Abstract
The goal of this study was to determine the process or processes most likely to be involved in reaction-time costs for spatially cued bimanual reaching. We used reaction time to measure the cost of bimanual symmetric movements compared to unimanual movements (a bimanual symmetric cost) and the cost for bimanual asymmetric movements compared to symmetric movements (a bimanual asymmetric cost). The results showed that reaction times were comparable for all types of movements in simple reaction time; that is, there was neither a bimanual symmetric cost nor an asymmetric cost. Therefore, unimanual, bimanual symmetric, and bimanual asymmetric movements have comparable complexity during response initiation. In choice conditions, there was no bimanual symmetric cost but there was a bimanual asymmetric cost, indicating that the preparation of asymmetric movements is more complex than symmetric movements. This asymmetric cost is likely the result of interference during response programming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bimanual movements are typically thought to require more complex movement preparation than their unimanual counterparts (Swinnen and Wenderoth 2004). This complexity may stem from concurrent selection of more than one response (Diedrichsen et al. 2001), from concurrent assembly of multiple motor commands (Heuer and Klein 2006b; Spijkers et al. 1997; Spijkers and Heuer 1995; Stelmach et al. 1988), or from some combination of these. Whatever the source of the complexity, a reasonable assumption is that more complex movement preparation requires more processing time; therefore, increased reaction time in a reach task can be used as an indicator of increased complexity.
The majority of studies that have investigated the preparation of bimanual movements have compared bimanual symmetric movements (i.e. the arms travel equivalent distances in the same directions) to unimanual movements in simple reaction-time tasks with spatial cues (Anson and Bird 1993; Di Stefano et al. 1980; Hughes and Franz 2007; Nagelkerke 2002; Ohtsuki 1981; Shen and Franz 2005; Taniguchi 1999a, b). The results from these studies have been inconsistent; some have shown simple reaction-time costs for bimanual symmetric movements (Anson and Bird 1993, finger extension; Di Stefano et al. 1980; Hughes and Franz 2007; Ohtsuki 1981; Shen and Franz 2005; Taniguchi 1999b) while others have not (Anson and Bird 1993, elbow flexion; Nagelkerke 2002; Taniguchi 1999a). Furthermore, other studies did not statistically compare reaction times of bimanual symmetric movements to unimanual movements (Fowler et al. 1991; Kawabe 1989; Kelso et al. 1979; Marteniuk et al. 1984; Norrie 1964, 1967; Steenbergen et al. 1996).
Studies that have measured choice reaction times with spatial cues (Blinch et al. 2011; Diedrichsen et al. 2001, 2006; Heuer and Klein 2006b; Stelmach et al. 1988; Weigelt and Cardoso de Oliveira 2003) have focused on the movement preparation of bimanual asymmetric movements (i.e. the arms travel different distances or directions) compared to symmetric movements. Diedrichsen et al. (2001) compared symmetric and asymmetric movements that were cued by spatial and symbolic cues. They found a large bimanual asymmetric cost with symbolic cues (55 ms in Experiment 1 and 94 ms in Experiment 2) and no cost with spatial cues. They argued that the asymmetric cost was the result of greater processing demands on response selection when two different symbolic cues required translation, which has been supported by other studies (reviewed by Wenderoth and Weigelt 2009).
Recent research has questioned whether there might be a small asymmetric cost with spatial cues. Heuer and Klein (2006b) and Diedrichsen et al. (2006) found small asymmetric costs (15–55 ms) and suggested the asymmetric cost is attenuated, but not eliminated, with spatial cues. They argued that the large and small asymmetric costs with symbolic and spatial cues revealed two forms of interference. The small cost with spatial cues might be the result of greater processing demands on response programming for asymmetric movements. The large cost with symbolic cues includes the small cost and the larger interference from the translation of two different symbolic cues. We focused on bimanual costs with spatial cues in this study. In regard to spatial cues, the results from previous studies on the asymmetric cost have been inconsistent. Some have shown choice reaction-time costs for bimanual asymmetric movements (Diedrichsen et al. 2006; Heuer and Klein 2006b; Stelmach et al. 1988, young participants; Weigelt and Cardoso de Oliveira. 2003, non-transformed conditions) while others have not (Blinch et al. 2011; Diedrichsen et al. 2001). The current study also examined potential bimanual symmetric costs in choice reaction-time conditions, which have not been looked at in great detail (Diedrichsen et al. 2001, 2006; Stelmach et al. 1988).
The bimanual symmetric and asymmetric reaction-time costs deserve further investigation, as these effects have been inconsistent and difficult to detect due to the small sample sizes used in the majority of previous studies on bimanual costs (Maxwell 2004). An examination of bimanual symmetric and bimanual asymmetric costs across simple and choice reaction-time tasks within a single study and with sufficient power is needed if we want to isolate the processes of movement preparation where bimanual reaches produce interference.
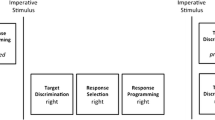
Our logic for isolating these processes in the current study was the following. Simple reaction time reflects the time required to recognise the imperative stimulus and initiate a response, assuming that participants fully program their movements prior to the imperative stimulus (Fig. 1). Furthermore, stimulus recognition is unlikely to be longer for bimanual tasks (which involve two visual onsets and therefore, more stimulus energy) than for unimanual ones. Therefore, if we find a bimanual cost in simple reaction-time tasks, then it is probably the result of greater processing during response initiation. Choice reaction time, on the other hand, reflects the time required to recognise the imperative stimulus, discriminate the targets, select an appropriate response, program that response, and initiate the response (Fig. 1). With spatial targets, we eliminate or attenuate the effects of response selection (Diedrichsen et al. 2001; Goodman and Kelso 1980). Furthermore, we can reasonably assume that imperative stimulus recognition and response initiation demands are equivalent between simple and choice reaction-time tasks. Therefore, if there is a bimanual cost in choice reaction time, but not in simple reaction-time tasks, then it is probably the result of target discrimination, response programming, or both processes.
Method
Participants
Twenty participants were tested from the university community (mean age of 25.1 years, 8 females). All participants reported being right-handed and had normal or corrected-to-normal vision. The research ethics board at the University of British Columbia approved the study, and participants gave written informed consent before participation.
Apparatus
Participants were seated at a table in a dimly lit room (Fig. 2). Virtual stimuli were projected on the surface of the table by an LCD monitor positioned over the table. Midway between the table and the monitor was a half-silvered mirror; it created the illusion that the stimuli appeared on the table. Participants were seated so that their mid-sagittal plane was in the middle of a three-row by two-column array of circle outlines displayed on the table. The circles in the closest row were the two home positions, and they had a radius of 3.4 mm. The remaining four circles were the short- and long-distance targets for the left and right arms. Each target had a radius of 15.3 mm, and the short and long targets were 100 and 200 mm from the home positions. A fixation cross was displayed in the middle of the four targets. Filling any of the circle outlines cued that target; this is subsequently referred to as illuminating a target (Fig. 2a). A light was always on under the mirror so that the participants could see their arms and the hand-held styli that they moved to the targets without obstructing the stimuli.
Apparatus and display. Participants reached to target positions on the table surface beneath the mirror. The mirror set-up allowed for the unobstructed presentation of visual stimuli. A light was always on under the mirror so that the participants’ arms and the hand-held styli were visible during the entire experiment. a The stimuli and the imperative stimuli seen by the participants on bimanual asymmetric Long–Short trials
Procedure
Bimanual trials began with the outlines of the four potential targets and the two home positions appearing on the surface of the table. The home positions were illuminated as a cue for the participants to press the tips of the hand-held styli in them. Participants could see their arms, the styli, and two small circles that were displayed on the table surface to represent the locations of tips of the styli in real time. Once the styli were in the home positions, the real-time feedback disappeared and the 1–2s variable foreperiod began. A fixation cross appeared between the four targets, and participants were instructed to fixate on it until after they reached for the targets. In simple reaction-time blocks, the outlines of the correct targets would change colour (from blue to yellow) during the foreperiod to encourage movement preparation before the imperative stimulus. The eight different types of unimanual and bimanual movements were tested in eight, separate simple reaction-time blocks.
Two targets, one for each arm, were illuminated (by filling the circle in with blue) as the imperative stimuli. Participants were instructed to “hit the targets as fast as possible”. At the end of the bimanual movement (the tips of the styli were pressed down on the surface), the fixation disappeared and the endpoint of each movement was displayed. Each endpoint and the correct target were displayed in green for a target hit and red for a target miss. Participants were encouraged to use this feedback to help them with subsequent trials. The trial was then labelled as “good” or “bad”. Bad trials were recycled to the end of the block. Examples of bad trials were target misses, anticipation (reaction time <100 ms), distraction (reaction time or movement time >1,000 ms), and asynchronous movement initiation (>60 ms reaction time difference between the pens). If it was a bad trial, then a message explained the problem, but the participants were not told that these trials were recycled. In total, 4.5 % of all the trials were recycled. Every trial ended with a blank screen, and then the next trial would begin when the participants lifted the styli.
Unimanual blocks were similar except that only one home position was illuminated and the imperative stimulus consisted of illuminating one target. The participants held only the required stylus for each unimanual block.
Design
The experiment consisted of testing unimanual, bimanual symmetric, and bimanual asymmetric movements in simple and 2-choice reaction-time conditions. Each combination of these conditions was tested in a separate block, which resulted in 12 blocks (Table 1). The order of these blocks was counterbalanced first by the type of reaction time (simple or 2-choice) and then by the type of movement (unimanual or bimanual). The order of the blocks that fell within this counterbalance was randomised; for example, the order of the four unimanual simple blocks (left long, right long, right short, right long) was randomised.
A block consisted of a practice phase and a test phase. In simple reaction time, there were 9 practice trials (1 was a catch trial with no imperative stimulus) and 36 test trials (4 were catch trials). The number of trials was doubled in 2-choice reaction time (18 practice, 72 test trials). There were two potential movements in 2-choice blocks, so half of the trials were one type of movement. The order of these movements was randomised in each block, and the same randomisation was used for each participant.
Two important considerations for choice conditions are the number and kind of movement choices. It is important to have the same number of choices in unimanual and bimanual conditions as the number of choices can influence reaction time (Hick 1952; Hyman 1953; cf. Favilla 1996; Wright et al. 2007). However, there may be an unequal number of choices when comparing unimanual movements in 4-choice to bimanual symmetric and asymmetric movements in 4-choice. Unimanual 4-choice requires the participant to select the arm that will move (left or right) as well as the distance of the movement (long or short). The arms do not need to be selected in bimanual 4-choice because both arms are required on all trials. In other words, unimanual 4-choice requires one arm to be selected after the imperative stimulus, whereas both arms can be selected before the imperative stimulus in bimanual 4-choice. This difference could lengthen reaction times in unimanual 4-choice and eliminate a potential bimanual symmetric cost. Our solution was to test unimanual and bimanual movements in 2-choice blocks (unimanual left, unimanual right, bimanual symmetric, bimanual asymmetric). This ensured that the arm (unimanual blocks) or arms (bimanual blocks) could be selected before the imperative stimulus in all blocks.
As for the kind of movement choice, if both symmetric and asymmetric bimanual movements are tested in one 4-choice block, then participants may strategically prepare for the harder, asymmetric movements. That is, participants might always prepare asymmetric movements in order to decrease the potential reaction time difference between movement types. Strategically preparing for the worst-case scenario has been shown when movements are made with or without visual feedback (cf. Elliott and Allard 1985; Zelaznik et al. 1983). We did not alter visual feedback in the current study, but the principle of preparing for the worst-case scenario may still apply. Therefore, we tested symmetric and asymmetric movements in separate 2-choice blocks.
Data acquisition and analysis
A microswitch in each stylus was sampled at 250 Hz to determine when the stylus tip was pressed against the table and when it was lifted. The signal from the microswitch was used to calculate reaction time (time from the imperative stimulus to stylus lift).
Mean reaction times for each participant were calculated for each arm, distance, and movement type in the twelve blocks. These were then collapsed into three movement types (unimanual, bimanual symmetric, bimanual asymmetric) for the two reaction-time conditions (simple, choice). The bimanual symmetric and asymmetric costs were investigated by statistical analysis on reaction times in simple and choice conditions with a 3 movement type (unimanual, bimanual symmetric, and bimanual asymmetric) repeated measures ANOVA. When local sphericity was violated (as indicated by Mauchly’s test, p < .10), the Huynh–Feldt correction was used when the ε was greater than or equal to .75 and the Greenhouse–Geisser correction was used otherwise. The uncorrected degrees of freedom and the ε values were reported (Huynh–Feldt εHF, Greenhouse–Geisser εGG). When the effect of movement type was significant, two a priori comparisons were performed: unimanual movements were compared to bimanual symmetric movements and symmetric movements were compared to asymmetric movements. The Sidak correction was used to control the familywise error rate.
The analyses on reaction times were also performed on the probabilities of target misses. Probabilities were normalised with the arcsine square-root transformation before statistical analysis; the data reported are percentages. Between-arm movement time and amplitude correlations were calculated for the four types of bimanual movements (Long–Long, Short–Short, Long–Short, and Short–Long). These correlations were transformed with Fisher’s r to z transformation before analysis with movement type (Long–Long, Short–Short, Long–Short, and Short–Long) ANOVAs. Simple and choice conditions were analysed with separate ANOVAs. The data reported are r values.
There may be a bias when comparing the mean choice reaction time of bimanual asymmetric movements to symmetric movements. Choice reaction times are influenced by the duration of the movement, with long duration movements resulting in longer reaction times than short duration movements (Klapp 1995, 2003). As Long–Long movements have longer movement times than Short–Short movements, we predicted that Long–Long movements would also have longer reaction times. For asymmetric movements, temporal assimilation occurs that results in movement times for the long- and short-distance movements that are similar to Long–Long movements (Kelso et al. 1979). If we consider only the influence of movement times on reaction times, then Long–Long, Long–Short, and Short–Long movements should have comparable reaction times that are longer than Short–Short movements. These differences could result in a bimanual asymmetric cost, as Short–Short movements have shorter reaction times than the other movements. We, therefore, compared the reaction times of only Long–Long movements to asymmetric movements with a repeated measures t test. This isolated an asymmetric cost that is the result of greater processing demands for asymmetric movements compared to symmetric movements (and not the result of the influence of movement duration on reaction times). Values are reported as the mean ± standard error.
Results
Reaction times in simple and choice conditions for unimanual, bimanual symmetric, and asymmetric movements are shown in Fig. 3. One obvious difference is that reaction times were longer in 2-choice (287 ± 7.0 ms) than simple conditions (258 ± 6.1 ms). Movement times are shown in Fig. 4; these data were collapsed across choice (simple, 2-choice) and the unimanual data were also collapsed across arm (left, right). The movement times of unimanual long (311 ± 11.4 ms) and bimanual Long–Long movements (334 ± 12.9, 328 ± 12.3 ms) were substantially longer than unimanual short (234 ± 9.0) and bimanual Short–Short movements (260 ± 10.7, 252 ± 10.2 ms). Asymmetric Long–Short (331 ± 12.8, 306 ± 12.1 ms) and Short-Long movements (309 ± 12.4, 319 ± 11.7 ms) had movement times that were more like bimanual Long–Long movements than Short–Short movements. This last result was indicative of temporal assimilation for asymmetric movements.
No costs in simple reaction time
In simple reaction time, the effect of movement type was not significant, F(2, 38) = 2.4, p = .11. Thus, reaction times were not significantly different between unimanual (261 ± 6.0 ms), bimanual symmetric (254 ± 6.7 ms), and asymmetric movements (259 ± 6.3 ms; Fig. 3). These results suggested that there was neither a bimanual symmetric cost nor a bimanual asymmetric cost in simple reaction-time tasks.
Bimanual asymmetric cost in choice reaction time
In 2-choice reaction time, there was a tendency for reaction times to increase from unimanual (276 ± 8.1 ms), to bimanual symmetric (284 ± 6.5 ms), to asymmetric movements (300 ± 8.1 ms; Fig. 3). Statistical analysis showed that the effect of movement type was significant, F(2, 38) = 11.0, p < .001. The a priori comparisons showed that reaction times were not significantly different between unimanual and bimanual symmetric movements (p = .21), which suggest that there was no bimanual symmetric cost. There was support for an asymmetric cost, as asymmetric movements had significantly longer reaction times compared to symmetric movements (p < .01). We controlled for the influence of movement duration on the asymmetric cost in choice conditions by comparing only Long–Long movements to asymmetric movements and found that asymmetric movements (300 ± 8.1 ms) had longer reaction times than Long–Long movements (289 ± 6.6 ms), t(19) = 2.4, p = .03.
Target misses, movement amplitudes, and between-arm correlations
Statistical analysis on target misses revealed that they were not significantly different for unimanual, symmetric, and asymmetric movements in simple and choice conditions (ps > .23; Table 2). This supported that the reaction times were not influenced by a speed-accuracy trade-off.
Movement amplitudes were relatively consistent in all conditions; long amplitudes ranged from 200.2 ± 0.40 to 202.0 ± 0.43 mm and short amplitudes ranged from 97.1 ± 0.38 to 99.8 ± 0.41 mm. The amplitudes showed no trend towards amplitude assimilation, which is more often seen when fast reversal movements are made without emphasis on accuracy.
Between-arm movement time and amplitude correlations were also examined (Table 3). These correlations were larger for movement times compared to amplitudes. The movement time correlations were not significantly different for any of the bimanual movements in simple or choice conditions (ps > .26). Amplitude correlations in simple and choice conditions showed the typical result that symmetric movements (Long–Long, Short–Short) had larger correlations than asymmetric movements (Long–Short, Short–Long).
Discussion
The goal of this study was to determine the process or processes most likely to be involved in reaction-time costs for spatially cued bimanual reaching. The advantage of this experiment, compared to previous experiments, was that bimanual symmetric costs and bimanual asymmetric costs were examined in simple and choice reaction-time tasks within a single study. The simple reaction-time task allowed us to isolate bimanual symmetric and asymmetric costs related to response initiation, and the choice reaction-time task allowed us to isolate bimanual costs related to target discrimination, response selection, and response programming.
In simple reaction time, there was neither a bimanual symmetric cost nor a bimanual asymmetric cost. This suggests that the total duration of imperative stimulus recognition and response initiation was comparable for unimanual, symmetric, and asymmetric movements. The first novel finding of this experiment is, therefore, that the duration of response initiation is comparable for unimanual, bimanual symmetric, and asymmetric movements. In this regard, unimanual movements are as complex as bimanual movements.
In choice reaction time, there was no bimanual symmetric cost but there was a bimanual asymmetric cost. Importantly, there was still a bimanual asymmetric cost when we controlled for the influence of movement duration on reaction times. Thus, the asymmetric cost is likely the result of greater processing demands for asymmetric movements compared to symmetric movements. This asymmetric cost suggests a form of bimanual interference. We should, more accurately, refer to this interference as bimanual asymmetric interference, as there was no bimanual symmetric cost.
Why did we find a small but significant bimanual asymmetric cost when previous studies (e.g. Diedrichsen et al. 2001; Hazeltine et al. 2003) have suggested that there is no asymmetric cost with spatial cues? Diedrichsen et al. (2001) found a large bimanual asymmetric cost (~100 ms) with symbolic cues and they did not find an asymmetric cost with spatial cues. The cost with symbolic cues has been attributed to greater processing demands on response selection in several studies (e.g. Albert et al. 2007; Diedrichsen et al. 2003; Weigelt et al. 2007). Two recent studies found a small but significant asymmetric cost with spatial cues (Diedrichsen et al. 2006; Heuer and Klein 2006b). They showed that the asymmetric cost is attenuated, but not eliminated, with spatial cues. Both studies argued that the large and small asymmetric costs reveal two components of interference. The large cost with symbolic cues is mostly caused by greater processing demands on response selection, and the small cost with spatial cues is caused by greater processing demands on response programming. Heuer and Klein suggested that the small cost with spatial cues escaped discovery in previous studies because of its small effect size. This present study confirms that there is a small but significant asymmetric cost with spatial cues.
Diedrichsen et al. (2006) and Heuer and Klein (2006b) reasoned that the asymmetric cost with spatial cues was caused by greater processing demands on response programming, but this has not been tested. The asymmetric cost could be the result of increased processing demands on any process or processes of movement preparation (imperative stimulus recognition, target discrimination, response selection, response programming, and response initiation). From the present results, we now have evidence that it is unlikely that imperative stimulus recognition and response initiation contributed to the bimanual asymmetric cost. This is supported by a comparable total duration of imperative stimulus recognition and response initiation for unimanual, symmetric, and asymmetric movements in simple reaction time. Therefore, the bimanual asymmetric cost is most likely the result of one or more preparation processes that are unique to choice conditions. These processes are target discrimination, response selection, and response programming.
Discrimination of symmetric targets may take less time than asymmetric targets as symmetric targets can become grouped as a gestalt because of their horizontal alignment and closer proximity than asymmetric targets (Han et al. 1999). However, it has been shown that the perceptual similarity or dissimilarity of the bimanual targets does not affect reaction time (Albert et al. 2007). Given that perceptually dissimilar targets are unlikely to be grouped as a gestalt, this argues against a role for target discrimination in the bimanual asymmetric cost.
For response selection, symmetric movements involve the selection of the same response for both arms. This is either a long-distance movement (Long–Long) or a short movement (Short–Short). Asymmetric movements involve the selection of a different response for each arm (Long–Short or Short–Long). It has been argued that there is a savings to response selection when selecting identical responses for both arms (Albert et al. 2007; Diedrichsen et al. 2003; Hazeltine et al. 2003). This, however, has been shown when the targets are cued symbolically. An example of a symbolic cue is presenting the word “Long” or “Short” to indicate a long- or short-distance target. Symbolic cues result in greater processing demands on response selection compared to the direct, spatial cues used in this experiment (Diedrichsen et al. 2001). It has been shown that preparation costs with symbolically cued movements are either eliminated or attenuated with spatial cues (Diedrichsen et al. 2001; Goodman and Kelso 1980). As we used spatial cues that place minimal demands on response selection, it is unlikely that response selection results in the bimanual asymmetric cost observed in this experiment.
It has been suggested that there is a savings to response programming when programming identical responses for both arms (Spijkers et al. 1997), so response programming of two movements with identical distances (symmetric) may be shorter than two movements with different distances (asymmetric). Stelmach et al. (1988) and Heuer and Klein (2006b) also argued that the asymmetric cost was the result of interference during response programming. Stelmach et al. (1988) reasoned that the programming of asymmetric movements are more complex than symmetric movements, as asymmetric movements require the programming of two different amplitudes and symmetric movements require the programming of the same amplitude twice. Heuer and Klein (2006b) argued that concurrently programming two different amplitudes resulted in the asymmetric cost, a process they referred to as “transient parametric coupling during amplitude specification” (pp. 238; cf. Heuer 1986). Therefore, the bimanual asymmetric cost that we observed is most likely the result of greater processing demands on response programming.
The greater processing demands on response programming are related to the movement parameters for asymmetric movements compared to symmetric movements. Compared to symmetric movements, the asymmetric movements used in this study had asymmetric movement amplitudes and asymmetric target locations. Thus, asymmetric amplitudes, asymmetric targets, or both could have caused the asymmetric cost. Two studies have attempted to determine the contributions of various asymmetric movement parameters to the bimanual asymmetric cost (Heuer and Klein 2006a; Weigelt 2007). The issue with these studies is that the changes in reaction times with symmetric and asymmetric parameters were confounded by other changes that influenced reaction times. In the study by Heuer and Klein, the number of movement choices depended on the starting locations of the movements. This is a problem because reaction times can increase with the number of movement choices (Hick 1952; Hyman 1953; cf. Favilla 1996; Wright et al. 2007). In the study by Weigelt, the movements were cued symbolically, so movements with two different symbolic cues had longer reaction times than movement with two identical cues. Further studies are required to determine the contribution of various asymmetric movement parameters to the asymmetric cost.
In summary, we observed neither a bimanual symmetric cost nor an asymmetric cost in simple reaction times. We can conclude that unimanual, bimanual symmetric, and bimanual asymmetric movements have comparable complexity for response initiation. There was also no bimanual symmetric cost for choice reaction-time tasks, so unimanual and bimanual symmetric movements have comparable preparation complexity. We did observe a bimanual asymmetric cost in choice reaction times, so the preparation of asymmetric movements is more complex than symmetric movements. This bimanual asymmetric cost in a choice task could be the result of greater processing demands on target discrimination, response selection, or response programming. Our results support that the most likely source of the asymmetric cost is response programming. The strength of our study was its use of spatial targets and a simple reaction-time task control condition, for they allowed us to examine bimanual reach costs that were independent of response selection and response initiation costs. However, further experiments are needed to confirm that response programming is the source of the asymmetric cost.
References
Albert NB, Weigelt M, Hazeltine E, Ivry RB (2007) Target selection during bimanual reaching to direct cues is unaffected by the perceptual similarity of the targets. J Exp Psychol Hum Percept Perform 33(5):1107–1116
Anson JG, Bird YN (1993) Neuromotor programming: bilateral and unilateral effects on simple reaction time. Hum Mov Sci 12(1–2):37–50
Blinch J, Cameron BD, Franks IM, Chua R (2011) Bimanual reaches with symbolic cues exhibit errors in target selection. Exp Brain Res 212(4):541–554
Di Stefano M, Mortelli M, Marzi CA, Berlucchi G (1980) Hemispheric control of unilateral and bilateral movements of proximal and distal parts of the arms as inferred from simple reaction time to lateralized light stimuli in man. Exp Brain Res 38(2):197–204
Diedrichsen J, Hazeltine E, Kennerley S, Ivry RB (2001) Moving to directly cued locations abolishes spatial interference during bimanual actions. Psychol Sci 12(6):493–498
Diedrichsen J, Ivry RB, Hazeltine E, Kennerley S, Cohen A (2003) Bimanual interference associated with the selection of target locations. J Exp Psychol Hum Percept Perform 29(1):64–77
Diedrichsen J, Grafton S, Albert N, Hazeltine E, Ivry RB (2006) Goal-selection and movement-related conflict during bimanual reaching movements. Cereb Cortex 16(12):1729–1738
Elliott D, Allard F (1985) The utilization of visual feedback information during rapid pointing movements. Q J Exp Psychol Hum Exp Psychol 37(3):407–425
Favilla M (1996) Reaching movements: programming time course is independent of choice number. NeuroReport 7(15–17):2629–2634
Fowler B, Duck T, Mosher M, Matheison B (1991) The coordination of bimanual aiming movements: evidence for a progressive desynchronization. Q J Exp Psychol A 43(2):205–221
Goodman D, Kelso JA (1980) Are movements prepared in parts? Not under compatible (naturalized) conditions. J Exp Psychol Gen 109(4):475–495
Han S, Humphreys GW, Chen L (1999) Uniform connectedness and classical Gestalt principles of perceptual grouping. Percept Psychophys 61(4):661–674
Hazeltine E, Diedrichsen J, Kennerley SW, Ivry RB (2003) Bimanual cross-talk during reaching movements is primarily related to response selection, not the specification of motor parameters. Psychol Res 67(1):56–70
Heuer H (1986) Intermanual interactions during programming of finger movements: transient effects of ‘homologous coupling’. In: Heuer H, Fromm C (eds) Generation and modulation of action patterns. Springer, Berlin, pp 87–101
Heuer H, Klein W (2006a) Intermanual interactions related to movement amplitudes and endpoint locations. J Mot Behav 38(2):126–138
Heuer H, Klein W (2006b) The influence of movement cues on intermanual interactions. Psychol Res 70(4):229–244
Hick WE (1952) On the rate of gain of information. Q J Exp Psychol 4(1):11–26
Hughes CM, Franz EA (2007) Experience-dependent effects in unimanual and bimanual reaction time tasks in musicians. J Mot Behav 39(1):3–8
Hyman R (1953) Stimulus information as a determinant of reaction time. J Exp Psychol 45(3):188–196
Kawabe S (1989) Effects of force output and preparatory set on premotor time of simultaneous bilateral responses. Percept Mot Skills 68(2):619–625
Kelso JA, Southard DL, Goodman D (1979) On the coordination of two-handed movements. J Exp Psychol Hum Percept Perform 5(2):229–238
Klapp ST (1995) Motor response programming during simple and choice reaction time: the role of practice. J Exp Psychol Hum Percept Perform 21(5):1015–1027
Klapp ST (2003) Reaction time analysis of two types of motor preparation for speech articulation: action as a sequence of chunks. J Mot Behav 35(2):135–150
Marteniuk RG, MacKenzie CL, Baba DM (1984) Bimanual movement control: information processing and interaction effects. Q J Exp Psychol Hum Exp Psychol 36(2):335–365
Maxwell SE (2004) The persistence of underpowered studies in psychological research: causes, consequences, and remedies. Psychol Methods 9(2):147–163
Nagelkerke P (2002) Bimanual limb interaction. Unpublished master’s thesis, University of British Columbia, Vancouver, Canada
Norrie ML (1964) Timing of two simultaneous movements of arms and legs. Res Q 35(4):511–522
Norrie ML (1967) Effects of unequal distances and handedness on timing patterns for simultaneous movements of arms and legs. Res Q 38(2):241–246
Ohtsuki T (1981) Increase in simple reaction time of knee extension induced by simultaneous bilateral performance. Percept Mot Skills 53(1):27–30
Shen YC, Franz EA (2005) Hemispheric competition in left-handers on bimanual reaction time tasks. J Mot Behav 37(1):3–9
Spijkers W, Heuer H (1995) Structural constraints on the performance of symmetrical bimanual movements with different amplitudes. Q J Exp Psychol A 48(3):716–740
Spijkers W, Heuer H, Kleinsorge T, van der Loo H (1997) Preparation of bimanual movements with same and different amplitudes: specification interference as revealed by reaction time. Acta Psychol 96(3):207–227
Steenbergen B, Hulstijn W, de Vries A, Berger M (1996) Bimanual movement coordination in spastic hemiparesis. Exp Brain Res 110(1):91–98
Stelmach GE, Amrhein PC, Goggin NL (1988) Age differences in bimanual coordination. J Gerontol 43(1):18–23
Swinnen SP, Wenderoth N (2004) Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci 8(1):18–25
Taniguchi Y (1999a) Effect of practice in bilateral and unilateral reaction-time tasks. Percept Mot Skills 88(1):99–109
Taniguchi Y (1999b) Right hemisphere contribution to motor programming of simultaneous bilateral response. Percept Mot Skills 88(3c):1283–1290
Weigelt M (2007) Re-examining structural constraints on the initiation of bimanual movements: the role of starting locations, movement amplitudes, and target locations. Hum Mov Sci 26(2):212–225
Weigelt C, Cardoso de Oliveira S (2003) Visuomotor transformations affect bimanual coupling. Exp Brain Res 148(4):439–450
Weigelt M, Rieger M, Mechsner F, Prinz W (2007) Target-related coupling in bimanual reaching movements. Psychol Res 71(4):438–447
Wenderoth N, Weigelt M (2009) Visual cues influence motor coordination: behavioral results and potential neural mechanisms mediating perception-action coupling and response selection. Prog Brain Res 174:179–188
Wright CE, Marino VF, Belovsky SA, Chubb C (2007) Visually guided, aimed movements can be unaffected by stimulus-response uncertainty. Exp Brain Res 179(3):475–496
Zelaznik HZ, Hawkins B, Kisselburgh L (1983) Rapid visual feedback processing in single-aiming movements. J Mot Behav 15(3):217–236
Acknowledgments
We would like to thank two anonymous reviewers for their insightful critiques. The Natural Sciences and Engineering Research Council of Canada supported this research with a postgraduate scholarship awarded to Jarrod Blinch and a discovery grant awarded to Romeo Chua. Brendan Cameron was supported by a Juan de la Cierva postdoctoral fellowship (JCI-2011-09664) and a Marie Curie CIG (618407). The views expressed here reflect those of the authors only and not those of the funders.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blinch, J., Cameron, B.D., Cressman, E.K. et al. Comparing movement preparation of unimanual, bimanual symmetric, and bimanual asymmetric movements. Exp Brain Res 232, 947–955 (2014). https://doi.org/10.1007/s00221-013-3807-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3807-7