering then to PRE value at POST30. Since MVIC remained reduced at POST30 in spite of a VAL recovery, peripheral alterations were involved in the delayed loss of torque following exercise. The reduction in peak twitch torque evoked by single and doublet (100 Hz) stimulations at POST and POST30 in the downhill condition revealed an impairment of the excitation–contraction coupling process (Girard et al. 2013). Peak twitches torque of the single and doublet stimulations were unchanged following the uphill exercise. This finding may reflect a postactivation potentiation related to an increase of the muscle–tendon stiffness (Lazzer et al. 2015), or a greater sensitivity of the Ca2+ with contractile proteins (Babault et al. 2008).
The present finding suggested that walking exercises performed at different slopes (+ 15% vs − 15%) but for the same duration (45 min) and at the same speed induced a similar level of neuromuscular fatigue. However, if the voluntary drive of the knee extensors was reduced to the same extent after each exercise, contractile properties were only impaired in the downhill condition. In accordance with isolated muscle contractions (Garnier et al. 2019), the present results suggest that the mode of muscle contraction did not modulate the amount of neuromuscular fatigue of different walking exercises performed at a similar amount of absolute work (Alexander et al. 2017), but might impact its etiology.
Corticospinal excitability changes following exercise
Figure 4 depicts the EMG responses in the investigated muscles to TMS in one representative subject. Immediately after and 30 min after downhill exercise, MEP area increased in the vastus lateralis muscle, while no change occurred after the uphill condition. The way a prolonged locomotor exercise affects corticospinal excitability in contracting muscles remains debated, being either unchanged (Goodall et al. 2012; Girard et al. 2013; O’Leary et al. 2015) or increased (Fernandez-del-Olmo et al. 2013; Jubeau et al. 2014; Temesi et al. 2014). The previous authors proposed that exercise duration (Temesi et al. 2014), or intensity (O’Leary et al. 2015) may represent likely factors involved in these changes. Our findings showed, for the first time, that the type of locomotor exercise (uphill versus downhill), and, consequently, the mode of muscle contraction (concentric versus eccentric), modulates corticospinal excitability changes. In the present study, TMS was applied during brief submaximal knee extensor contractions corresponding to 20% MVIC recorded at PRE. The muscle activity during submaximal knee extensors contraction did not change after exercise, and thus, MEP area increase could not be explained by EMG activity change with muscle fatigue (Goodall et al. 2009). Contrary to the VL muscle, no corticospinal excitability change occurred for the RF muscle. Specific corticospinal changes for two synergistic muscles have already been reported between the gastrocnemius and the soleus muscles (Duclay et al. 2011). The greater contribution of the RF muscle during downhill walking (Franz and Kram 2012) associated with its greater fatigability (Akima and Saito 2013) would, thus, probably reduce spinal excitability of the RF muscle (Racinais et al. 2007), masking the increase of corticospinal excitability reported in the VL muscle.
The previous findings from Mazerie et al. (2012) reported greater cortical activations during downhill compared to uphill walking. One likely explanation for this behaviour is related to the greater cortical demands necessary to control locomotor patterns compared to a more common task as uphill walking (Kurz et al. 2012). On the other hand, downhill walking primarily involves the knee extensors under the eccentric mode of muscle contraction, which was showed to increase cortical activity as a consequence of the great magnitude of afferent feedback elicited from lengthening muscles (Fang et al. 2001). Interestingly, afferent input from contracting muscles is a powerful factor increasing excitability of the primary motor cortex area, and affecting intracortical connectivity between this area and the primary sensory cortex area (Schabrun et al. 2012). To further determine whether intracortical connectivity would be affected by the mode of muscle contraction, intracortical inhibition was also assessed in the knee extensors after exercise.
Cortical inhibition changes following exercise
Whatever the walking condition, CSP duration did not change following the exercise for both RF and VL muscles. In accordance with the previous studies that also investigated CSP changes after locomotor exercise (Goodall et al. 2012; Fernandez-del-Olmo et al. 2013; Girard et al. 2013; Jubeau et al. 2014), our findings suggested that the cortical GABAB inhibition was unchanged after walking exercise. Sidhu and colleagues showed that the apparent increase of cortical inhibition that occurred during a moderate-intensity cycling exercise recovered quickly at exercise cessation (Sidhu et al. 2013). Thus, one may argue that the absence of CSP change may relate to the delay of measurement (~ 1.5 min) at exercise cessation. Another explanation could stand for an insufficient exercise intensity to observe persistent changes after exercise, since one previous study showed that severe-intensity, but not a moderate-intensity cycling exercise, reduced CSP duration (O’Leary et al. 2015). In addition to single pulse, SICI was performed using paired-pulse TMS to investigate whether exercise modulated intracortical inhibition. Our findings showed no change in SICI for both the VL and the RF muscles after uphill or downhill exercise.
The stability of the CSP and of the SICI of the VL muscle after the downhill exercise occurred along with a rise in MEP area. In the present study, the intensities used for single- and paired-pulse TMS at POST and POST30 were the same as used at PRE. Both the duration of the CSP (Orth and Rothwell 2004) and the magnitude of SICI (Lackmy and Marchand-Pauvert 2010; Temesi et al. 2017) increase with increasing MEP area. Thus, increasing MEP area in the VL muscle at POST and POST30 in the downhill condition would have led to increase CSP and to increase SICI. However, the lack of change of the CSP duration, and that in the ratio between the conditioned and the non-conditioned MEP area for the VL muscle suggest a decrease in intracortical inhibition after downhill exercise. These findings are in accordance with the reduction of SICI after fatiguing concentric (Benwell et al. 2006) or eccentric contractions, while MEP remained stable (Pitman and Semmler 2012), or the unchanged CSP in the presence of an increased MEP amplitude (Temesi et al. 2014). However, they denote from the increase in SICI with unchanged MEP amplitude following a severe-intensity cycling exercise (O’Leary et al. 2015). Considering that only the downhill condition impaired the contractile properties of the knee extensors in the present study, the apparent decrease in cortical inhibition in this condition would likely reflect a specific strategy aiming to facilitate the selective activation of the muscles involved in the action (Ortu et al. 2008). Consistently with the previous findings (O’Leary et al. 2015), the absence of clear intracortical inhibition change may result from an insufficient exercise intensity used in the present study.
Conclusion
The present study evidences that the knee extensors mode of muscle contraction primarily involved during a walking exercise can modulate both corticospinal excitability and intracortical inhibition after exercise. Specifically, the eccentric mode of muscle contraction during downhill walking increased corticospinal excitability, and, although not clearly evidenced, may reduce intracortical inhibition. These results suggest that the neural control of muscular contraction during a walking exercise, including cortical activities from movement preparation and execution and/or peripheral afferent feedback from muscles, is a potential factor for neuroplastic changes.
References
Abe D, Fukuoka Y, Muraki S, Yasukouchi A, Sakaguchi Y, Niihata S (2011) Effects of load and gradient on energy cost of running. J Physiol Anthropol 30:153–160
Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19:126–130
Akima H, Saito A (2013) Activation of quadriceps femoris including vastus intermedius during fatiguing dynamic knee extensions. Eur J Appl Physiol 113:2829–2840
Alexander N, Strutzenberger G, Ameshofer LM, Schwameder H (2017) Lower limb joint work and joint work contribution during downhill and uphill walking at different inclinations. J Biomech 61:75–80
Babault N, Maffiuletti NA, Pousson M (2008) Postactivation potentiation in human knee extensors during dynamic passive movements. Med Sci Sports Exerc 40:735–743
Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW (2006) Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res 170:191–198
Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Muller JFM, Ziemann U (2007) Functional connectivity between secondary and primary motor areas underlying hand-foot coordination. J Neurophysiol 98:414–422
Davranche K, Temesi J, Verges S, Hasbroucq T (2015) Transcranial magnetic stimulation probes the excitability of the primary motor cortex: a framework to account for the facilitating effects of acute whole-body exercise on motor processes. J Sport Heal Sci 4:24–29
Duclay J, Pasquet B, Martin A, Duchateau J (2011) Specific modulation of corticospinal and spinal excitabilities during maximal voluntary isometric, shortening and lengthening contractions in synergist muscles. J Physiol 589:2901–2916
Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH (2001) Greater movement-related cortical potential during human eccentric versus concentric muscle contractions. J Neurophysiol 86:1764–1772
Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH (2004) Distinct brain activation patterns for human maximal voluntary eccentric and concentric muscle actions. Brain Res 1023:200–212
Fernandez-del-Olmo M, Rodriguez FJ, Marquez G, Iglesias X, Marina M, Benitez A, Vallejo L, Acero R (2013) Isometric knee extensor fatigue following a Wingate test: peripheral and central mechanisms. Scand J Med Sci Sport 23:57–65
Franz JR, Kram R (2012) The effects of grade and speed on leg muscle activations during walking. Gait Posture 35:143–147
Garnier YM, Lepers R, Stapley PJ, Papaxanthis C, Paizis C (2017) Changes in cortico-spinal excitability following uphill versus downhill treadmill exercise. Behav Brain Res 317:242–250
Garnier YM, Lepers R, Dubau Q, Pageaux B, Paizis C (2018) Neuromuscular and perceptual responses to moderate-intensity incline, level and decline treadmill exercise. Eur J Appl Physiol 118:2039–2053
Garnier YM, Paizis C, Lepers R (2019) Corticospinal changes induced by fatiguing eccentric versus concentric exercise. Eur J Sport Sci 19:166–176
Girard O, Bishop DJ, Racinais S (2013) Neuromuscular adjustments of the quadriceps muscle after repeated cycling sprints. PLoS One 8:1–9
Goodall S, Romer LM, Ross EZ (2009) Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp Physiol 94:995–1004
Goodall S, González-Alonso J, Ali L, Ross EZ, Romer LM (2012) Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590:2767–2782
Jubeau M, Rupp T, Perrey S, Temesi J, Wuyam B, Levy P, Verges S, Millet GY (2014) Changes in voluntary activation assessed by transcranial magnetic stimulation during prolonged cycling exercise. PLoS One 9:1–10
Karvonen J, Vuorimaa T (1988) Heart rate and exercise intensity during sports activities. Practical application. Sport Med 5:303–311
Kurz MJ, Wilson TW, Arpin DJ (2012) Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 59:1602–1607
Lackmy A, Marchand-Pauvert V (2010) The estimation of short intra-cortical inhibition depends on the proportion of spinal motoneurones activated by corticospinal inputs. Clin Neurophysiol 121:612–621
Lazzer S, Salvadego D, Taboga P, Rejc E, Giovanelli N, Di Prampero PE (2015) Effects of the Etna uphill ultramarathon on energy cost and mechanics of running. Int J Sports Physiol Perform 10:238–247
Mazerie J, Bourdillon N, Derosiere G, Alexandre F, Perrey S (2012) Larger brain oxygenation changes during downhill than uphill walking. Fundam Clin Pharmacol 26:61
McDonnell MN, Orekhov Y, Ziemann U (2006) The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173:86–93
McDonnell MN, Buckley JD, Opie GM, Ridding MC, Semmler JG (2013) A single bout of aerobic exercise promotes motor cortical neuroplasticity. J Appl Physiol 114:1174–1182
Minetti AE, Moia C, Roi GS, Susta D, Ferretti G (2002) Energy cost of walking and running at extreme uphill and downhill slopes. J Appl Physiol 93:1039–1046
O’Leary TJ, Morris MG, Collett J, Howells K (2015) Central and peripheral fatigue following non-exhaustive and exhaustive exercise of disparate metabolic demands. Scand J Med Sci Sports 26:1287–1300
Orth M, Rothwell JC (2004) The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol 115:1076–1082
Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC (2008) Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586:5147–5159
Pitman BM, Semmler JG (2012) Reduced short-interval intracortical inhibition after eccentric muscle damage in human elbow flexor muscles. J Appl Physiol 113:929–936
Racinais S, Girard O, Micallef JP, Perrey S (2007) Failed excitability of spinal motoneurons induced by prolonged running exercise. J Neurophysiol 97:596–603
Rojas Vega S, Strüder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W (2006) Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res 1121:59–65
Schabrun SM, Ridding MC, Galea MP, Hodges PW, Chipchase LS (2012) Primary sensory and motor cortex excitability are co-modulated in response to peripheral electrical nerve stimulation. PLoS One 7:1–7
Sidhu SK, Lauber B, Cresswell AG, Carroll TJ (2013) Sustained cycling exercise increases intracortical inhibition. Med Sci Sports Exerc 45:654–662
Singh AM, Duncan RE, Neva JL, Staines WR (2014a) Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci Med Rehabil 6:23–33
Singh AM, Neva JL, Staines WR (2014b) Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Exp Brain Res 232:3675–3685
Strojnik V, Komi PV (1998) Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol 84:344–350
Temesi J, Rupp T, Martin V, Arnal PJ, Verges S, Millet GY (2014) Central fatigue assessed by transcranial magnetic stimulation in ultratrail running. Med Sci Sport Exerc 46:1166–1175
Temesi J, Ly SN, Millet GY (2017) Reliability of single- and paired-pulse transcranial magnetic stimulation for the assessment of knee extensor muscle function. J Neurol Sci 375:442–449
Acknowledgements
This work was supported by the French Ministry of Higher Education, Research and Innovation and by the Region Bourgogne Franche-Comté (Grant no. 2017-BFCO-SR-P51.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garnier, Y.M., Paizis, C., Martin, A. et al. Corticospinal excitability changes following downhill and uphill walking. Exp Brain Res 237, 2023–2033 (2019). https://doi.org/10.1007/s00221-019-05576-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05576-1