Abstract
Flexible and stable postural control requires adaptation to changing environmental conditions, a process which requires re-weighting of multisensory stimuli. Recent studies, as well as predictions from a computational model, have indicated a reciprocal re-weighting relationship between modalities when a sensory stimulus changes amplitude. As one modality is down-weighted, another is up-weighted to compensate (and vice versa). The purpose of this study was to investigate the dynamics of intra- and inter-modality re-weighting process by examining postural responses to manipulation of proprioception and visual modalities simultaneously. Twenty-two young adults were placed in a visual cave and stood on a variable-pitch platform for thirteen trials of 250 s apiece. The platform was rotated at constant frequency of 0.4 Hz and amplitudes of 0.3 (low) or 1.5 (high) degrees. Platform amplitude was manipulated in two conditions: low-to-high or high-to-low. The visual stimulus was displayed at constant frequency of 0.35 Hz and amplitude of 0.08 degrees. The results showed both fast and slow changes in center of mass (CoM) response to the switch in platform amplitude. On both timescales, CoM response changed in a reciprocal manner relative to platform amplitude. When the platform amplitude increased (low-to-high condition), CoM response decreased relative to the platform and increased relative to the visual stimulus, indicating both intra-modality and inter-modality sensory re-weighting. In the high-to-low condition, however, there was no change in CoM response relative to visual stimulus, indicating that re-weighting may also be dependent on the absolute level of gain. Sway variability at frequencies other than the stimulus frequency also showed a reciprocal relationship with CoM gain relative to platform. Overall, these results indicate that dynamics of multisensory re-weighting is clearly more complicated than the schemes proposed by current adaptive models of human postural control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adaptation is a signature feature of flexible and stable postural control (Forssberg and Nashner 1982). Cues from visual, vestibular and somatosensory systems must be integrated to properly provide information in order for the postural system to adapt to any perturbation (Horak and Macpherson 1996). One example of such adaptation is the modification of postural responses as environmental conditions change (e.g., stepping onto a slippery surface). Many studies have established that such changes are partially driven by adaptively re-weighting sensory information (Horak and Macpherson 1996; Oie et al. 2002; Peterka 2002; Oie et al. 2005; Maurer et al. 2006; Rinaldi et al. 2009). For example, a common experimental technique is for subjects to stand within a visual “moving room,” in which the walls of the laboratory move sinusoidally but the floor remains motionless, creating conflicts between vision and the other senses (e.g., proprioception and vestibular). The visual motion of the walls is initially small, making it difficult to distinguish self-motion from the motion of the room, resulting in a strong postural response. However, if the amplitude of the sinusoidal visual input is doubled, a linear system would double the amplitude of the postural response at the frequency of the input so that gain (sway amplitude/visual input amplitude) remains constant. What in fact happens is that the postural control system responds by “down-weighting” vision, meaning that the system relies less on vision, indicated by a reduction in gain, due to a postural response that increases less than that of the visual amplitude, indicating a nonlinear process (e.g., Oie et al. 2002; Peterka 2002; Rinaldi et al. 2009).
During standing posture, however, multiple modalities provide information about self-motion, begging the question of how these inputs are re-weighted continuously. By manipulating the amplitude of one sensory modality while the amplitude of a second modality remains constant, recent studies have demonstrated a reciprocal trade-off: As one modality is down-weighted, an alternative sensory input is up-weighted to compensate, indicating an inter-modal relationship (Oie et al. 2002; Allison et al. 2006; Bonfim et al. 2006; Cenciarini and Peterka 2006). Such reciprocity suggests that re-weighting of different sensory modalities is necessarily linked, providing an important constraint for adaptive models of multisensory fusion and human postural control (van der Kooij et al. 2001; Carver et al. 2006).
This reciprocal inter-modal relationship was observed in a model by Carver et al. (2005, 2006). The model has an adaptive Kalman filter with a single adaptive parameter θ that simultaneously controls the weights of a visual input and a lumped non-visual input. The adaptive scheme consists of adjusting θ to minimize mean squared ankle torque. The model was originally developed to account for changes in steady-state gain as visual motion amplitude changed, but was found to also account for changes in gain when subjects are exposed to abrupt changes in visual motion amplitude (Barela et al. unpublished data; Jeka et al. 2008).
Here, we test whether adaptive behavior of the Carver et al.’s model is consistent with the inter-modal re-weighting relationship between two sensory stimuli. Two sensory stimuli (vision and proprioception) were manipulated in a continuous fashion to determine how inter- and intra-modality re-weighting evolves in time. Intra-modality re-weighting refers to change in gain of a sensory modality (e.g. vision) when amplitude of motion of this sensory modality is changed. Inter-modality re-weighting refers to change in gain of a sensory modality (e.g., vision) when amplitude of motion of a different sensory input (e.g., proprioception) is changed.
The primary question addressed by this study is whether there is a fixed reciprocal relationship between the gains of two modalities, as predicted by the model of Carver et al. (2005, 2006) in which adaptation is described by a single parameter. Specifically, when platform amplitude is increased, CoM gain relative to platform amplitude is predicted to decrease and CoM gain relative to visual amplitude is predicted to increase. When platform amplitude is decreased, CoM gain relative to platform is predicted to increase and CoM gain relative to vision is predicted to decrease. Our interest was to determine whether the reciprocal gain relationship was consistently observed when stimulus amplitude increased or decreased.
Additionally, it is important to recognize that gain reflects the postural response only at the frequency of the sensory input. There are many other frequencies in the postural response, and a change in environmental (e.g., vision) motion is also known to change sway variability at non-stimulus frequencies. This effect has been shown for subjects viewing a moving visual scene or lightly touching a moving surface with the fingertip (Allison et al. 2006; Jeka et al. 2006). The Carver et al.’s (2005, 2006) adaptive model predicts that any increase in gain at the stimulus frequency should be matched by a decrease in sway variability at non-stimulus frequencies (and vice versa). This provides a second prediction (along with the reciprocal gain relationship stated above) to test whether the sensory re-weighting process can be explained with a single adaptive parameter from the Carver et al.’s model. The results did not support a fixed reciprocal relationship between the CoM gain relative to vision and platform, in contradiction to the model of Carver et al. (2005, 2006).
Materials and methods
Participants
Twenty-two young, healthy adults (mean age, 21.9 (±2.84 SD) years of age, 11 females and 11 males), students at the University of Maryland, participated in this study. All students reported no known musculoskeletal or neurological disorders that might affect their ability to maintain balance and had normal and corrected-to-normal vision during all test. They were informed about the experimental procedures and asked to sign the written consent form approved by the Institutional Review Board at University of Maryland.
Experimental setup
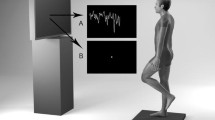
The participants stood upright quietly on a moveable platform inside a visual cave. The cave consists of three translucent screens (2.5 m × 2.0 m) (Mechdyne, Inc, Marshalltown, Iowa, USA) positioned one in front and two on either side of the participants. Subjects faced the front screen in the middle of the cave, approximately 1 m from frontal screen and 1.5 m from either side screen. Three JVC projectors (Model: DLA-M15U, Victor Company, Japan) were used to display a random pattern of 500 white triangles (3.4 × 3.4 × 3 cm) on a black background, with a resolution of 1,024 × 768 pixels and a frame rate of 60 Hz. In order to avoid aliasing effect in the foveal region (Dijkstra et al. 1994), no triangles were displayed in the frontal “wall” within a horizontal band of ±5 degree around the subject’s eye height. Subjects were asked to maintain their gaze on the blank area during all trials. The apparatus was controlled by a PC workstation using specific software (CaveLib, Mechdyne, Inc) to generate the visual display (triangles) written in Visual C++.
A variable-pitch platform (40 × 60 cm) was also used to provide sensory mechanical stimulation through rotation about the ankle joint. Participants were placed barefoot in the middle of the platform, feet 6.5 cm apart, with their medial malleolus ankle coincident with the rotational axis of the platform. The platform has a controller (Compumotor—GV6K-U12E G) and a servomotor (Compumotor—BE343LJ-K10NBE). Custom software (Compumotor—Motion Architect) generated platform rotations in different amplitudes. Data collection used custom LabVIEW programs (National Instruments, Inc).
Data acquisition
Kinematic data and platform motion were recorded using a motion analysis system (Optotrak 3020, Northern Digital, Inc) at a sampling rate of 120 Hz. Four wireless IRED markers were placed on the ankle (right lateral malleolus), knee (right lateral epicondyle), hip (right greater trochanter) and shoulder (right acromion process). Three markers were attached on a small triangle board (6 × 6 × 6 cm) with each marker fixed in one corner of this board and placed on the participant’s head (inion). Another three markers were placed on the moveable platform in order to measure its displacement.
Muscle activity was also recorded from the right lateral gastrocnemius, soleus, tibialis anterior, biceps femoris, rectus femoris, rectus abdominus, erector spinae muscles of the lumbar spine and neck extensors using a telemetric EMG system (Noraxon Telemyo 900). Electrodes were placed parallel to muscle fibers 2.5 cm apart and bandage with elastic bands in order to avoid the cable movement. EMG signals were recorded at a sampling rate of 1,080 Hz and bandwidth of 16–500 Hz. EMG data were collected for future analysis and will be presented in a subsequent manuscript.
The participants wore a safety harness and stood upright on the variable-pitch platform inside the virtual room for thirteen trials of 250 s apiece. The surrounding visual environment and the platform were stationary in the first trial (no motion). In the following randomized trials, both visual display and platform were rotated simultaneously at different amplitudes and frequencies. The platform was rotated at a constant frequency of 0.4 Hz and amplitude of 0.3 or 1.5 degrees (mean-to-peak), which was switched after 80 s from low-to-high or high-to-low amplitudes of platform motion, making a total of 12 trials, six in each condition (six low-to-high and six high-to-low). The visual stimulus was displayed at a constant frequency of 0.35 Hz and amplitude of 0.08 degrees (mean-to-peak). In half of the trials (three trials of each condition), the visual scene and platform rotated in the same direction at the beginning of the trial; in the other half of the trials, they rotated in opposite directions. This procedure was employed so that after averaging across trials, the cross-correlation function between the visual-scene and platform rotations would be zero, which insured that sway responses to platform and visual-scene motion could be measured independently (see "Data analysis" below). Four-minute rests were taken after every two trials to minimize fatigue effects during the test.
Data analysis
The displacement trajectories of the ankle, knee, hip and shoulder markers were used to estimate the center of mass (CoM) trajectories in the AP, medial–lateral and vertical directions based upon a three-segment model (Winter et al. 1990). The CoM angle with respect to vertical was then determined using the AP and vertical displacements of the CoM and the ankle marker. Therefore, CoM refers to the angle of the body in the sagittal plane with respect to the ankle position.
To verify the postural response amplitude to the visual scene and to the platform motion, a frequency response function (FRF) was obtained. FRF was calculated dividing the Fourier transform of the estimated CoM trajectory by the Fourier transform of each stimulus (visual and platform motion) trajectory, separately. This analysis was calculated in 2.5-s intervals with platform motion and 2.857-s intervals with visual motion for each trial (cycle-by-cycle FRF). The FRF was averaged across trials for each subject and then across subjects. After averaging across trials, the FRF for platform rotation was not artificially biased by the FRF for visual-scene rotation and vice versa, because the mean cross-correlation function between the visual-scene and platform rotations was zero (see “Data acquisition” above).
Four variables were calculated: gain, phase, position variability and velocity variability. Gain was computed as the absolute value of the mean FRF, indicating 1 when CoM angular displacement matched the stimulus amplitude. CoM angular displacement relative to the visual (platform) stimulus was referred to as “CoM gain relative to visual (platform) amplitude.” Phase was computed as the argument of the FRF indicating the temporal relationship between CoM displacement and the sensory stimuli, calculated in radians and converted to degrees. Positive and negative phase values indicate that the postural response led or lagged behind the sensory stimulus, respectively. For each cycle, 95 % confidence intervals for gain and phase were computed by constructing a 95 % confidence region for the FRF in the complex plane (Kiemel et al. 2006).
To calculate position and velocity variability, power was removed by subtracting sinusoids corresponding to the CoM Fourier transform at the visual and platform stimuli frequencies (Jeka et al. 2000). CoM trajectories were computed over six 40-s segments, with a time step of 0.1 s. CoM position and velocity variability were then defined as the standard deviation of the residual CoM trajectories, namely, at frequencies other than visual and platform stimulus. CoM position and velocity variability provide information about postural sway at frequencies other than the visual and platform stimuli frequencies, which provides a more complete description of the effect of sensory re-weighting (see “Discussion”).
Analysis of gain and phase
Analysis of gain and phase was performed through separate analyses of FRFs for the visual and platform perturbations. Each analysis consisted of two steps: (1) linear least-squares fits of changes in FRFs over time in which the dependent variables were the real and imaginary parts of the FRFs, and (2) a nonlinear analysis in which the dependent variables were the gains and phases at the endpoints of the least-squares fits from the first step. We used this two-step approach because: (1) a linear analysis is appropriate for the real and imaginary parts of the FRFs but not for gain and phase, as explained below, and (2) it is easier to interpret results in terms of gain and phase than in terms of the real and imaginary parts of the FRF, but there is a nonlinear relationship between the two sets of variables.
Except at the beginning of the trial and immediately after the switch in platform amplitude, changes in FRFs were slow and approximately linear across time. To describe these changes, we computed least-squares fits of the real and imaginary parts of the FRF as a linear function of the perturbation cycle index. For each subject and condition, we fit the FRF over two time intervals: one from the third cycle of the trial (denoted as b 1) to the last cycle before the amplitude switch (b 2), and one from the third cycle after the switch (a 1) to the last cycle of the trial (a 2). Linear fits were not applied to the first two cycles of the trial and the first two cycles after the switch in order to avoid fast nonlinear changes in the FRF. Linear fits were evaluated at the endpoints b 1, b 2, a 1 and a 2, resulting in fitted FRF values H cps, where c ∈ {1, 2} denotes condition, p ∈ {b 1, b 2, a 1, a 2} denotes endpoint, and s ∈ {1, 2,…,22} denotes subject.
We used a nonlinear multivariate statistical model to analyze gain and phase based on the fitted endpoint FRF values H cps (Jeka et al. 2008, 2010):
where γ cp is group gain and ϕ cp is group phase. Variation across subjects was described by the random variables δ cps and ε cps, which were assumed to have a zero-mean multivariate normal distribution. Because of our statistical model is based on the distribution of FRF values, not gains, it avoids biases in gain due to side-lobe leakage (Jeka et al., 2008). The gains γ cp and phases ϕ cp were estimated by maximizing the model’s log-likelihood (Seber and Wild 2003). The maximum-likelihood estimates of γ cp and ϕ cp are the absolute value and argument, respectively, of the mean of H cps across subjects.
To test a null hypothesis H about gains γ cp or phases ϕ cp, we maximized the model’s log-likelihood with parameters constrained by the null hypothesis. We then compared the constrained maximum log-likelihood, L H , to the unconstrained maximum log-likelihood, L, using a F test applied to Wilks’ Λ = exp (2(L H −L)/n) (Seber 1984; Polit 1996), using the same degrees of freedom ν 1 and ν 2 as in the corresponding linear case.
For both conditions, we tested for slow changes in gain and phase both before the switch (cycles b 1 vs. b 2) and after the switch (cycles a 1 vs. a 2). We refer to these changes as “slow,” because the changes were between cycles separated by more than 70 s. In addition, we tested for fast changes in gain and phase after the change in the platform amplitude, the gain and phase values immediately before the switch (cycle b 2) was compared to the first fitted gain and phase values after the switch (cycle a 1). We refer to these changes as “fast,” because the changes were between cycles separated by only 7.5 s for platform motion and 8.6 s for visual-scene motion. For gain, we tested whether these changes in FRF values summed to zero for the two conditions (\( \gamma_{{1,b_{2} }} - \gamma_{{1,a_{1} }} - \gamma_{{2,b_{2} }} + \gamma_{{2,a_{1} }} = 0 \)), which indicates whether the rate of these changes is similar at the two amplitude switches (low-to-high and high-to-low conditions). The α-level for all analyses was 0.05.
Analysis of variability
Two repeated measures MANOVAs were performed to verify the differences in the position and velocity variability in each condition. Post hoc analyses with a Bonferroni adjustment were used. The α-level for all analyses was 0.05.
Results
Figure 1 shows exemplars of CoM motion from one participant and the concurrent visual and platform motion at low-to-high (Fig. 1a) and high-to-low (Fig. 1b) platform amplitude conditions. CoM sway was induced by the visual-scene and platform motion in both low-to-high and high-to-low platform amplitude conditions. Furthermore, changes in CoM amplitude are evident when platform amplitude switched from low-to-high or high-to-low. Table 1 summarizes gain and phase values between visual and CoM sway and platform and CoM sway used to compare postural responses before, during, and after platform switch.
Low-to-high platform amplitude condition
Figure 2 depicts the mean gain and phase across subjects and trials relative to the visual stimulus and platform motion in the low-to-high platform amplitude condition. The third and last values labeled in Table 1 refer to first (actual 3rd value) and last fitted values used to compare the CoM responses before (pre) and after (post) platform amplitude change. Also, the last fitted value before and the first fitted value (actual 3rd value) after the platform amplitude change was used to verify the CoM response during the transition.
Mean cycle-to-cycle gain and phase of CoM angular displacement relative to the visual stimulus (circles) and platform motion (squares) at low-to-high condition across subjects and trials. Error bars denote 95 % confidence intervals. The third and last values (gray circles and squares) refer to first (actual third value) and last fitted values used to compare the CoM responses before (pre), during and after (post) platform amplitude change
During the first 80 s of the trial when the platform was at low amplitude (slow change), CoM gain relative to platform increased, F (1, 21) = 27.76, p = 0.0001, and CoM gain relative to vision decreased F (1, 21) = 26.83, p = 0.0001. However, these gains quickly changed with an abrupt increase in platform amplitude (fast change). Comparisons of gains before and after the amplitude transition revealed that CoM gain relative to platform decreased, F (1, 21) = 73.81, p = 0.0001, and CoM gain relative to vision increased, F (1, 21) = 15.25, p = 0.0005. After the transition (following 160 s), these gain values were maintained (slow change).
CoM phase relative to platform was higher than CoM phase relative to vision, F (1, 21) = 5.04, p = 0.03. CoM phase relative to platform increased just after the transition (fast change) and decreased during the last 160 s (slow change), F (1, 21) = 6.47, p = 0.02. Phase was constant for visual stimulus (p > 0.05).
High-to-low platform amplitude condition
Figure 3 depicts the mean gain and phase across subjects and trials to the visual stimulus and platform motion in the high-to-low platform condition. The first (actual third value) and last fitted values before and after platform transition are also depicted as seen in Fig. 2.
Mean cycle-to-cycle gain and phase of CoM angular displacement relative to the visual stimulus (circles) and platform motion (squares) at high-to-low condition across subjects and trials. Error bars denote 95 % confidence intervals. The third and last values (gray circles and squares) refer to first (actual third value) and last fitted values used to compare the CoM responses before (pre), during and after (post) platform amplitude change
In the first 80 s when platform amplitude was high, CoM gain relative to platform increased, F (1, 21) = 10.249, p = 0.004, and CoM gain relative to vision decreased, F (1, 21) = 5.92, p = 0.02. The slow change in gain to the constant visual stimulus when platform amplitude changed suggests inter-modality re-weighting of the sensory stimuli (see “Discussion”).
After the amplitude switch from high-to-low, CoM gain relative to platform increased (fast change), F (1, 21) = 91.7, p = 0.0001, which was maintained during the last 160 s of the trial (slow change), F (1, 21) = 0.51, p = 0.82. CoM gain relative to vision did not change when the CoM gain relative to platform increased abruptly (fast change), F (1, 21) = 0.62, p = 0.43, but then slowly decreased until the end of the trial (slow change). The last CoM gain relative to vision fitted value after the platform switch was significantly lower than the first fitted value (slow change), F (1, 21) = 8.60, p = 0.008.
Negative phase values indicated that the CoM response was temporally lagging the visual and platform motion. In addition, phase was slightly more positive in the first 80 s of the trial for both visual, F (1, 21) = 12.63, p = 0.001, and platform motion (slow change), F (1, 21) = 16.86, p = 0.0005. The temporal relationship between postural sway and the visual stimulus, F (1, 21) = 1.54, p = 0.22, and platform motion, F (1, 21) = 0.02, p = 0.87, did not change after the platform amplitude switch (fast change), remaining constant for the last 160 s of the trial (slow change).
The summation of changes in FRFs at the amplitude platform switch in both conditions was tested whether they differed from zero. Significant differences from zero for CoM gain relative to vision (p = 0.0085) and CoM gain relative to platform (p = 0.0027) were found. Changes in CoM gain relative to vision were larger when the platform motion increased rather than decreased after the amplitude transition. Changes in CoM gain relative to platform were larger when platform motion decreased.
Position and velocity variability
Figure 4a–d depicts mean position and velocity variability across subjects and trials at both conditions. In general, sway variability increased when the platform amplitude was high and decreased when platform amplitude was low. A MANOVA revealed differences between the six 40-s segments, Wilks’ Lambda = 0.057, F (2, 10) = 19.93, p < 0.001 in both the high-to-low and the low-to-high (Wilks’ Lambda = 0.093, F (2, 10) = 11.74, p < 0.001) conditions. Univariate analyses showed that these effects were found in both conditions for position and velocity variability, ps < 0.001. Consistent with predictions, post hoc tests indicated a decrease in position and velocity variability after the amplitude transition in the high-to-low condition and an increase in position and velocity variability after the amplitude transition in the low-to-high condition.
Discussion
The purpose of this study was to investigate the dynamics of intra- and inter-modality re-weighting process by examining postural responses to manipulation of two sensory modalities simultaneously. Our present results clearly showed both intra- and inter-modality re-weighting, but in addition, the dynamics of re-weighting is characterized by both fast and slow changes, consistent with the previous results (Jeka et al. 2008). Our results argue for an adaptive scheme that is driven by more than a single adaptive parameter.
Inter- and intra-modality re-weighting
Multisensory re-weighting was demonstrated by changes in CoM response relative to platform and visual manipulation. Intra-modality re-weighting is reflected by the change in CoM gain relative to platform in response to a change in the amplitude of platform motion. Inter-modality re-weighting is reflected by the change in CoM gain relative to vision whose amplitude remained constant as the platform amplitude was changed. Our results showed both intra- and inter-modality re-weighting.
Intra-modality re-weighting was observed on both a fast and slow time scale. CoM gain relative to platform changed quickly when the amplitude of the platform changed from low-to-high and high-to-low. However, intra-modality re-weighting was also observed over a longer time scale, in the first 80 s of each condition, as CoM gain relative to platform slowly increased, most dramatically in the low-to-high condition. The slow increase is due to the onset of platform motion from a stationary position. Body sway couples most strongly to a stationary sensory stimulus, which provides the best reference to estimate self-motion. As soon as the sensory stimulus begins to move, rapid down-weighting occurs. Close inspection of Figs. 2 and 3 shows that the first measurement of CoM gain relative to platform at the beginning of the trial is higher than the first fitted value, indicating down-weighting. Pairwise comparisons indicated significant differences between these two measurements for both conditions (p < 0.0001). This was followed by a slow increase in gain, indicating up-weighting. The initial rapid down-weighting suggests that the nervous system interprets any movement of the environment as a large movement, an over-reaction to the initial movement of the platform, and then slowly up-weights the platform stimulus to readjust it to appropriate levels. This rapid drop in CoM gain relative to platform (down-weighting) is observed again as the platform changes from low-to-high amplitude. However, slow up-weighting is not observed after the amplitude switch, suggesting that the slow changes observed earlier in the trial may be a function of the absolute level of gain in addition to the change in stimulus amplitude.
Inter-modality re-weighting is also observed in the low-to-high condition in Fig. 2, as changes in CoM gain relative to vision were observed when platform amplitude changed. The slow up-weighting of CoM gain relative to platform is matched by slow down-weighting of CoM gain relative to vision in the first 80 s of the low-to-high condition, even though the visual stimulus oscillated at constant amplitude. The slow visual down-weighting could also be due to the onset of the platform movement at the beginning of the trial, as discussed above. Just as the platform stimulus seems to be down-weighted too much at the trial onset, the visual stimulus seems up-weighted too much, followed by a period of adjustment to bring both weights to appropriate levels. Equally striking is that after the platform switches to high amplitude, vision is again up-weighted rapidly with no further down-weighting over the longer time scale to the end of the trial, similar to the behavior of CoM gain relative to platform. Thus, both intra- and inter-modality processes are intertwined to adjust the coupling strength to each stimulus to achieve the most precise estimate of self-motion.
In the high-to-low condition, intra-modality effects were qualitatively similar to the low-to-high condition, as CoM gain relative to platform displayed an abrupt increase after the platform amplitude switch. Slow changes in gain were also evident. However, inter-modality effects were not as striking for the visual stimulus gain. CoM gain relative to vision did not display a change in gain after the platform amplitude decreased. Instead, a slow decrease in CoM gain relative to vision was observed across the entire trial.
Modeling
The gain changes observed here are only partially consistent with a model proposed by Carver et al. (2005, 2006). Because the model has a single adaptive parameter, it cannot account for a change in CoM gain relative to platform that is not accompanied by a change in CoM gain relative to vision, such as we observed when the platform amplitude was decreased during the trial (Fig. 3). This suggests that the model should be modified to have a separate adaptive parameter for each sensory modality. Such a modified model would not have a fixed inverse relationship between CoM gain relative to vision and CoM gain relative to platform, although there would still be a tendency for the gains to move in opposite directions in response to a change in platform amplitude in order to minimize ankle torque (or some other critical parameter).
Position and velocity variability
The preceding discussion has focused on the effects of sensory re-weighting on gains, which describe postural sway at the frequencies of the visual and platform stimuli. As described in the introduction, sensory re-weighting is also thought to effect sway at non-stimulus frequencies. For example, when subjects view a moving visual scene or lightly touch a moving surface with the fingertip, increasing environmental motion not only decreases the gain to environmental motion but also increases sway variability at non-stimulus frequencies (Allison et al. 2006; Jeka et al. 2006). In this study, we found the analogous result for platform motion. Sway variability was higher when the platform motion was high than when it was low.
In Jeka et al. (2006) we showed that the link between environmental motion and sway variability occurs in the Carver et al.’s (2005, 2006) adaptive model and is the result of a trade-off between reducing sway at stimulus frequencies and reducing sway at non-stimulus frequencies. The essential idea is the hypothesis that the nervous system adjusts the sensory weights of different modalities to (approximately) minimize overall sway variability, that is, the total variability at both stimulus and non-stimulus frequencies. When environmental motion is small, postural responses to the motion are small and sensory weights primarily reflect the goal of minimizing sway variability. However, as environmental motion increases, postural responses to environmental motion become a substantial contribution to overall sway variability and the nervous system down-weights those modalities most affected by the environmental motion to reduce sway at the stimulus frequency. However, sensory weights are no longer optimal to reduce sway at non-stimulus frequencies as well, so sway variability at non-stimulus frequencies increases. Thus, down-weighting a sensory modality has both positive and negative effects (i.e., a trade-off). The positive effect is to diminish the effect of environmental motion that is biasing the nervous system’s estimate of self-motion. The negative effect is that the nervous system has lost information it needs to precisely estimate body dynamics. Thus, the greater the down-weighting, the greater the reduction in sway at stimulus frequencies, but also the greater the increase in sway at non-stimulus frequencies. In the Carver et al.’s (2005, 2006) model, and presumably in humans, the sensory weights chosen by the nervous system are a trade-off between these two factors.
Limitations
Under normal conditions, flexible control of stance is achieved by continuous re-weighting of visual, somatosensory and vestibular inputs to provide convergent information about body dynamics (e.g., Peterka 2002; Jeka et al. 2000; Maurer et al. 2006). Here, we manipulated and quantitatively observed responses of visual and somatosensory inputs without reference to vestibular contributions. Considering the challenges of controlling and measuring transient changes of gain with two sensory inputs, a novelty of this study, adding a third input in form of galvanic stimulation, was not considered feasible, but clearly needs to be addressed in future studies. Even though the contribution of the vestibular system during quiet or mildly perturbed stance is considered noisy and weak (Mergner et al. 1993), recent advances in the understanding of galvanic input clearly suggest that it may be manipulated effectively (Day et al. 2011). In addition, the choice of amplitude and frequency of stimulus manipulation may also constitute a critical issue. Our choices on amplitudes and frequencies of the stimuli (vision and platform) were based on the previous studies which observed strong responses to the chosen parameters and on practical limitations of our equipment. Although our results provide important knowledge about the re-weighting process, further investigations will determine the generality of these results to other experimental conditions.
Conclusion
The current results indicate that the dynamics of multisensory re-weighting, especially inter-modality, is clearly more complicated than the schemes proposed by current adaptive models of human postural control (van der Kooij et al. 2001; Carver et al. 2006). The signature feature of current schemes is for modalities to be re-weighted in a reciprocal fashion to compensate for changing environmental conditions. This reciprocity was certainly observed here, but in a manner that suggested more than a single parameter to guide the re-weighting process.
References
Allison LK, Kiemel T, Jeka JJ (2006) Multisensory reweighting of vision and touch is intact in healthy and fall-prone older adults. Exp Brain Res 175:342–352
Bonfim TR, Polastri PF, Barela JA (2006) Efeito do toque suave e da informação visual no controle da posição em pé de adultos. Ver Bras Educ Fís Esporte 20:15–25
Carver S, Kiemel T, van der Kooij H, Jeka JJ (2005) Comparing internal models of the dynamics of the visual environment. Biol Cybern 92:147–163
Carver S, Kiemel T, Jeka JJ (2006) Modeling the dynamics of sensory reweighting. Biol Cybern 95:123–134
Cenciarini M, Peterka RJ (2006) Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol 95:2733–2750
Day BL, Ramsay E, Welgampola MS, Fitzpatrick RC (2011) The human semicircular canal model of galvanic vestibular stimulation. Exp Brain Res 210(3–4):561–568
Dijkstra TMH, Schöner G, Giese MA, Gielen CCAM (1994) Frequency dependence of the action-perception cycle for postural control in a moving visual environment: relative phase dynamics. Biol Cybern 71:489–501
Forssberg H, Nashner LM (1982) Ontogenetic development of postural control in man: adaptation to altered support and visual conditions during stance. J Neurosci 2:545–552
Horak FB, Macpherson JM (1996) Postural orientation and equilibrium. In: Rowell LB, Shepard JT (eds) Handbook of physiology. Oxford University Press, New York, pp 255–292
Jeka JJ, Oie KS, Kiemel T (2000) Multisensory information for human postural control: integrating touch and vision. Exp Brain Res 134:107–125
Jeka JJ, Allison L, Saffer M, Zhang Y, Carver S, Kiemel T (2006) Sensory reweighting with translational visual stimuli in young and elderly adults: the role of state-dependent noise. Exp Brain Res 174:517–527
Jeka JJ, Oie KS, Kiemel T (2008) Asymmetric adaptation with functional advantage in human sensorimotor control. Exp Brain Res 191:453–463
Jeka JJ, Allison LK, Kiemel T (2010) The dynamics of visual reweighting in healthy and fall-prone older adults. J Mot Behav 42:197–208
Kiemel T, Oie KS, Jeka JJ (2006) Slow dynamics of postural sway are in the feedback loop. J Neurophysiol 95:1410–1418
Maurer C, Mergner T, Peterka R (2006) Multisensory control of human upright stance. Exp Brain Res 171:231–250
Mergner T, Hlavacka F, Schweigart G (1993) Interaction of vestibular and proprioceptive inputs. J Vestib Res 3:41–57
Oie KS, Kiemel T, Jeka JJ (2002) Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cogn Brain Res 14:164–176
Oie KS, Kiemel T, Barela JA, Jeka JJ (2005) The dynamics of sensory reweighting: a temporal asymmetry. Gait Posture 21:S29
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88:1097–1118
Polit DF (1996) Data analysis and statistics for nursing research. Appleton & Lange, Stamford
Rinaldi NM, Polastri PF, Barela JA (2009) Age-related changes in postural control sensory reweighting. Neurosci Lett 467:225–229
Seber GAF (1984) Multivariate observations. Wiley, New York
Seber GAF, Wild CJ (2003) Nonlinear regression. Wiley, Hoboken
van der Kooij H, Jacobs R, Koopman B, van der Helm F (2001) An adaptive model of sensory integration in a dynamic environment applied to human stance control. Biol Cybern 84:103–115
Winter DA, Patla AE, Frank JS, Walt SE (1990) Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther 70:340–347
Acknowledgments
The support of this research was provided by RO1 NS35070—J. Jeka PI. P. F. Polastri was sponsored by Conselho Nacional de Desenvolvimento Científico (PDEE-CNPq) to develop part of her dissertation at Cognitive Motor Neuroscience Laboratory (UMD-College Park, USA). Predoctoral Fellowship: Brazilian Government. Process 3133-05/2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polastri, P.F., Barela, J.A., Kiemel, T. et al. Dynamics of inter-modality re-weighting during human postural control. Exp Brain Res 223, 99–108 (2012). https://doi.org/10.1007/s00221-012-3244-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3244-z