Abstract
Processing of predictive contextual cues of an impending perturbation is thought to induce adaptive postural responses. Cueing in previous research has been provided through repeated perturbations with a constant foreperiod. This experimental strategy confounds explicit predictive cueing with adaptation and non-specific properties of temporal cueing. Two experiments were performed to assess those factors separately. To perturb upright balance, the base of support was suddenly displaced backwards in three amplitudes: 5, 10 and 15 cm. In Experiment 1, we tested the effect of cueing the amplitude of the impending postural perturbation by means of visual signals, and the effect of adaptation to repeated exposures by comparing block versus random sequences of perturbation. In Experiment 2, we evaluated separately the effects of cueing the characteristics of an impending balance perturbation and cueing the timing of perturbation onset. Results from Experiment 1 showed that the block sequence of perturbations led to increased stability of automatic postural responses, and modulation of magnitude and onset latency of muscular responses. Results from Experiment 2 showed that only the condition cueing timing of platform translation onset led to increased balance stability and modulation of onset latency of muscular responses. Conversely, cueing platform displacement amplitude failed to induce any effects on automatic postural responses in both experiments. Our findings support the interpretation of improved postural responses via optimized sensorimotor processes, at the same time that cast doubt on the notion that cognitive processing of explicit contextual cues advancing the magnitude of an impending perturbation can preset adaptive postural responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent investigation has suggested that balance control requires intervention of higher order sensorimotor processing in the cerebral cortex (see Bolton 2015; Jacobs 2014 for reviews). Participation of higher order levels of processing in balance control has been named “central set” (Horak et al. 1989; Horak and Nashner 1986; Prochazka 1989), conveying the notion that automatic postural responses (APRs) are regulated in accordance with contextual cues provided by predictive sensory information indicating one or more characteristics of an impending perturbation of body balance stability. More specifically, predictive contextual cues processed at a cognitive level of control are thought to induce improved postural responses based on feedforward signals from higher to lower levels of postural control (Horak et al. 1989; Horak and Nashner 1986; Prochazka 1989). Cognitive processing of contextual cues might provide information about spatial components of an impending perturbation, like direction and magnitude of forces possibly leading to destabilization of the upright posture. From this conceptualization, in conditions that explicit contextual cues of a postural perturbation are available one would predict that APRs lead to reduced body balance destabilization in comparison with conditions of uncertainty about the characteristics of an impending perturbation.
Although the concept of “central set” has been firmly established in the literature on postural control, experimental evidence for the effect of cueing characteristics of an impending balance perturbation on the generation of APRs is controversial (Diener et al. 1991; Fujio et al. 2016; Horak et al. 1989; Silva et al. 2015; Smith et al. 2012). Preliminary data on this matter were provided by Horak et al. (1989) in experiments assessing the effect of cueing velocity or amplitude of translation of the supporting platform to perturb stance. Results revealed that cueing those parameters of the impending perturbation failed to affect latency of agonist and antagonist muscles activation onset. In further investigation, Diener et al. (1991) evaluated postural responses preceded by visual cueing of direction and/or amplitude of the forthcoming rotation of the support base. Results showed that cueing failed to affect muscular responses to recover body equilibrium. More recently, Silva et al. (2015) and Fujio et al. (2016) using rotation of the support base to perturb balance found that cueing direction of the impending perturbation failed to modulate muscular responses to restore stable upright stance (see also Maki and Whitelaw 1993).
Exceptions to lack of effect of cueing about an impending perturbation on the generation of APRs were found in experiments in which cueing amplitude of the base of support displacement was provided implicitly by means of the sequence of perturbations (Beckley et al. 1991; Gilles et al. 1999; Smith et al. 2012). In the experiment by Smith et al. (2012), for instance, in the cued condition the supporting platform was moved with the same amplitude (low or high) in all trials within a block, while in the uncued condition the amplitude of platform motion was randomly varied across trials. Results showed that the cued condition induced slower initial center of pressure (CoP) displacement following the perturbation. This finding was interpreted as evidence for participation of the cognitive level of processing (uncertainty reduction) in the generation of APRs by presetting lower levels of control responsible for the organization and scaling of postural responses in agreement with the predicted characteristics of the impending perturbation. However, an ambiguity in the interpretation of these results should be noticed: at the same time that repeated exposures to the same amplitude of displacement of the base of support cued the characteristics of the forthcoming perturbation, it also allowed for adaptation of postural responses over repeated trials with the same characteristics. As the uncued condition was implemented by means of a random sequence of trials, participants may have had increased difficulty to adapt to each amplitude of platform displacement by generating different responses from the previous one in every trial. Preceding studies have shown adaptation of postural responses to a sequence of trials repeating the characteristics of the perturbation, leading to improved recovery of body equilibrium (Chong et al. 2000; Horak et al. 1989; Horak and Nashner 1986; Mierau et al. 2015; Oude Nijhuis et al. 2009; Tang et al. 2012; Welch and Ting 2014). Welch and Ting (2014), for instance, found body balance adaptation from exposure to the same postural perturbation through translation of the support base, as featured by decreasing center of mass (CoM) displacement amplitude and velocity over repeated trials. At the muscular level, adaptation was found to take place in long-latency responses, with reduction of magnitude of muscular activation as a consequence of repeated postural perturbations. These results suggest that sensory feedback from prior perturbations is used to optimize sensorimotor responses in ensuing trials with similar characteristics in the search for minimization of body destabilization and energy expenditure. From these findings, we pose the possibility that the assumed effect of cueing characteristics of an impending perturbation through repeated trials on APRs, suggesting participation of a higher order cognitive level of processing, may have been due to adaptation taking place at a lower non-cognitive level of control.
In the present investigation, we aimed to assess the individual effects of cueing the characteristics of an impending postural perturbation and adaptation to repeated exposures to the same perturbation. Stance was perturbed by means of unanticipated backward displacement of the base of support in three amplitudes. To evaluate the effect of cueing, we provided visual cues indicating the amplitude of the impending perturbation in part of the trials. Effect of adaptation was evaluated by comparing blocked trials with the same amplitude of platform displacement against a sequence of trials with random variation of amplitudes of platform displacement across trials. Based on the proposition of “central set” (Horak et al. 1989; Horak and Nashner 1986; Prochazka 1989), one could hypothesize that cueing amplitude of base of support displacement leads to adaptive postural responses. Conversely, if the effect of cueing a postural perturbation by previous repeated trials (e.g., Smith et al. 2012) is due to adaptation rather than uncertainty reduction, a competing hypothesis is that adaptive responses are achieved from blocked trials but not from explicit amplitude cueing.
Experiment 1
Methods
Participants
Twelve university students (7 women), age range 19–35 years (M = 23.83 years, SD = 6.26), height range 1.52–1.90 m (M = 1.74 m, SD = 0.11), weight range 52–101 kg (M = 71.39 kg, SD = 15.07), participated in this experiment. Inclusion criteria were having no neurological, sensorial or musculoskeletal diseases possibly affecting balance control, as declared by the participant. Participants provided informed consent and experimental procedures were approved by the Institutional Review Board of the University of São Paulo in accordance with the declaration of Helsinki.
Task and equipment
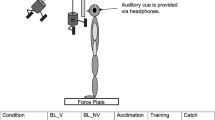
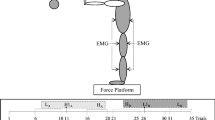
Participants were tested in upright posture with the feet positioned hip-width apart and slightly oriented sideward (self-selected as preferred), keeping their arms folded in front of the chest. The task consisted of recovering stable body equilibrium following unanticipated backward displacement of the supporting platform, keeping the feet in place to respond to the perturbation. The platform was moved in one of three amplitudes: 5, 10 and 15 cm, with respective displacement times of 450, 650 and 950 ms.Footnote 1 For the three platform displacement amplitudes, peak velocity was 20 cm/s and peak acceleration was 100 cm/s2. The moving platform was custom-built having a force plate (AMTI, model OR6) as the base of support. Platform displacement was controlled by means of software elaborated in LabVIEW (National Instruments). Kinematics of postural responses were evaluated by means of four optoelectronic cameras (Vicon, model T10), tracking passive spherical markers (14 mm in diameter) attached at joints of interest. Electromyography (EMG) of posterior muscles was recorded through wireless surface electrodes (Delsys Inc., Boston, MA, model Trigno). A pulse of 5 V generated at the onset of the force platform displacement was used to synchronize signals from the force plate, cameras and EMG at Vicon Nexus.
Experimental design and procedures
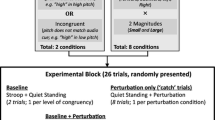
We used a single group experimental design, testing participants in four conditions resulting from the factorial combination of cueing and sequence of platform displacement amplitudes. For displacement amplitude cueing, we provided visual cues through upward vertical arrows on a monitor screen positioned in front of the participants at their eyes height. The 5-, 10- and 15-cm-long platform displacements were cued by arrows 6, 12 and 18 cm high (2 cm wide), respectively. In the cued trials, the arrow signaling the respective displacement amplitude on the current trial was shown to the participant on the monitor screen for 2 s. In the uncued condition, a directionally neutral 3-cm-sided square was presented for the same duration. Immediately after this period, a 3-cm-diameter circle was presented on the monitor screen as a warning signal. The image of the circle was maintained on the screen until the end of the trial, and the participant kept gazing at it while recovering balance following platform displacement. Following the onset of circle presentation, onset of platform displacement was randomly varied within a time window of 500–2000 ms. For manipulation of sequence of amplitudes of platform displacement, in one condition each amplitude was blocked in a single series of sequential trials. In the other condition for this factor, the three displacement amplitudes were pseudorandomly varied across trials, with the rule of no more than two trials in sequence with the same displacement amplitude. Combination of the factors’ cue and sequence resulted then in four testing conditions: cued in a block sequence, cued in a random sequence, uncued in a block sequence and uncued in a random sequence.
Participants were instructed about the following: (a) meaning of the visual cues and preparatory signals presented on the monitor screen, (b) importance of focusing attention on the amplitude cueing provided by the arrow length in the cued trials, (c) refraining from trying to anticipate the platform displacement and (d) that only feet-in-place responses should be used to recover balance stability. For evaluation, feet positions were marked with adhesive tape on the force plate to ensure the same base of support throughout the experiment. Preceding the testing trials, we evaluated participants’ baseline CoP position in quiet stance and provided participants with familiarization trials in the specific experimental condition. For the block sequence condition, participants performed one block of five familiarization trials for each displacement amplitude. In the random sequence condition, participants performed a single block of 15 familiarization trials in a random sequence across the three amplitudes (five trials each). Immediately after the respective familiarization trials, evaluation of postural responses was made through three trials for each platform displacement amplitude in the four experimental conditions. Participants performed, then, 36 testing trials in total. The order of experimental conditions was counterbalanced across participants.
For testing, CoP position was visually monitored in the period immediately preceding platform displacement to prevent anticipatory postural adjustments. If participants failed to position their CoP about the same location as observed in quiet stance (based on subjective visual evaluation), they were oriented by the experimenter to adjust body position to assume the approximate baseline CoP positioning. Following the perturbing backward platform displacement, participants stayed on the platform while it was slowly returned to the initial position. Inter-trial intervals within a block endured about 10 s, while an interval of 5 min was provided after half of the testing trials. For this latter interval participants rested sat on a chair.
Following skin trichotomy and asepsis, the electrodes were positioned over the muscles gastrocnemius medialis (GM), biceps femoris (BF) and erector spinae (ES) of the right side of the body. Electrodes were positioned following the recommendations from the European project of surface EMG for non-invasive assessment of muscles (http://www.seniam.org/). Spherical kinematic markers were attached at the following anatomical points on the right side of the body: fifth metatarsophalangeal joint, lateral malleolus, lateral knee joint center, greater trochanter and at the approximate axis of shoulder rotation.
Data collection and analysis
Data were extracted and processed through MATLAB (Mathworks, Natick, MA) routines, following preliminary visual inspection of signals for individual trials. Data sampling frequency was set at 2000 Hz for EMG and at 200 Hz for kinematics and ground reaction forces. EMG signals were amplified with a gain of 1000, and digitally band-pass filtered between 20 and 400 Hz. Kinematic and ground reaction forces data were digitally low-pass filtered with a cut-off frequency of 10 Hz. Signals were filtered through a dual-pass fourth-order Butterworth filter. Estimation of center of mass (CoM) displacement was based on the anthropometric model proposed by Winter (1991), assuming symmetric displacement between both body sides.
The following dependent variables were analyzed: peak values for (a) amplitude and (b) velocity of CoP displacement, (c) amplitude of CoM displacement, and amplitude of (d) hip and (e) ankle rotation following platform motion onset. (f) Stability of automatic postural responses (SPR), calculated by integrating the difference between the CoP and CoM time series (normalized to the participant’s height) over 150 ms following 50 ms of the GM muscle activation onset; this variable provides a measure of the margin of stability of the postural response (Winter et al. 1998). High values of SPR are interpreted to indicate increased postural stability. (g) Latency of muscular activation onset having as the criterion the time that increasing EMG in response to the perturbation reached the value of two standard deviations above the EMG average in the interval of 200 ms preceding platform displacement onset. (h) Magnitude of muscular activation, estimated by means of root mean square (RMS) of the EMG envelope in the interval of 75 ms following muscular activation onset; raw values were normalized to the respective individual maximum value in the interval of interest across experimental conditions.
Statistical analysis was made for the 5-cm amplitude of platform displacement, using the other displacement amplitudes for manipulation of sequence of displacement amplitudes. As several reported effects from different analyses were observed in the 5-cm but not in the 10- and 15-cm displacement amplitudes,Footnote 2 results presented here should not be generalized for all amplitudes of platform translation. Requirements for use of parametric statistics were evaluated by means of the Kolmogorov–Smirnov test. Data were analyzed through two-way 2 (cue: cued × uncued) × 2 (sequence: block × random) ANOVAs with repeated measures on both factors. Post hoc comparisons were made through the Duncan test. Significant effects (p ≤ 0.05) are reported for F 1,11 degrees of freedom, accompanied by the respective effect sizes given by the partial eta squared (η 2 p ).
Results
Stepping responses to recover balance were not observed across participants over the different amplitudes of platform displacement. Figure 1 shows single trial signals for the characteristic postural responses to platform translation comparing the four experimental conditions. In that figure, we present different dimensions of postural responses: CoP (B) displacement and (C) velocity, (D) CoM displacement, and activation of the (F) GM, (G) BF and (H) ES muscles; with time of onset of platform translation indicated by vertical dotted lines, while the upper panels show the characteristics of (A) displacement and (E) velocity of platform translation.
Representative single trial signals contrasting the conditions of cued (C) and uncued (U) perturbations for the random (R) and block (B) sequences. The following signals are represented for the 5-cm amplitude of platform displacement: CoP b displacement and c velocity, d CoM displacement, and activation of the muscles f GM, g BF, and h ES. The upper panels show the characteristics of a displacement and e velocity of platform translation, while dashed vertical lines represent the onset of platform displacement
Across analyses, significant main effects of sequence were found in different variables (indicated by asterisk in the figures), while neither significant main effects of cueing nor interactions were observed. For analysis of peak amplitude of CoP displacement the significant main effect of sequence, F = 6.71, p = 0.025, η 2 p = 0.38, was due to lower values for the block (M = 7.09 cm, SE = 0.53) in comparison with the random (M = 7.90 cm, SE = 0.44) sequence (Fig. 2a). For analysis of CoP peak velocity the significant sequence effect, F = 6.89, p = 0.02, η 2 p = 0.39, was due to lower velocities in the block (M = 52.92 cm/s, SE = 6.79) than in the random (M = 61.49 cm/s, SE = 6.01) sequence (Fig. 2b). Analysis of peak amplitude of CoM displacement indicated also the significant sequence effect, F = 6.44, p = 0.03, η 2 p = 0.37, due to lower values in the block (M = 5.49 cm, SE = 0.49) than in the random (M = 6.43 cm, SE = 0.42) sequence (Fig. 2c). Figure 2d shows the representation of the relation between CoP and CoM displacements following onset of platform motion (vertical dashed line), with the epoch used for calculation of stability of the postural response indicated by the shaded area. Analysis revealed the significant sequence effect, F = 4.98, p = 0.05, η 2 p = 0.32, with higher values for the block, (M = 3.54, SE = 0.36) than for the random (M = 3.12, SE = 0.28) sequence (Fig. 2e).
Average values (standard errors in vertical bars) across experimental conditions (cueing x sequence), showing, respectively, CoP a peak amplitude and b peak velocity, c CoM peak displacement, d representative curves of CoP (thin line) and CoM (thick line) and the interval used for calculation of stability of postural responses (SPR, shaded area) following onset of platform motion (vertical dashed line), with the corresponding e SPR values, and f hip peak rotation amplitude following postural perturbation. Asterisks represent significant sequence effects
Analysis of peak amplitude of hip rotation indicated the significant main effect of sequence, F = 10.72, p = 0.007, η 2 p = 0.49, due to lower values for the block (M = 2.91°, SE = 0.37) than for the random (M = 3.70°, SE = 0.48) sequence (Fig. 2f). Analysis of peak amplitude of ankle rotation showed a similar trend, but it did not reveal any significant effects, p values > 0.2 (mean across conditions = 2.38″, SE = 0.32).
Analysis of latency of muscular activation onset indicated the significant main effect of sequence for GM, F = 7.34, p = 0.02, η 2 p = 0.40, due to higher values for the block (M = 103.45 ms, SE = 7.26) than for the random (M = 99.42 ms, SE = 8.15) sequence (Fig. 3a). The sequence effect in the analysis of ES, F = 4.32, p = 0.05, η 2 p = 0.28, revealed a different relation, with significantly lower values in the block (M = 171.43 ms, SE = 10.72) than in the random (M = 189.09 ms, SE = 11.49) sequence (Fig. 3e). Analysis of BF failed to reveal any significant effects, p values > 0.1 (Fig. 3c). To evaluate whether the differential effect of sequence between the GM and ES muscles affected the inter-muscular activation timing, we analyzed the delta between GM and ES activation onsets. Results indicated the significant main effect of sequence, F = 6.69, p = 0.03, η 2 p = 0.28, due to lower values for the block (M = 66.04 ms, SE = 8.03) than for the random (M = 89.08 ms, SE = 9.71) sequence.
Average values (standard errors in vertical bars) across experimental conditions (cueing x sequence) for latency of activation onset and normalized magnitude of activation, respectively, for the muscles a, b gastrocnemius medialis (GM), c, d biceps femoris (BF), and e, f erector spinae (ES). Asterisks represent significant sequence effects
Analysis of magnitude of muscular activation in the period preceding platform displacement showed no significant effects across the evaluated muscles, p values > 0.1. Results for magnitude of muscular activation in response to platform motion indicated the sequence effect for GM, F = 9.49, p = 0.01, η 2 p = 0.46, with lower values for the block (M = 0.55, SE = 0.07) than for the random (M = 0.68, SE = 0.07) sequence (Fig. 3b). Analysis of activation magnitude of BF (Fig. 3d) and ES (Fig. 3f) failed to reveal any significant effects, p values > 0.3.
Discussion
Results from Experiment 1 showed across different variables the effect of sequence of trials but not of explicit cueing. The block in comparison with the random sequence of platform displacement amplitudes led to lower peak amplitudes of CoP and CoM displacements and of hip rotation, lower CoP peak velocity, and increased stability of postural responses following the perturbation. In the analysis of muscular responses, the block sequence induced longer latency of activation onset in association with lower magnitude of activation for the muscle GM, and lower latency of activation onset for the muscle ES. Although amplitude cueing is inherent to blocked trials, lack of effect of explicit cueing indicates that the diverse modulations of postural responses as a function of trials sequence were not due to increased predictability of the characteristics of the upcoming trial provided explicitly by the visual cue.
Adaptation of APRs from repeated exposure
Modulation of postural responses in the block sequence was found to be adaptive, with increased balance stability following perturbation as indicated by lower amplitude of CoM displacement and increased stability of postural responses. An intriguing facet of the results was that the improved balance stability in the block sequence was associated with reduced peak amplitude and velocity of CoP displacement. This finding indicates that body equilibrium was recovered in the block condition by means of a reduced rate of torque exertion at the ankles following platform motion. It is plausible that reduced torque at the ankles in the block condition is associated with the lower magnitude of activation of the muscle GM, one of the agonists for ankle plantar flexion. These data suggest that repeated trials with the same amplitude induced body balance recovery not only with increased stability but also with lower energy consumption in comparison with the random sequence. Improved balance stability allied to reduced energetic cost from repeated perturbations with the same parameters was expected from previous findings (Blouin et al. 2003; Marigold and Patla 2002; Welch and Ting 2014), and indicates that a sequence of trials with the same magnitude of perturbation optimizes postural responses. Optimization of postural responses to a series of perturbations with the same characteristics has been proposed to be a consequence of the sensorimotor system adjusting the gain of the different feedback sources signaling loss of body stability from responses produced in previous trials (Mierau et al. 2015; Welch and Ting 2014). From this perspective, repeated perturbations seem to lead to the formation of a sensorimotor set, acting in a feedforward manner to adjust response parameters like joint stiffness and muscle viscosity (Kim et al. 2009; Schuurmans et al. 2011) in the ensuing perturbations. A random sequence of perturbations, on the other hand, seems to prevent fast optimal adaptation by requiring different scaling of postural responses every trial. As in this experimental condition the preceding trial requires specification of different response parameters to recover balance stability, the time available for detecting the perturbation magnitude and scaling the respective response may be insufficient for response optimization in situations that the sequence of perturbations is randomized.
A further point in our results that might contribute to understanding adaptation of postural responses from repeated perturbations is the difference of inter-muscular activation onset delays between the block and random sequences. In the block sequence muscular activation onset was earlier for the muscle ES and later for the muscle GM in comparison with the random sequence. With that modulation of the timing of muscular responses the delay of inter-muscular activation onset was shorter between important posterior muscles for recovery of balance stability in the block as compared to the random sequence. Considering that reduction of the delay of inter-muscular activation onset was associated with increased stability of balance recovery in the block sequence, it is possible that the observed modulation of the timing of muscular activation leads to increased effectiveness of the muscular synergy employed to recover stable balance at the same time that might make the response energetically more economic. We hypothesize that such an optimization of muscular responses is mediated by feedforward processes available in the block sequence only.
Perturbation magnitude cueing
Our results revealed that explicitly cueing amplitude of the impending platform displacement failed to modulate postural responses to balance perturbation. Considering that magnitude of the early component of muscular responses is based on feedforward control (Horak et al. 1996), lack of effect of cueing on early muscular activation suggests that predictability of the characteristics of the impending perturbation was unable to modulate the early component of postural responses. Additionally, lack of effect of cueing on peak CoM and CoP displacements suggests that perturbation predictability was also unable to affect later components of APRs. Then, our results are contradictory to the notion that cognitive processing of contextual cues reducing uncertainty about an impending postural perturbation could adjust the central set to generate adaptive APRs (cf. Prochazka 1989). From the current and previous findings (e.g., Diener et al. 1991), it becomes apparent that APRs to a sudden perturbation are impermeable to cognitive processing of the magnitude of an impending balance perturbation. On the other hand, as the adaptive behavior was found in the blocked trials, causing a predictable perturbation on balance stability, it is plausible that repeating the same perturbation over sequential trials has been used by the postural control system as an implicit cue favoring predictive mechanisms at a non-cognitive level (see Bubic et al. 2010). In this case, perturbation predictability at a non-cognitive level might induce adjustments mediated by feedforward processes, leading to increased stance stability of automatic postural responses.
An argument that could be made against the interpretation of lack of effect of predictive cueing on APRs is that we produced postural perturbations in the condition of temporal uncertainty. Previous investigation has shown that adaptive postural responses to a predictable perturbation were observed in a context in which timing of perturbation onset was also cued by means of a constant foreperiod (Jacobs et al. 2008; Mochizuki et al. 2010; Smith et al. 2012). At the neurological level, it has been shown that cortical activation in the pre-perturbation epoch is associated with temporal predictability of events leading to balance perturbation (Jacobs et al. 2008; Maeda and Fujiwara 2007; Mochizuki et al. 2008). In line with these results, Mochizuki et al. (2010) pose the possibility that predictive cues about characteristics of an impending perturbation could be useful for generation of APRs only in the context of full predictability of a perturbation, with anticipation of its direction, magnitude and onset time. On the other hand, cueing simultaneously the perturbation characteristics and its timing leads to an ambiguous interpretation. Certainty of perturbation timing could favor the generation of the response, regardless of predictability of perturbation direction. Some support for this notion has been provided by results showing that cueing timing of a postural perturbation leads to reduction of the latency of muscular activation onset (McChesney et al. 1996; Silva et al. 2015) and of magnitude of muscular activation (Fujio et al. 2016), but not predictive directional cueing. From these results, it is possible that the assumed effect of processing of contextual cues advancing the required scaling of APRs to a specific perturbation (Jacobs et al. 2008; Mochizuki et al. 2010; Smith et al. 2012) be due to processes associated with temporal predictability of perturbation onset rather than to processing of the physical characteristic of the impending perturbation. In Experiment 2, we aimed to evaluate separately the effects of cueing the amplitude of an impending balance perturbation and cueing the timing of perturbation onset. We hypothesized that if modulation of postural responses by cueing magnitude and timing of a perturbation (Jacobs et al. 2008; Mochizuki et al. 2010; Smith et al. 2012) is due to reduction of temporal uncertainty, only conditions provided with perturbation timing cueing lead to adaptive APRs.
Experiment 2
Methods
The method employed in the current experiment was the same as that described for Experiment 1, except for the points presented below.
Participants
Healthy university students (n = 13, 11 women), different from those participating of the Experiment 1, volunteered for this experiment. Age range 18–28 years (M = 20.31, SD = 2.63), height range 1.52–1.83 m (M = 1.64 m, SD = 0.08), and weight range 45–79 kg (M = 58.16 kg, SD = 10.43). Inclusion criteria were having no neurological, sensorial or musculoskeletal diseases possibly affecting balance control, as declared by the participant. Participants provided informed consent and experimental procedures were approved by the Institutional Review Board of the University of São Paulo in accordance with the declaration of Helsinki.
Experimental design and procedures
A single group experimental design was employed to evaluate the effect of cueing perturbation amplitude and timing individually and in combination. Trials were performed in a random sequence of amplitudes of platform translation (5, 10 and 15 cm). In one condition, we cued amplitude of platform displacement (AMP), using the same procedures as described for Experiment 1 for the random-cued condition. For cueing perturbation timing (TIM), during the period of the warning visual stimulus display participants were provided with three beeps (through loudspeakers) at constant intervals of 1 s. Computer-controlled platform translation was initiated in coincidence with the third beep of that series. In the other cued condition, participants were given both amplitude and timing cues (CAT). As a control condition, neither amplitude nor timing cueing was provided (NoC). In the conditions in which timing was not cued (AMP, NoC), platform displacement was initiated in the interval of 500–2000 ms following the appearance of the warning stimulus.
The experiment was initiated by instructing participants about the cueing signals. For familiarization with cueing signals, participants performed five trials for each amplitude of platform displacement in a random sequence, receiving both amplitude and timing cues. An extra set of nine familiarization trials (three for each amplitude, random sequence) was performed in each experimental condition immediately before the respective testing trials. Intervals of 5 min were offered between trials for each condition, during which participants rested sat on a chair.
Analysis
Data were analyzed through one-way (cueing: time × amplitude × amplitude and time × uncued) ANOVA for repeated measures. Significant effects are reported for F 3,36 degrees of freedom.
Results
In Fig. 4 are shown single trial signals representing the profiles found in each experimental condition for CoP (B) displacement and (C) velocity, (D) CoM displacement, and activation of the (F) GM, (G) BF and (H) ES muscles, with platform (A) displacement and (E) velocity displayed at the top. This figure represents the relationship found in different variables associated with postural responses to the sudden platform translation. Namely, the two conditions provided with timing cueing (TIM, CAT) induced similar adaptive modulation of postural responses (no significant differences between these conditions across analyses), while the condition of translation amplitude cueing (AMP) led to non-significantly different responses in comparison with the uncued condition across the variables analyzed. That relationship between experimental conditions was found in the analyses of peak amplitude of CoP displacement, F = 6.92, p = 0.001, η 2 p = 0.37 (Fig. 5a); peak amplitude of CoM displacement, F = 3.06, p = 0.04, η 2 p = 0.20 (Fig. 5b); stability of postural responses, F = 11.92, p = 0.001, η 2 p = 0.50 (Fig. 5c). Analyses of CoP peak velocity, F = 1.72, p = 0.18 (mean across conditions = 63.71 cm/s, SE = 5.22), and peak rotation amplitude at the hip, F = 1.26, p = 0.30 (mean across conditions = 4.85°, SE = 0.72), and ankle, F = 1.90, p = 0.15 (mean across conditions = 2.95″, SE = 0.40) failed to indicate significant differences across experimental conditions.
Single trial signals are shown for representative profiles of the 5-cm amplitude of platform displacement in the conditions cueing perturbation amplitude (AMP), timing (TIM) and both (CAT), in comparison with no cueing (NoC). The following signals are represented: CoP b displacement and c velocity, d CoM displacement, and activation of the muscles f GM, g BF and h ES. The upper panels show the characteristics of a displacement and e velocity of platform translation, while dashed vertical lines represent the onset of platform displacement
Average values (standard errors in vertical bars) across experimental conditions (AMP platitude cueing; TIM timing cueing; CAT both; NoC no cueing) showing peak amplitude for a CoP and b CoM, c stability of postural responses, and latency of activation onset for the muscles d gastrocnemius medialis (GM), e biceps femoris (BF), and f erector spinae (ES) following the postural perturbation. Asterisks represent significant differences of TIM-CAT in comparison with AMP-NoC
Analysis of magnitude of muscular activation in the period preceding platform displacement showed no significant differences across conditions for the three muscles evaluated, p values > 0.6. EMG analysis in the period following perturbation showed that at both conditions cueing timing of platform translation (TIM, CAT) led to increased latency of muscular activation onset in comparison with the AMP and NoC conditions, with no significant differences between the latter: GM, F = 4.98, p = 0.005, η 2 p = 0.29 (Fig. 5d); BF, F = 23.96, p = 0.001, η p 2 = 0.69 (Fig. 5e); and ES, F = 17.08, p = 0.001, η p 2 = 0.63 (Fig. 5f). Analyses of activation magnitude for GM (mean across conditions = 0.57, SE = 0.07), BF (mean across conditions = 0.49, SE = 0.08) and ES (mean across conditions = 0.51, SE = 0.08) showed no significant effects, p values > 0.3.
Discussion
Results from Experiment 2 showed that at both conditions cueing timing of platform translation onset led to adaptive postural responses, while no effect was found for exclusive cueing of amplitude of platform translation. Our data revealed that predictability of perturbation onset time induced improved postural responses. The main result showing improved postural responses in the two conditions receiving perturbation timing cueing was lower peak amplitude of CoM displacement. That finding indicates reduced body sway following the perturbation, and then reduced risk of disequilibrium as a result of timing cueing. That result was associated with reduced peak amplitude of CoP displacement, indicating that in the conditions receiving the perturbation timing cueing CoP displacement was farther from reaching the balance stability limits on the support base in comparison with the conditions not receiving the timing cueing. Given that improved postural responses from perturbation timing cueing were observed in the condition of uncertainty about amplitude of platform displacement (TIM), these results suggest that awareness of the kind of the impending perturbation was unable to modulate APRs generation.
A particularly interesting finding was that improved postural responses in the conditions providing cueing on perturbation timing was delayed activation onset in the muscles participating in the response synergy. This effect was observed in the lower–upper leg and trunk muscles the same way in both conditions offering temporal cueing, and it was consistent with the delayed activation onset of the GM muscle in the blocked condition in Experiment 1. This result could be considered counterintuitive given that temporal cueing has been shown to lead to early onset times of muscular activation in response to balance perturbations produced by rotation of the support platform (McChesney et al. 1996; Silva et al. 2015). Responses to balance perturbation induced by platform translation, however, may have been optimized by a distinct response strategy. Platform rotation at the ankle requires a fast recovery of body vertical orientation to prevent a fall. In this case, fast muscular response may be thought to favor keeping upright balance. Responses to short backward platform translation, on the other hand, seem to be improved by inhibiting the ill-controlled startle reflex burst of muscular activation (cf. Oude Nijhuis et al. 2010), while giving place to a more efficient response with increased participation of higher levels of control. In a similar reasoning, Fujio et al. (2016) have proposed that when predictive cueing of perturbation timing is available the central nervous system modulates the reflex gain triggered by the stretched muscles, leading to optimization of postural responses. From this perspective, APRs would be downregulated adaptively and integrated into the motor output in a functional way at a task level of control (Safavynia and Ting 2013; Weerdesteyn et al. 2008; Welch and Ting 2014). In line with this interpretation, Mihara et al. (2008) showed that providing a constant time between a directionally neutral warning signal and a postural perturbation onset led to activation of the right posterior parietal cortex and supplementary motor area. The posterior parietal cortex receives multimodal inputs from different sensory organs potentially providing information relevant for scaling of postural responses (Andersen et al. 1997). Additional evidence has shown that the posterior parietal cortex is involved in detecting postural instability (Slobounov et al. 2006), and in the dynamic representation of the body schema (Pellijeff et al. 2006). Recent results have shown that the locus of the cortical response to a sudden postural perturbation is at the supplementary motor area (Ferraye et al. 2014; Fujimoto et al. 2014; Marlin et al. 2014; Mierau et al. 2015), and that it is correlated with magnitude of postural sway and respective muscular activation (Mierau et al. 2015). From these findings, we conjecture that cueing timing of a postural perturbation leads to timely activation of cortical areas responsible for selecting and scaling APRs in consonance with sensory feedback signaling destabilization of body balance.
General discussion
Findings from both Experiments 1 and 2 converged to show that explicitly cueing the amplitude of an impending balance perturbation fails to induce adaptive postural responses. Similar background muscular activation in the epoch immediately preceding stance perturbation supports the assumption that muscular responses were reactive rather than associated with anticipatory postural adjustments. Results from Experiment 1 lead to the interpretation that the effect of using blocked trials to make perturbation characteristics predictable might be due to adaptation based on optimization of feedforward and feedback mechanisms from repeated exposure to the same perturbation over trials. In Experiment 2, we showed that cueing timing of perturbation onset is sufficient to induce improved postural responses, which were not changed by providing explicit cueing of perturbation amplitude. Our findings, therefore, suggest that automatic postural responses are unaffected by cognitive processing of contextual cues making predictable at the cognitive level the magnitude of an impending perturbation.
Our results suggesting the null effect of explicit contextual cues of amplitude of the base of support displacement on APRs could be thought to be contradictory to evidence of participation of cortical structures in the generation of postural responses to unanticipated perturbations (Bolton 2015, for a review). Cortical activation following balance perturbation has been suggested to be associated with sensory processing (Dietz et al. 1985; Quant et al. 2004a, b), error detection of upright balance (Adkin et al. 2006), and more recently to magnitude of postural sway and muscular activation following a sudden balance perturbation (Mierau et al. 2015). However, it should be noticed that cortical activation is not necessarily related with task-specific cognitive processing in balance control, like predicting the postural sway (and the required response) provoked by a mechanical perturbation when provided with a contextual cue signaling perturbation parameters (e.g., direction, magnitude). Interaction between cortical and subcortical structures in the generation of APRs (e.g., Ferraye et al. 2014; Ouchi et al. 1999) could take place at a higher order organization of the response without calling upon cognitive processing of the anticipated consequences of an impending perturbation. From this perspective, participation of cortical structures in the generation of APRs is not incompatible with the proposition of dissociation between cognitive processing of predictive cues of the magnitude of an impending perturbation and automatic postural responses. As an alternative counterpoint to our interpretation, one could argue that results showing impairment of APRs by performing a secondary cognitive task (Coelho et al. 2016; Little and Woollacott 2014, 2015; Norrie et al. 2002) represent evidence for cognitive participation in the production of APRs. In this case, performance of a secondary cognitive task might be conceived to affect balance recovery because of shared attentional resources between the two tasks rather than interference in task-specific cognitive processing. Then, findings of cortical involvement and attentional requirements for APRs generation could not be argued as evidence for penetrability of cognitive processing of contextual cues into automatic postural responses.
Core in the conceptualization of “central set” is the notion that processing of contextual cues at the cognitive level induces adaptive postural responses based on feedforward control (Horak et al. 1989; Horak and Nashner 1986; Prochazka 1989). Adaptive postural responses from repeated exposure to the same perturbation (Experiment 1) have led to the interpretation that prior perturbations are used to adjust the sensitivity of feedback-based sensorimotor processes controlling postural responses in ensuing trials (Welch and Ting 2014). From this perspective, muscular responses to cutaneous–muscular receptors on the feet soles (Meyer et al. 2004a, b; Thompson et al. 2011), and in muscle spindles of the lower leg (Thompson et al. 2011) and more proximally in the upper leg or trunk (Bloem et al. 2000, 2002) would be modulated over repeated trials in the search for optimization of APRs to preserve body balance stability. However, adaptation from repeated perturbations over trials in Experiment 1 might also be explained by considering that repetition of the same perturbation over a series of trials might play the role of an implicit cue (Bubic et al. 2010), priming a suitable postural response through feedforward processes (Horak et al. 1989; Horak and Nashner 1986; Prochazka 1989). Results from Experiment 2 allowed for a more direct conclusion about this issue, given that we used exclusively random sequences of perturbations across the testing conditions. The finding that adaptive responses were observed from the timing cueing in perturbations different from the preceding one suggests that APRs optimization was based on feedback processes instead of priming a specific postural response via feedforward control.
Observation of muscular activation onset latencies in the range of 90–200 ms across conditions and experiments suggests that adaptation of postural responses relied on long-latency responses, mediated by high levels of movement control rather than peripheral medullary reflexes. Previous results have been interpreted to indicate that APRs to unanticipated perturbations are characterized by early automatic (stereotyped) and late higher order processing epochs (Maki and McIlroy 2007). Our results, conversely, showed that the early component of muscular responses was modulated in magnitude (Experiment 1, GM) and timing (Experiment 1, GM and ES; Experiment 2, GM, BF and ES). Thus, the current findings support the conclusion that early components of APRs were affected by sequence of perturbations and timing cueing. Interestingly, we found that the major adaptation across experiments was longer activation onset latencies for the block sequence (Experiment 1: GM) and perturbation time cueing (Experiment 2: GM, BF and EE), while activation delay was diminished in the muscle ES in the block sequence (Experiment 1). These results may indicate that timing of activation can be differentially adjusted across muscles by the central nervous system in the search for optimization of postural responses. The finding that altered early muscular activation was associated with improved balance stability in both experiments suggests that not only the initial muscular burst but also the entire postural response was affected by repetition and timing cueing of a balance perturbation.
One of the main conclusions from our results was that the explicit cueing of platform displacement amplitude failed to induce any effects on the generation of APRs. Thus, adaptive postural responses as result of blocked trials do not seem to be associated with processing of the perturbation characteristics at a cognitive level but with optimization of postural responses over repeated trials having the same response requirements. Provision of cueing of perturbation timing is supposed to favor feedback-based processes inducing adaptive postural responses. Consequently, our findings are contradictory to the theorization proposing that processing of explicit contextual cues advancing the magnitude of an impending perturbation (uncertainty reduction) can preset adaptive postural responses leading to improved balance recovery (Horak et al. 1989; Horak and Nashner 1986; Prochazka 1989). Then, the findings presented in the current investigation suggest a limitation of cognitive processing to affect automatic postural responses.
Notes
Graphical representation of displacement and velocity for the three amplitudes of platform translation is presented as Supplementary Material.
Responses to the 5-cm displacement amplitude induced clearer differences between the experimental conditions. It is possible that the shorter time to complete the platform displacement in the 5-cm amplitude prevented more extensive online feedback-based adjustments following the early response component, being then more clearly related to APRs. The data for the 10- and 15-cm displacement amplitudes are presented as Supplementary material.
References
Adkin AL, Quant S, Maki BE, McIlroy WE (2006) Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res 172:85–93
Andersen RA, Snyder LH, Bradley DC, Xing J (1997) Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20:303–330
Beckley DJ, Bloem BR, Remler MP, Roos RA, Van Dijk JG (1991) Long latency postural responses are functionally modified by cognitive set. Electroencephalogr Clin Neurophysiol 81:353–358
Bloem BR, Allum JH, Carpenter MG, Honegger F (2000) Is lower leg proprioception essential for triggering human automatic postural responses? Exp Brain Res 130:375–391
Bloem BR, Allum JH, Carpenter MG, Verschuuren JJ, Honegger F (2002) Triggering of balance corrections and compensatory strategies in a patient with total leg proprioceptive loss. Exp Brain Res 142:91–107
Blouin JS, Descarreaux M, Belanger-Gravel A, Simoneau M, Teasdale N (2003) Attenuation of human neck muscle activity following repeated imposed trunk-forward linear acceleration. Exp Brain Res 150:458–464
Bolton DA (2015) The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci Biobehav Rev 57:142–155
Bubic A, von Cramon DY, Schubotz RI (2010) Prediction, cognition and the brain. Front Hum Neurosci 4:25
Chong RK, Horak FB, Woollacott MH (2000) Parkinson’s disease impairs the ability to change set quickly. J Neurol Sci 175:57–70
Coelho DB, Bourlinova C, Teixeira LA (2016) Higher order balance control: distinct effects between cognitive task and manual steadiness constraint on automatic postural responses. Hum Mov Sci 50:62–72
Diener HC, Horak F, Stelmach G, Guschlbauer B, Dichgans J (1991) Direction and amplitude precueing has no effect on automatic posture responses. Exp Brain Res 84:219–223
Dietz V, Quintern J, Berger W (1985) Afferent control of human stance and gait: evidence for blocking of group I afferents during gait. Exp Brain Res 61:153–163
Ferraye MU, Debû B, Heil L, Carpenter M, Bloem BR, Toni I (2014) Using motor imagery to study the neural substrates of dynamic balance. PLoS ONE 9:e91183
Fujimoto H, Mihara M, Hattori N et al (2014) Cortical changes underlying balance recovery in patients with hemiplegic stroke. Neuroimage 85:547–554
Fujio K, Obata H, Kawashima N, Nakazawa K (2016) The effects of temporal and spatial predictions on stretch reflexes of ankle flexor and extensor muscles while standing. PLoS ONE 11:e0158721
Gilles M, Wing AM, Kirker SG (1999) Lateral balance organisation in human stance in response to a random or predictable perturbation. Exp Brain Res 124:137–144
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55:1369–1381
Horak FB, Diener HC, Nashner LM (1989) Influence of central set on human postural responses. J Neurophysiol 62:841–853
Horak FB, Frank J, Nutt J (1996) Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol 75:2380–2396
Jacobs JV (2014) Why we need to better understand the cortical neurophysiology of impaired postural responses with age, disease, or injury. Front Integr Neurosci 8:69
Jacobs JV, Fujiwara K, Tomita H, Furune N, Kunita K, Horak FB (2008) Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophysiol 119:1431–1442
Kim S, Horak FB, Carlson-Kuhta P, Park S (2009) Postural feedback scaling deficits in Parkinson’s disease. J Neurophysiol 102:2910–2920
Little CE, Woollacott M (2014) Effect of attentional interference on balance recovery in older adults. Exp Brain Res 232:2049–2060
Little CE, Woollacott M (2015) EEG measures reveal dual-task interference in postural performance in young adults. Exp Brain Res 233:27–37
Maeda K, Fujiwara K (2007) Effects of preparatory period on anticipatory postural control and contingent negative variation associated with rapid arm movement in standing posture. Gait Posture 25:78–85
Maki BE, McIlroy WE (2007) Cognitive demands and cortical control of human balance-recovery reactions. J Neural Transm 114:1279–1296
Maki BE, Whitelaw RS (1993) Influence of expectation and arousal on center-of-pressure responses to transient postural perturbations. J Vestib Res 3:25–39
Marigold DS, Patla AE (2002) Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol 88:339–353
Marlin A, Mochizuki G, Staines WR, McIlroy WE (2014) Localizing evoked cortical activity associated with balance reactions: does the anterior cingulate play a role? J Neurophysiol 111:2634–2643
McChesney JW, Sveistrup H, Woollacott MH (1996) Influence of auditory precuing on automatic postural responses. Exp Brain Res 108:315–320
Meyer PF, Oddsson LI, De Luca CJ (2004a) Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp Brain Res 157:526–536
Meyer PF, Oddsson LI, De Luca CJ (2004b) The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res 156:505–512
Mierau A, Hulsdunker T, Struder HK (2015) Changes in cortical activity associated with adaptive behavior during repeated balance perturbation of unpredictable timing. Front Behav Neurosci 9:1–12
Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S (2008) Role of the prefrontal cortex in human balance control. Neuroimage 43:329–336
Mochizuki G, Sibley KM, Esposito JG, Camilleri JM, McIlroy WE (2008) Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clin Neurophysiol 119:1626–1637
Mochizuki G, Boe S, Marlin A, McIlRoy WE (2010) Perturbation-evoked cortical activity reflects both the context and consequence of postural instability. Neuroscience 170:599–609
Norrie RG, Maki BE, Staines WR, McIlroy WE (2002) The time course of attention shifts following perturbation of upright stance. Exp Brain Res 146:315–321
Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M (1999) Brain activation during maintenance of standing postures in humans. Brain 122(Pt 2):329–338
Oude Nijhuis LB, Allum JH, Borm GF, Honegger F, Overeem S, Bloem BR (2009) Directional sensitivity of “first trial” reactions in human balance control. J Neurophysiol 101:2802–2814
Oude Nijhuis LB, Allum JH, Valls-Sole J, Overeem S, Bloem BR (2010) First trial postural reactions to unexpected balance disturbances: a comparison with the acoustic startle reaction. J Neurophysiol 104:2704–2712
Pellijeff A, Bonilha L, Morgan PS, McKenzie K, Jackson SR (2006) Parietal updating of limb posture: an event-related fMRI study. Neuropsychologia 44:2685–2690
Prochazka A (1989) Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33:281–307
Quant S, Adkin AL, Staines WR, Maki BE, McIlroy WE (2004a) The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neurosci 5:18
Quant S, Adkin AL, Staines WR, McIlroy WE (2004b) Cortical activation following a balance disturbance. Exp Brain Res 155:393–400
Safavynia SA, Ting LH (2013) Long-latency muscle activity reflects continuous, delayed sensorimotor feedback of task-level and not joint-level error. J Neurophysiol 110:1278–1290
Schuurmans J, van der Helm FC, Schouten AC (2011) Relating reflex gain modulation in posture control to underlying neural network properties using a neuromusculoskeletal model. J Comput Neurosci 30:555–565
Silva MB, Coelho DB, de Lima-Pardini AC, Martinelli AR, da Silva Baptista T, Ramos RT, Teixeira LA (2015) Precueing time but not direction of postural perturbation induces early muscular activation: comparison between young and elderly individuals. Neurosci Lett 588:190–195
Slobounov S, Wu T, Hallett M (2006) Neural basis subserving the detection of postural instability: an fMRI study. Mot Control 10:69–89
Smith BA, Jacobs JV, Horak FB (2012) Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Exp Brain Res 222:455–470
Tang KS, Honegger F, Allum JH (2012) Movement patterns underlying first trial responses in human balance corrections. Neuroscience 225:140–151
Thompson C, Belanger M, Fung J (2011) Effects of plantar cutaneo-muscular and tendon vibration on posture and balance during quiet and perturbed stance. Hum Mov Sci 30:153–171
Weerdesteyn V, Laing AC, Robinovitch SN (2008) Automated postural responses are modified in a functional manner by instruction. Exp Brain Res 186:571–580
Welch TD, Ting LH (2014) Mechanisms of motor adaptation in reactive balance control. PLoS ONE 9:e96440
Winter DA (1991) The biomechanics and motor control of human gait: normal, elderly and pathological. University of Waterloo Press, Waterloo
Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K (1998) Stiffness control of balance in quiet standing. J Neurophysiol 80:1211–1221
Acknowledgements
This work was supported by the Brazilian Council of Science and Technology (CNPq, Grant Number 302628/2013-4), and by the São Paulo Research Foundation (FAPESP, Grant Number 2011/20265-3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coelho, D.B., Teixeira, L.A. Cognition and balance control: does processing of explicit contextual cues of impending perturbations modulate automatic postural responses?. Exp Brain Res 235, 2375–2390 (2017). https://doi.org/10.1007/s00221-017-4980-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-4980-x