Abstract
Purpose
To examine corticospinal excitability and neuromuscular function following the completion of eccentric (ECC) or concentric (CON) maximal exercises of same mechanical work.
Methods
Ten males (29.9 ± 11.8 years) performed maximal isokinetic knee extensor contractions in four experimental sessions. The two first sessions (one in ECC and one in CON) ended with a dynamic peak torque loss of 20%. The work completed in each contraction type was then achieved in the other contraction type. Neuromuscular function- maximal voluntary isometric contraction (MVIC), voluntary activation level (VAL), potentiated doublet (Dt), M-wave- and corticospinal excitability- motor evoked potential (MEP) amplitude and silent period (SP)—were assessed in the vastus lateralis (VL) and rectus femoris (RF) muscles at 20% MVIC before and immediately after exercise.
Results
To lose 20% of dynamic peak torque subjects performed 1.8 times more work in ECC than CON (P = 0.03), inducing a non-different decline in MVIC (P = 0.15). VAL dropped after the ECC sessions only (− 8.5 ± 6.7%; all P < 0.027). Only, the CON session featuring the greatest work affected Dt amplitude (− 9.4 ± 23.8%; P = 0.047). In both muscles, MEP amplitude decreased (all P < 0.001) and MEP SP stayed constant (all P > 0.45), irrespective of contraction type (all P > 0.15).
Conclusion
Same-work maximal ECC and CON exercises induced similar fatigue level but from different origins (preferentially central for ECC vs peripheral for CON). Yet, net corticospinal excitability did not depend on contraction type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eccentric (ECC) and concentric (CON) contractions are the two types of dynamic muscle actions, both ubiquitous in daily life. ECC refers to an active lengthening of the muscle (e.g., the quadriceps while walking downstairs), whereas CON denotes a shortening of it (e.g., walking upstairs). ECC has been increasingly utilized for rehabilitation purposes thanks to the possibility of developing high levels of force at a low energetic demand it provides (Abbott et al. 1952) while inducing limited muscle damage after a habituation period (LaStayo et al. 2013; Lovering and Brooks 2013).
The impacts of ECC exercise on neuromuscular function are often described in comparison to CON exercise realized at the same torque or mechanical power output, the same heart rate or the same oxygen consumption. The criteria picked largely influence the outcomes of the comparison (Clos et al. 2019). It notably affects exercise-induced neuromuscular fatigue (labeled “fatigue” in this manuscript), defined as an “acute impairment of performance” (Enoka and Stuart 1992), reflected by a loss of maximal force/torque developed during a maximal voluntary isometric contraction (MVIC). For instance, when exercises comprising maximal ECC or CON contractions are matched for a given subsequent MVIC decrease, participants complete more mechanical work in ECC than CON (Souron et al. 2018). Conversely, if the work completed- and torque- is similar in the two contractions types, CON is expected to elicit more MVIC loss than ECC. Fatigue affects other neuro-physiological parameters such as muscle activity assessed via electromyography (EMG; Enoka and Stuart 1992). It is notably accompanied by a depressed corticospinal excitability—reflected by motor evoked potential (MEP) amplitude (Davranche et al. 2015; Goodall et al. 2014)—and a longer absence of EMG signal following a stimulation (silent period—SP; Goodall et al. 2018)—assessed at constant EMG (Martin et al. 2006; McNeil et al. 2011a; Weavil et al. 2016). We hence assume that an ECC task would specifically alter corticospinal excitability and thus the neural command to the muscle.
The presence of differences in the neural command during maximal ECC and CON contractions (Duchateau and Enoka 2016) is another reason to expect particular adjustments of corticospinal excitability following ECC exercise (Goodall et al. 2014). A lower EMG amplitude (Aagaard et al. 1998; Amiridis et al. 1996; Kellis and Baltzopoulos 1998; Komi et al. 2000; Tesch et al. 1990; Westing et al. 1991), and a lower voluntary activation level (Amiridis et al. 1996; Westing et al. 1990) during maximal ECC contractions indicate an incapacity of the voluntary command to fully activate the muscle in untrained subjects. Moreover, while corticospinal excitability is depressed during ECC contractions, authors have reported an enhanced corticospinal excitability, as indicated by a shortened SP (Duclay et al. 2014) and an improved activity of facilitatory neural networks (Howatson et al. 2011).
Löscher and Nordlund (2002) and Latella et al. (2018) compared the effects of single-joint ECC with CON repeated maximal elbow flexions on corticospinal excitability measured after the task. Both found no effect of contraction type on MEP size of agonist muscles. In addition, Löscher and Nordlund (2002) observed unchanged SP after both contraction types, suggesting no modification in corticospinal inhibition. Nevertheless, these similar outcomes appeared after the completion of a likely greater total mechanical work in ECC than CON, since participants carried-out a number of maximal repetitions in each contraction type. Yet, performing more work might depress corticospinal excitability (McNeil et al. 2011a), but also trigger mechanisms enhancing it such as a rise in sympathetic activity (Buharin et al. 2013), or body temperature (Périard et al. 2011). Only in Garnier et al. (2018) did volunteers complete the same work in each knee extensor contraction type. In their study, for a similar MEP depression, SP lasted longer after the CON condition in the rectus femoris muscle (RF), indicating greater corticospinal inhibition following CON but not ECC bouts. Contractions were, however, performed at 80% MVIC, which corresponds to a lower relative intensity in ECC than CON. Then the modifications in corticospinal excitability reported might have concerned a smaller population of motoneurons in ECC (Duchateau and Enoka 2016; Gandevia 2001). Finally, these three studies showed various extent of MVIC loss adding a confound to the comparison of their results: − 13% in Garnier et al. (2018); − 31% in Latella et al. (2018); and − 44% in Löscher and Nordlund (2002).
In this context, the main goal of this study was to shed light on how contraction type (ECC versus CON) affects net corticospinal excitability after the completion of an exercise matched for both mechanical work and intensity. It was also critical to monitor fatigue for the reasons mentioned above. To avoid the confound of the greater neural drive at higher exercise intensities (Lockyer et al. 2018; McDonnell et al. 2013; Weavil et al. 2015) participants realized maximal contractions in all conditions. A familiarization session warranted their ability to genuinely exercise at maximal intensity. We hypothesized that for the same exercise quantity (i.e. same mechanical work) and intensity (set as maximal), contraction type would not significantly affect post-exercise MEP size.
Methods
Participants
Ten healthy males (29.9 ± 11.8 years, 73.9 ± 9.5 kg, 179 ± 5 cm) participated in the study and signed an informed consent form. A medical checkup assured that none of them presented a history of neurological disorder or injury. The study was conducted in accordance with the Declaration of Helsinki (2008).
Protocol design
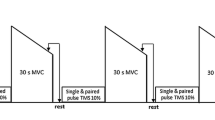
Figure 1 depicts an overview of the experimental protocol. Volunteers visited the laboratory five times: the first session was devoted to familiarizing participants with the experimental procedures and followed 1 week later by the first of four experimental sessions, separated by at least 72 h. Each experimental session started with a 10-min warm-up on a cycle-ergometer followed by ten submaximal CON knee extensions. Then, subjects performed either maximal ECC or CON knee extensor contractions until reaching a targeted dynamic peak torque loss, or a given total mechanical work at the end of exercise. Knee extensor neuromuscular function and corticospinal excitability were assessed before (PRE), and immediately after (POST) the exercise. The participants performed first one of the two sessions targeting a dynamic peak torque loss (randomized order), then carried-out either the same session or completed the same work in the other contraction type (randomized order), and finished with the last session aiming at a given work.
ECCF ECC contractions performed until 20% of dynamic torque loss, CONF CON contraction performed until 20% of dynamic torque loss, ECCW ECC contractions performed until having completed the same work as in CONF, CONW ECC contractions carried-out until having completed the same work as in ECCF, MVIC Maximal voluntary isometric contraction, PNS Peripheral nerve stimulation, VAL Voluntary activation level, Dt Double twitch, MEP Motor evoked potential
Familiarization session
Subjects were familiarized to transcranial magnetic stimulation (TMS), femoral nerve stimulation, maximal voluntary isometric contraction (MVIC, N.m), and notably isokinetic CON and ECC knee extensor contractions. This session served to set the participant’s position on the isokinetic ergometer (System Pro 4, Biodex Medical System, New York, NY), replicated during the experimental sessions. In addition, the participants completed three sets of twelve maximal ECC contractions to trigger a repeated-bout effect (McHugh 2003), allowing a fair comparison between the responses to the subsequent CON and ECC exercise sessions.
Conditions targeting a dynamic peak torque loss
On two separate days, the participants performed two sets of maximal contractions in CON (+ 60° s−1) (CONF) or ECC (− 60° s−1) (ECCF) matched for dynamic peak torque loss. The baseline value was the average of the dynamic peak torques of the three first contractions of each session. The set of contractions terminated when the torque declined by 20% for three contractions in a row.
Conditions targeting a total work
At two other occasions, the subjects completed maximal ECC (ECCW) or CON (CONW) contractions, with the objective to match the total work (J) achieved during CONF and ECCF sessions, respectively.
Measurements
MVIC
The subjects carried-out MVICs at a 70° knee extension angle (0° being complete knee extension). Baseline MVIC was the best peak torque of two trials. A third MVIC was performed if the difference between the two first was more than 5%. One MVIC was performed after each exercise and served to adjust the submaximal contraction force developed during TMS and femoral nerve stimulations.
Dynamic contractions
Participants contracted their right knee extensors on an isokinetic dynamometer (System Pro 4, Biodex Medical System, New York, NY) at an angular speed of 60° s−1 over a 90° range of motion, with 15° below complete knee extension as the upper limit. They were seated with a 90° hip flexion, the knee joint axis in line with the rotation axis of the dynamometer, and the leg strapped to the lever arm 2 cm above the malleoli. In all conditions, the subjects were instructed to execute each contraction at the maximal of their capabilities in an all-out fashion (i.e., regardless of exercise duration).
Electromyography
The experimenter shaved and cleaned the skin above the muscle bellies of the vastus lateralis (VL) and the rectus femoris (RF) muscles with alcohol swabs before placing self-adhesive pre-gelled bipolar electrodes (10 mm) (based on SENIAM guidance, seniam.org), and a reference electrode on the patella of the contralateral limb. EMG and torque signals were sampled at 2 kHz and amplified from 15 Hz to 2 kHz using an MP150 unit and stored for offline analysis in the Acqknowledge 4.2 software (Bipoac Systems Inc., Santa Barbara, CA, USA). EMG RMS (root mean square) at rest was below 0.05 mV.
Peripheral nerve stimulation
Electrical stimulations were administered using a constant-current stimulator (Digitimer DS7, Hertfordshire, UK). The device was connected to a stylus held on the femoral nerve by the experimenter, and to a self-adhesive rectangular anode (10 × 5 cm) on the gluteus maximus muscles. The stimulation intensity used (135 ± 30 mA, 1000 μs) was 120% of that above which higher stimulation intensities elicited no further increase in M-wave amplitude (MMAX) at rest. Our goal was to ensure the recruitment of all the motor units in the muscle studied. One MMAX was recorded during isometric knee extensions at 20% MVIC before and after the exercise of each session.
A superimposed electrical doublet at 167 Hz at the MVIC plateau (Dtsup, 120% of MMAX), was followed 5 s later by a potentiated doublet (Dt) in the relaxed muscle. The voluntary activation level (VAL) was obtained by comparing the mechanical responses to the superimposed and potentiated Dt. The correction of Strojnik and Komi (1998) was applied to include the torque value at the moment of the stimulation (MIVCstim). Behrens et al. (2017) demonstrated the reliability of this formula for the knee extensor muscles.
Transcranial magnetic stimulation
TMS was administered on the motor cortex area contralateral to the knee extensors, using a double-coned coil (Magstim, Whitland, Dyfed, UK) inducing a posterior to anterior direction current flow. The experimenter held the coil and sent all TMS pulses manually during isometric knee extensions corresponding to 20% MVIC (maintained for and separated by about 4 s). A tape was placed on the skull at the location of the hotspot. The active motor threshold was the “lowest TMS intensity that elicited a clearly visible response in at least three of five stimulations” (Sidhu et al. 2014). The stimulator intensity was set so as to obtain an MEP amplitude of about 50% of the maximal raw value (mV) in both the RF and the VL muscles (Garnier et al. 2019). Ten MEP were delivered at PRE and POST, as this number of stimulation is sufficient to provide consistent between-session reliability in MEP method (Cavaleri et al. 2017).
Data analysis
During the exercise
Raw torque data were used to screen for intra-session differences. To account for the baseline gap between ECC and CON, the average peak torque of the three last contractions was expressed as a percentage of the average of the three first contractions.
PRE and POST measurements
The average EMG RMS was calculated over 100 ms around the MVIC peak force and normalized to MMAX amplitude (EMGMAX RMS/MMAX). Along with VAL it indicates modifications beyond the neuromuscular junction (i.e. central fatigue), while changes in Dt (peak value) and MMAX amplitude imply peripheral modifications (Millet and Lepers 2004).
MEP and MMAX amplitudes and areas were measured with a cursor so as to take into account their varying shapes (Martin et al. 2006; Sidhu et al. 2009). As amplitude and area did not change differently, the only amplitude is reported. The average MEP amplitude from each set of ten waves (PRE and POST) was expressed as a percentage of MMAX amplitude to consider modifications in muscle excitability. The background EMG RMS of MMAX and MEP was analyzed over 25 ms before the stimulation artefact (Škarabot et al. 2018), and normalized to MMAX amplitude recorded at 20% MVIC as well. We measured the SP manually from the stimulation artefact to the return of normal EMG (O’Leary et al. 2015), averaged the values from each set of ten waves, and calculated MEP SP/ MEP amplitude ratio (Orth and Rothwell 2004). The SP was scrutinized by analyzing the SP following the MMAX (Cox and Cafarelli 1999) in both VL and RF muscles, in an attempt to gauge changes in spinal inhibitory mechanisms (Taylor et al. 1999). Moreover, we calculated the ratio between the superimposed torques following MEP and MMAX as an indirect indicator of the motoneuronal population recruited.
Statistical analysis
All the data are expressed as mean ± standard deviation unless stated. All the data of an array that were more than two standard deviations below or above the mean value were removed (1.5% of the data). A Shapiro-Wilk test determined whether the data followed a normal distribution, which was the case of dynamic peak torque loss (% start), MVIC, VAL, Dt, MMAX amplitude in the RF muscle, MEP SP, and M-wave SP in the RF muscle. We applied a logarithmic transformation to abnormal data (number of contractions per set, EMG MAX RMS/MMAX ratio, MMAX amplitude in the RF muscle, MEP amplitude, MEP SP/MEP amplitude ratio, MMAX amplitude and MEP background EMG, M-wave SP in the VL muscle, superimposed MEP torque/superimposed MMAX torque ratio). The total work completed and the dynamic peak torque remained abnormal despite a logarithmic transformation. These variables were thus treated using Friedman’s non-parametric ANOVA (analysis of variance), followed-up by Wilcoxon’s matched pairs test. Two-way repeated measures ANOVA served to assess the effect of condition (ECCF, CONW, CONF, ECCW), time (PRE, POST), and interaction (time × condition) on all the parametric variables listed above, except the number of contraction and the average dynamic peak torque. The two latter variables were analyzed using a one-way ANOVA. Tukey’s HSD test followed significant ANOVA results. Greenhouse-Geisser’s correction served to fix the P value when the parametric data did not confirm the assumption of sphericity. Cohen’s dz (2013) completed the P value of non-parametric and followed-up analyses (G*Power software version 3.1.9.4; Kiel University, Kiel Germany). Pearson’s correlation coefficients were computed between Dt loss (N.m) and VAL loss (%), and MVIC loss (N.m) in CON and ECC.
Results
Knee extensor dynamic contractions
Table 1 indicates the number of contractions, the work completed, and the average dynamic peak torque corresponding to each session. To elicit the same dynamic peak torque loss (− 22.2 ± 4.5%, P = 0.96, dz = 0.12) subjects performed 1.8 times more work in ECCF than CONF (P = 0.03; dz = 0.85). When completing a given work, the average dynamic peak torque was greater in ECC than CON (all P < 0.01; all dz > 1.7). The number of contractions was not significantly different between the sessions matched for work (all P > 0.59) despite a medium effect size (all dz < 0.78).
Fatigue during the dynamic exercise
Figure 2 shows the dynamic peak torque loss (% start) throughout all conditions. Dynamic peak torque was higher in ECC than CON at the start of the exercise by 81 ± 49 N.m (P = 0.005; all dz = 1.7), and after the completion of the same work by 100 ± 71.4 N.m (all P < 0.01; all dz > 1.4). The difference was accentuated after the contractions (P < 0.001, dz = 0.82). Dynamic peak torque loss was more pronounced in CON than ECC for the completion of the same work. It was 15.0 ± 14.6% lower in CONW than ECCF (P = 0.02; dz = 1.02), and 13.4 ± 7.0% lower in CONF than ECCW (P < 0.001; dz = 1.92).
The panel a shows the decline in dynamic peak torque from the three first to the three last contractions of the exercise. The values are expressed as a percentage of those of the three first contractions of the first set of the same session. Sessions matched for work are illustrated in the same color (grey versus black). The panel b displays the evolution of the torque with knee angle during one maximal knee extension of a typical subject during an ECC and a CON contraction at the beginning and the end of the exercise. Different from the condition matched for work at a given time: $means P < 0.05; $$means P < 0.01. ECCF ECC contractions performed until 20% of dynamic torque loss, CONF CON contraction performed until 20% of dynamic torque loss, ECCW ECC contractions performed until having completed the same work as in CONF, CONW ECC contractions carried-out until having completed the same work as in ECCF. Work 1 (W1) corresponds to the 10.2 ± 5.3 × 103 J performed in ECCF and CONW, and Work 2 (W2) refers to the 5.8 ± 5.9 × 103 J completed in CONF and ECCW
Fatigue following the exercise
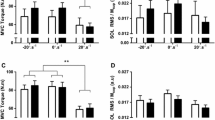
Figure 3 displays the MVIC, VAL and Dt values of all conditions. MVIC decreased with time (P < 0.001; \({\eta }_{p }^{2}\) = 0.70) but showed no interaction effect (P = 0.15; \({\eta }_{p }^{2}\) = 0.13). VAL and Dt showed an interaction effect (all P < 0.02; all \({\eta }_{p }^{2}\) > 0.23). VAL declined by 12.5 ± 14.3% in ECCF (P = 0.002; dz = 0.52), and by 7.5 ± 6.5% (P < 0.027) in ECCW, yet did not change in CONF nor CONW (all P > 0.87; all dz < 0.54). Dt decreased by 16.7 ± 16.4% (P = 0.02; dz = 0.78) in CONW, but remained unchanged in the other conditions (all P > 0.57; all dz < 0.65).
The figure represents the MVIC (maximal isometric contraction) (a), the VAL (voluntary activation level) (b), and the Dt (torque evoked via a double twitch) (c), before (PRE) and after (POST) the exercise. Sessions matched for work are illustrated in the same color (grey versus black). Different from PRE: *means P < 0.05; ***means P < 0.001, ECCF ECC contractions performed until 20% of dynamic torque loss, CONF CON contraction performed until 20% of dynamic torque loss, ECCW ECC contractions performed until having completed the same work as in CONF, CONW ECC contractions carried-out until having completed the same work as in ECCF. Work 1 (W1) corresponds to the amount of work performed in ECCF and CONW, and Work 2 (W2) to the work completed in CONF and ECCW
At PRE the average MMAX amplitude was 11.1 ± 5.5 mV in the VL muscle and 12.8 ± 6.7 mV in the RF muscle. ANOVA showed no effect of time nor condition on MMAX amplitude in either muscle (all P > 0.24; all \({\eta }_{p }^{2}\) < 0.04). MMAX background EMG was unaffected by time or condition (all P > 0.24; all \({\eta }_{p }^{2}\) < 0.11). ANOVA exhibited no change on EMGMAX RMS/MMAX ratio in the VL muscle (all P > 0.15; all \({\eta }_{p }^{2}\) < 0.49) while this ratio decreased with time (P = 0.001; \({\eta }_{p }^{2}\) = 0.35) in the RF muscle.
VAL decrease (%) was correlated to MVIC decrease (N.m) in ECC (r = 0.54; P = 0.013), but not in CON (r = 0.12; P = 0.63).
Corticospinal changes resulting from the exercise
MEP and MMAX background EMG remained stable during the sessions (all P < 0.06; all \({\eta }_{p }^{2}\) > 0.18), so did the ratio between the superimposed torques corresponding to MEP and MMAX (P = 0.29; \({\eta }_{p }^{2}\) = 0.12).
Figure 4 represents the MEP amplitude and MEP SP/MEP amplitude ratio for all conditions. MEP amplitude declined with time in both muscles (all P < 0.008; all \({\eta }_{p }^{2}\) > 0.18). At PRE, MEP amplitude was 21.5 ± 12.9% of MMAX in the VL muscle and 43.3 ± 26.5% of MMAX in the RF muscle. MEP amplitude decreased from PRE to POST in the VL muscle (− 3.8 ± 11.3% MMAX) (P < 0.01; dz = 0.42) and the RF muscle (− 13.3 ± 17.0% MMAX) (P < 0.01; dz = 0.67).
The figure shows MEP (motor evoked potential) amplitude and SP (silent period) normalized to MEP amplitude of the vastus lateralis (a and b), and of the rectus femoris muscles (c and d) at PRE and POST. Sessions matched for work are illustrated in the same color (grey versus black). ***Different from PRE (P < 0.001). ECCF ECC contractions performed until 20% of dynamic torque loss, CONF CON contraction performed until 20% of dynamic torque loss, ECCW ECC contractions performed until having completed the same work as in CONF, CONW ECC contractions carried-out until having completed the same work as in ECCF. Work 1 (W1) corresponds to the amount of work performed in ECCF and CONW, and Work 2 (W2) to the work completed in CONF and ECCW
At PRE, the average MEP SP duration at PRE was of 106 ± 18 ms in the VL muscle and of 103 ± 17 ms in the RF muscle. It was unaffected by condition (all P > 0.25; all \({\eta }_{p }^{2}\) < 0.12) or time (all P > 0.45; all \({\eta }_{p }^{2}\) < 0.11). The MEP SP /MEP amplitude ratio increased from PRE to POST (VL muscle: + 22.4 ± 46.9%; RF muscle: + 27.5 ± 48.4%) (all P < 0.001; all \({\eta }_{p }^{2}\) > 0.18).
The raw SP following the MEP and the MMAX in the RF muscle of a typical subject are illustrated in Fig. 5. M-wave SP in the RF muscle rose from 86 ± 17 to 92 ± 19 ms after the exercise (P = 0.01; \({\eta }_{p }^{2}\) = 0.19), while that in the VL muscle did not vary (P = 0.06; \({\eta }_{p }^{2}\) = 0.11).
The figure illustrates M-wave silent period (a) and MEP (motor evoked potential) silent period (b) from the rectus femoris muscle of a typical subject, before (PRE) and after the exercise (POST). The distance covered by each bidirectional arrow represents silent period duration. c and d Represent the silent period of M-wave and of MEP, respectively, of all participants. *Different from PRE (P < 0.05). Note that MEP amplitude was smaller at POST than PRE while the size of M-wave remained stable
Discussion
The goal of this work was to assess the influence of contraction type on neuromuscular fatigue and corticospinal excitability, after maximal dynamic contractions performed until having completed a given mechanical work. Despite the absence of effect of contraction type on the magnitude of fatigue (i.e. MVIC), we found peripheral fatigue after one CON session, and central fatigue subsequent to all ECC sessions. In accordance with our hypothesis, contraction type exerted no significant influence on MEP amplitude. MEP SP was not modulated either, although M-wave SP rose in the RF muscle, but not in the VL muscle.
Effect of work on neuromuscular fatigue
After having completed the same amount of work, dynamic peak torque dropped more with CON than ECC contractions (i.e. CONF vs ECCW and CONW vs ECCF). MVIC losses were, however, not different between the conditions at exercise termination. We hereby confirm the greater sensitivity of dynamic than isometric torque as an indicator of fatigue following maximal dynamic exercise (Place and Millet 2019).
VAL declined in both ECC sessions. Thus, when a sufficient amount of work was achieved, CON induced preferentially peripheral and ECC contractions provoked central fatigue, respectively. The moderate correlation between MVIC decline and VAL decline after ECC contractions corroborates the latter observation and is coherent with the findings of Souron et al. (2018). The absence of a decrease in VAL post CON exercise likely reflects a specificity of fatigue resulting from CON work. The decrease in the EMGMAX RMS/MMAX in the RF muscle suggests a weaker motor command during MVIC, which coincides with the lower VAL observed in ECC. The absence of modulations of the EMGMAX RMS/MMAX in the VL muscle seems to indicate no alteration of the motor command towards this muscle, which thus appears not to have been involved in the reduction of VAL with time. Nonetheless, it must be noted that the EMG RMS of a single muscle is a reductive indicator of the command sent to a muscle group such as the quadriceps, and even to the muscle itself because the activity of all the firing motor units cannot be fully detected (Farina et al. 2004).
Dt amplitude dropped in CONW but not in CONF, as more work was performed than in the former condition. Souron et al. (2018) found Dt amplitude to decrease only as from an hour after the end of ECC exercise. In our work, the twitch evoked by a high-frequency doublet remained unaffected by ECC work, indicating limited alterations of the contractile process. This is coherent with the findings of Souron et al. (2018) and of Garnier et al. (2018), who reported more pronounced evoked peak torque depression using a twitch at 10 Hz than at 100 Hz after ECC, revealing a larger contribution of impaired excitation-contraction coupling than damaged contractile proteins in peripheral fatigue (Verges et al. 2009). Although the participants of our study were familiar to ECC maximal contractions, this type of exercise is known for the sarcomere damage it causes even in trained subjects (Byrne et al. 2004). In fact, the unchanged high-frequency Dt amplitude we observed would indicate limited damage of the contractile proteins only if we had observed a more depressed low-frequency Dt amplitude, which we have not tested here. A potentiation effect (Froyd et al. 2018; Mador et al. 1994) might also have occurred due to repeated contractions, compensating for peripheral fatigue. Since exercise did not affect MMAX amplitude, peripheral fatigue should result from factors other than sarcolemmal excitability, namely impaired calcium release or contractile proteins damage as mentioned above (Allen et al. 2008).
Effect of work on corticospinal excitability
MEP amplitude decreased in both muscles after the completion of a given work, independently from contraction type. Fatigue would be responsible for this, impairing motoneuronal excitability (McNeil et al. 2011b), while the amount of work performed does not appear to have any influence. Yet we cannot exclude that contraction type influenced the excitability at the cortical and/or the spinal levels distinctly. An enhanced excitability at one level would counterbalance a depressed excitability at the other level, and lead to a similar MEP size. Studies that focused on a single contraction type depicted prolonged SP after CON (Goodall et al. 2018; Gruet et al. 2014; Kennedy et al. 2016; Williams et al. 2014) but not after ECC contractions (Goodall et al. 2017). Furthermore, ECC contractions tended to decrease short-interval intracortical inhibition (Pitman and Semmler 2012), which is coherent with the conclusions drawn based on SP. Those results are in line with those of Garnier et al. (2018), whose participants completed the same work in both contractions types. The fact that in their study the subjects performed contractions at a submaximal intensity while in ours they produced maximal efforts, likely explains the absence of effect of contraction type on SP we found. This stability with time suggests no variation in corticospinal inhibition (Inghilleri et al. 1993; Taylor et al. 1997). Yet, depressed MEP amplitude usually yields shorter SP for a given background EMG (Orth and Rothwell 2004), while here the increased ratio MEP SP/MEP amplitude showed a more pronounced relative corticospinal inhibition by the mechanisms underlying SP- the GABAB neurotransmitters at the cortical level. Then the intensity of the neural command through the corticospinal pathway would largely determine the corticospinal modulations remaining after the exercise. Data from Löscher and Nordlund (2002) strengthen this assumption as they observed no effect of- maximal- contraction type on SP either.
So as to estimate possible spinal influences on SP, we analyzed the SP following MMAX. Beforehand, it must be specified that M-wave SP concerned a larger population of motoneurons than that of MEP, evoked at a lower relative stimulation intensity. M-wave SP appears to be partly due to antidromic collisions (Renshaw 1941), which we assume were of similar importance prior to and after exercise given the constant MMAX background EMG and the same supramaximal stimulation used to evoke MMAX. As a matter of fact, the lengthened SP in the RF muscle would likely result from intrinsic physiological mechanisms, namely a depressed neural conduction velocity (Taylor et al. 1999), diminished muscle spindle discharges, or greater Golgi tendon organ inputs (Škarabot et al. 2019; Taylor et al. 1999; Yacyshyn et al. 2016). We verified that the superimposed torques associated to MEP and MMAX did not significantly vary across the sessions, supporting the assumption that the SP subsequent to each wave concerned a comparable population of motoneurons, and probably stimulated muscle spindle and Golgi tendon organs similarly- this does not guarantee the same firing from these afferents. Other spinal inhibitory mechanisms are likely to have occurred, including a longer motoneuronal after-hyperpolarization period, and especially, Renshaw’s recurrent inhibition (Ziemann et al. 1993). Given that MMAX and MEP were recorded during contractions of the same intensity, all the motoneurons silent following MMAX were most likely silent after MEP too. Then, the spinal inhibitory phenomena highlighted by the change in M-wave SP should also concern MEP SP. In the RF muscle, M-wave SP lengthened, signifying increased spinal inhibition. MEP SP would have remained constant due to a shift in the facilitation/inhibition balance towards facilitation at the cortical or the pyramidal nerve levels. In the VL muscle, neither M-wave SP nor MEP SP evolved, lets assume that either no phenomena such as those described above took place, or that inhibitory mechanisms were compensated for by, for example, an enhanced activity of the Na+–K+ pump (Hicks and McComas 1989). Interestingly, the lengthened RF muscle SP and unchanged VL muscle SP are consistent with the attenuated neural drive towards the former and the unchanged neural drive towards the latter, respectively (assessed via EMGMAX RMS/MMAX). Altogether, our findings suggest that spinal mechanisms would have inhibited the central command sent to the RF. We suggest this has to do with the distinct involvement of the two muscles in knee extension at low force levels. Namely, MEP and MMAX were elicited at 25% MVIC, an intensity at which the RF muscle is relatively less recruited than the VL muscle (Alkner et al. 2000). Then, a smaller proportion of the motoneurons innervating the RF muscle might have been activated during the stimulations.
Effect of fatigue etiology on corticospinal excitability
Fatigue attenuates spinal excitability for a given background EMG (Martin et al. 2006; McNeil et al. 2011a; Weavil et al. 2016), and can prolong MEP SP duration (Goodall et al. 2018). Fatigue is also known to be specific to the exercise performed (Enoka and Stuart 1992; Place and Millet 2019), visible in our experiment through its distinct etiology following CON and ECC exercises. While we found no difference in MEP amplitude decline between the conditions, it seems that fatigue origin could influence corticospinal excitability. For instance, if peripheral fatigue is caused by reduced calcium release from the sarcoplasmic reticulum (Allen et al. 2008) in response to a given motoneuronal firing, or by damaged contractile proteins, it will probably not affect MEP size- at least not taking into account inhibitory feedbacks. Presynaptic inhibition of muscle spindle discharges due to type III-IV afferents would depress corticospinal excitability (McNeil et al. 2011b; Sidhu et al. 2014). On the other hand, central fatigue, due to a shift in the balance between excitation-inhibition towards inhibition (Taylor and Gandevia 2008), generates depressed MEP area (Weavil et al. 2016).
One explanation for the constant MEP depression we found regardless of fatigue origin is that MEP amplitude and MEP SP are not very sensitive to fatigue etiology. In this case, the corticospinal pathway would adjust the excitability at its different levels to yield a given net excitability, depending notably on fatigue magnitude (McNeil et al. 2011a), but not- or little- on its origin. It should also be noted that VAL was assessed at maximal exercise intensity while MEP were evoked at 20% of that intensity, making it hard to relate one parameter to the other. Moreover, VAL reflects the central contribution in force production of several muscles while MEP focuses on one muscle only.
Conclusion
After the completion of a given amount of work with maximal ECC or CON knee extensors contractions, net corticospinal excitability decreased in both the VL and the RF muscles, independently from contraction type. Our data let infer that spinal inhibitory mechanisms affected the RF muscle, which we cannot ascertain regarding the VL muscle. Even though maximal isometric torque losses were similar after exercise, CON contractions provoked peripheral fatigue whereas ECC exercise-induced preferentially central fatigue, potentially affecting the origin of changes in corticospinal excitability. The impact of fatigue magnitude and its etiology on the responsiveness of the corticospinal pathway should thus be cautiously considered in future studies studying the modulations of corticospinal excitability in response to fatiguing exercise.
Change history
17 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00421-020-04578-0
Abbreviations
- ANOVA:
-
Analysis of variance
- CON:
-
Concentric
- CONF:
-
Concentric session aiming for 20% MVIC loss
- CONW:
-
Concentric session aiming for the work completed in ECCF
- Dt:
-
Double twitch
- Dtsup:
-
Superimposed double twitch
- ECC:
-
Eccentric
- ECCF:
-
Eccentric session aiming for 20% MVIC loss
- ECCW:
-
Eccentric session aiming for the work completed in CONF
- EMG:
-
Electromyography
- MVIC:
-
Maximal voluntary isometric contraction
- MVICstim:
-
Maximal voluntary isometric contraction when stimulated
- MMAX :
-
Maximal M-wave
- RF:
-
Rectus femoris
- RMS:
-
Root mean square
- SP:
-
Silent period
- TMS:
-
Transcranial magnetic stimulation
- VAL:
-
Voluntary activation level
- VL:
-
Vastus lateralis
- SENIAM:
-
Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles
References
Aagaard P, Simonsen E, Andersen J, Magnusson S, Halkjær-Kristensen J, Dyhre-Poulsen P (1998) Neural inhibition during eccentric and concentric quadriceps contraction: effects of resistance training. Med Sci Sports Exerc 30:207
Abbott BC, Bigland B, Ritchie JM (1952) The physiological cost of negative work. J Physiol 117:380–390. https://doi.org/10.1113/jphysiol.1952.sp004755
Alkner BA, Tesch PA, Berg HE (2000) Quadriceps EMG/force relationship in knee extension and leg press. Med Sci Sports Exerc 32:459–463. https://doi.org/10.1097/00005768-200002000-00030
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332. https://doi.org/10.1152/physrev.00015.2007
Amiridis IG, Martin A, Morlon B, Martin L, Cometti G, Pousson M, van Hoecke J (1996) Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur J Appl Physiol 73:149–156. https://doi.org/10.1007/BF00262824
Behrens M, Husmann F, Gube M, Felser S, Weippert M, Bruhn S, Zschorlich V, Mau-Moeller A (2017) Intersession reliability of the interpolated twitch technique applied during isometric, concentric, and eccentric actions of the human knee extensor muscles. Muscle Nerve 56:324–327. https://doi.org/10.1002/mus.25498
Buharin VE, Butler AJ, Rajendra JK, Shinohara M (2013) Enhanced corticospinal excitability with physiologically heightened sympathetic nerve activity. J Appl Physiol Bethesda Md 1985(114):429–435. https://doi.org/10.1152/japplphysiol.01586.2011
Byrne C, Twist C, Eston R (2004) Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med Auckl NZ 34:49–69. https://doi.org/10.2165/00007256-200434010-00005
Cavaleri R, Schabrun SM, Chipchase LS (2017) The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): a systematic review and meta-analysis. Syst Rev 6:48. https://doi.org/10.1186/s13643-017-0440-8
Clos P, Laroche D, Stapley PJ, Lepers R (2019) Neuromuscular and perceptual responses to sub-maximal eccentric cycling. Front Physiol. https://doi.org/10.3389/fphys.2019.00354
Cohen J (2013) Statistical power analysis for the behavioral sciences. Routledge. https://doi.org/10.4324/9780203771587
Cox DM, Cafarelli E (1999) The mixed nerve silent period is prolonged during a submaximal contraction sustained to failure. Muscle Nerve 22:320–328. https://doi.org/10.1002/(sici)1097-4598(199903)22:3<320::aid-mus4>3.0.co;2-1
Davranche K, Temesi J, Verges S, Hasbroucq T (2015) Transcranial magnetic stimulation probes the excitability of the primary motor cortex: a framework to account for the facilitating effects of acute whole-body exercise on motor processes. J Sport Health Sci 4:24–29. https://doi.org/10.1016/j.jshs.2014.09.001
Duchateau J, Enoka RM (2016) Neural control of lengthening contractions. J Exp Biol 219:197–204. https://doi.org/10.1242/jeb.123158
Duclay J, Pasquet B, Martin A, Duchateau J (2014) Specific modulation of spinal and cortical excitabilities during lengthening and shortening submaximal and maximal contractions in plantar flexor muscles. J Appl Physiol 117:1440–1450. https://doi.org/10.1152/japplphysiol.00489.2014
Enoka RM, Stuart DG (1992) Neurobiology of muscle fatigue. J Appl Physiol Bethesda Md 1985(72):1631–1648. https://doi.org/10.1152/jappl.1992.72.5.1631
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol Bethesda Md 1985(96):1486–1495. https://doi.org/10.1152/japplphysiol.01070.2003
Froyd C, Beltrami F, Noakes T (2018) Neuromuscular fatigue at task failure and during immediate recovery after isometric knee extension trials. Sports 6:156. https://doi.org/10.3390/sports6040156
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789. https://doi.org/10.1152/physrev.2001.81.4.1725
Garnier YM, Paizis C, Lepers R (2018) Corticospinal changes induced by fatiguing eccentric versus concentric exercise. Eur J Sport Sci. https://doi.org/10.1080/17461391.2018.1497090
Garnier YM, Paizis C, Martin A, Lepers R (2019) Corticospinal excitability changes following downhill and uphill walking. Exp Brain Res. https://doi.org/10.1007/s00221-019-05576-1
Goodall S, Howatson G, Romer L, Ross E (2014) Transcranial magnetic stimulation in sport science: a commentary. Eur J Sport Sci 14:S332–S340. https://doi.org/10.1080/17461391.2012.704079
Goodall S, Howatson G, Thomas K (2018) Modulation of specific inhibitory networks in fatigued locomotor muscles of healthy males. Exp Brain Res 236:463–473. https://doi.org/10.1007/s00221-017-5142-x
Goodall S, Thomas K, Barwood M, Keane K, Gonzalez JT, St Clair Gibson A, Howatson G (2017) Neuromuscular changes and the rapid adaptation following a bout of damaging eccentric exercise. Acta Physiol Oxf Engl 220:486–500. https://doi.org/10.1111/apha.12844
Gruet M, Temesi J, Rupp T, Levy P, Verges S, Millet GY (2014) Dynamics of corticospinal changes during and after high-intensity quadriceps exercise. Exp Physiol 99:1053–1064. https://doi.org/10.1113/expphysiol.2014.078840
Hicks A, McComas AJ (1989) Increased sodium pump activity following repetitive stimulation of rat soleus muscles. J Physiol 414:337–349. https://doi.org/10.1113/jphysiol.1989.sp017691
Howatson G, Taylor MB, Rider P, Motawar BR, McNally MP, Solnik S, DeVita P, Hortobágyi T (2011) Ipsilateral motor cortical responses to TMS during lengthening and shortening of the contralateral wrist flexors. Eur J Neurosci 33:978–990. https://doi.org/10.1111/j.1460-9568.2010.07567.x
Inghilleri M, Berardelli A, Cruccu G, Manfredi M (1993) Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466:521–534
Kellis E, Baltzopoulos V (1998) Muscle activation differences between eccentric and concentric isokinetic exercise. Med Sci Sports Exerc 30:1616–1623
Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL (2016) Effects of fatigue on corticospinal excitability of the human knee extensors. Exp Physiol 101:1552–1564. https://doi.org/10.1113/EP085753
Komi P, Linnamo V, Silventoinen P, Sillanpää M (2000) Force and EMG power spectrum during eccentric and concentric actions. Med Sci Sports Exerc 32:1757–1762
LaStayo P, Marcus R, Dibble L, Frajacomo F, Lindstedt S (2013) Eccentric exercise in rehabilitation: safety, feasibility, and application. J Appl Physiol 116:1426–1434. https://doi.org/10.1152/japplphysiol.00008.2013
Latella C, Goodwill AM, Muthalib M, Hendy AM, Major B, Nosaka K, Teo WP (2018) Effects of eccentric versus concentric contractions of the biceps brachii on intracortical inhibition and facilitation. Scand J Med Sci Sports. https://doi.org/10.1111/sms.13334
Lockyer EJ, Benson RJ, Hynes AP, Alcock LR, Spence A-J, Button DC, Power KE (2018) Intensity matters: effects of cadence and power output on corticospinal excitability during arm cycling are phase- and muscle-dependent. J Neurophysiol. https://doi.org/10.1152/jn.00358.2018
Lovering RM, Brooks SV (2013) Eccentric exercise in aging and diseased skeletal muscle: good or bad? J Appl Physiol 116:1439–1445. https://doi.org/10.1152/japplphysiol.00174.2013
Löscher WN, Nordlund MM (2002) Central fatigue and motor cortical excitability during repeated shortening and lengthening actions. Muscle Nerve 25:864–872. https://doi.org/10.1002/mus.10124
Mador MJ, Magalang UJ, Kufel TJ (1994) Twitch potentiation following voluntary diaphragmatic contraction. Am J Respir Crit Care Med 149:739–743. https://doi.org/10.1164/ajrccm.149.3.8118645
Martin PG, Gandevia SC, Taylor JL (2006) Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol 95:3512–3518. https://doi.org/10.1152/jn.01230.2005
McDonnell MN, Buckley JD, Opie GM, Ridding MC, Semmler JG (2013) A single bout of aerobic exercise promotes motor cortical neuroplasticity. J Appl Physiol Bethesda Md 1985(114):1174–1182. https://doi.org/10.1152/japplphysiol.01378.2012
McHugh MP (2003) Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports 13:88–97
McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL (2011a) Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589:3533–3544. https://doi.org/10.1113/jphysiol.2011.207191
McNeil CJ, Giesebrecht S, Khan SI, Gandevia SC, Taylor JL (2011b) The reduction in human motoneurone responsiveness during muscle fatigue is not prevented by increased muscle spindle discharge. J Physiol 589:3731–3738. https://doi.org/10.1113/jphysiol.2011.210252
Millet GY, Lepers R (2004) Alterations of neuromuscular function after prolonged running, cycling and skiing exercises. Sports Med Auckl NZ 34:105–116. https://doi.org/10.2165/00007256-200434020-00004
Orth M, Rothwell JC (2004) The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 115:1076–1082. https://doi.org/10.1016/j.clinph.2003.12.025
O’Leary TJ, Morris MG, Collett J, Howells K (2015) Reliability of single and paired-pulse transcranial magnetic stimulation in the vastus lateralis muscle. Muscle Nerve 52:605–615. https://doi.org/10.1002/mus.24584
Pitman BM, Semmler JG (2012) Reduced short-interval intracortical inhibition after eccentric muscle damage in human elbow flexor muscles. J Appl Physiol Bethesda Md 1985(113):929–936. https://doi.org/10.1152/japplphysiol.00361.2012
Place N, Millet GY (2019) Quantification of neuromuscular fatigue: What do we do wrong and Why? Sports Med. https://doi.org/10.1007/s40279-019-01203-9
Périard JD, Caillaud C, Thompson MW (2011) Central and peripheral fatigue during passive and exercise-induced hyperthermia. Med Sci Sports Exerc 43:1657–1665. https://doi.org/10.1249/MSS.0b013e3182148a9a
Renshaw B (1941) Influence of discharge of motoneurons upon excitation of neighboring motoneurons. J Neurophysiol 4:167–183. https://doi.org/10.1152/jn.1941.4.2.167
Sidhu SK, Bentley DJ, Carroll TJ (2009) Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J Appl Physiol Bethesda Md 1985(106):556–565. https://doi.org/10.1152/japplphysiol.90911.2008
Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M (2014) Spinal μ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592:5011–5024. https://doi.org/10.1113/jphysiol.2014.275438
Souron R, Nosaka K, Jubeau M (2018) Changes in central and peripheral neuromuscular fatigue indices after concentric versus eccentric contractions of the knee extensors. Eur J Appl Physiol 118:805–816. https://doi.org/10.1007/s00421-018-3816-0
Strojnik V, Komi PV (1998) Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol Bethesda Md 1985(84):344–350. https://doi.org/10.1152/jappl.1998.84.1.344
Taylor JL, Allen GM, Butler JE, Gandevia SC (1997) Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res 117:472–478
Taylor JL, Butler JE, Gandevia SC (1999) Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res 127:108–115. https://doi.org/10.1007/s002210050779
Taylor JL, Gandevia SC (2008) A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol Bethesda Md 1985(104):542–550. https://doi.org/10.1152/japplphysiol.01053.2007
Tesch PA, Dudley GA, Duvoisin MR, Hather BM, Harris RT (1990) Force and EMG signal patterns during repeated bouts of concentric or eccentric muscle actions. Acta Physiol Scand 138:263–271. https://doi.org/10.1111/j.1748-1716.1990.tb08846.x
Verges S, Maffiuletti NA, Kerherve H, Decorte N, Wuyam B, Millet GY (2009) Comparison of electrical and magnetic stimulations to assess quadriceps muscle function. J Appl Physiol 106:701–710. https://doi.org/10.1152/japplphysiol.01051.2007
Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M (2015) Intensity-dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. Am J Physiol-Regul Integr Comp Physiol 308:R998–R1007. https://doi.org/10.1152/ajpregu.00021.2015
Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M (2016) Fatigue diminishes motoneuronal excitability during cycling exercise. J Neurophysiol 116:1743–1751. https://doi.org/10.1152/jn.00300.2016
Westing SH, Cresswell AG, Thorstensson A (1991) Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol 62:104–108. https://doi.org/10.1007/BF00626764
Westing SH, Seger JY, Thorstensson A (1990) Effects of electrical stimulation on eccentric and concentric torque-velocity relationships during knee extension in man. Acta Physiol Scand 140:17–22. https://doi.org/10.1111/j.1748-1716.1990.tb08971.x
Williams PS, Hoffman RL, Clark BC (2014) Cortical and spinal mechanisms of task failure of sustained submaximal fatiguing contractions. PLoS ONE 9:e93284. https://doi.org/10.1371/journal.pone.0093284
Yacyshyn AF, Woo EJ, Price MC, McNeil CJ (2016) Motoneuron responsiveness to corticospinal tract stimulation during the silent period induced by transcranial magnetic stimulation. Exp Brain Res 234:3457–3463. https://doi.org/10.1007/s00221-016-4742-1
Ziemann U, Netz J, Szelényi A, Hömberg V (1993) Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett 156:167–171. https://doi.org/10.1016/0304-3940(93)90464-v
Škarabot J, Mesquita RNO, Brownstein CG, Ansdell P (2019) Myths and Methodologies: How loud is the story told by the transcranial magnetic stimulation-evoked silent period? Exp Physiol 104:635–642. https://doi.org/10.1113/EP087557
Škarabot J, Tallent J, Goodall S, Durbaba R, Howatson G (2018) Corticospinal excitability during shortening and lengthening actions with incremental torque output. Exp Physiol. https://doi.org/10.1113/EP087347
Author information
Authors and Affiliations
Contributions
All authors discussed the protocol design. PC and RL finalized the design. PC implemented the study and drafted the manuscript. YG, AM, and RL critically revised the manuscript. All authors approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Clos, P., Garnier, Y., Martin, A. et al. Corticospinal excitability is altered similarly following concentric and eccentric maximal contractions. Eur J Appl Physiol 120, 1457–1469 (2020). https://doi.org/10.1007/s00421-020-04377-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04377-7