Abstract
The properties of sensory reweighting for control of human upright stance have primarily been investigated through experimental techniques such as sinusoidal driving of postural sway. However, other forms of visual inputs that are commonly encountered, such as translation, may produce different adaptive responses. We directly compared sinusoidal and translatory inputs at stimulus parameters that made stimulus velocity comparable with each type of stimulus. Young healthy individuals were compared with healthy elderly and elderly designated as “fall-prone” to investigate whether the hypothesized basis for poor balance control in the “fall-prone” elderly is related to their ability to reweight sensory inputs appropriately. Standing subjects were presented with visual displays which moved in the medial–lateral direction either by (1) oscillating at different amplitudes or (2) simultaneously oscillating and translating at different speeds. All three subject groups showed that increasing the amplitude of the oscillations led to a decrease in gain. Increasing translation speed led to decreases in gain only at speeds above 1 cm/s. This suggests that the nervous system is processing more than just stimulus velocity to determine the postural response. A model implementing “state-dependent noise”, in which visual stimulus noise increases with relative speed, was developed to account for the difference between translation and oscillation. The weak group effects question the common view that the fall-prone elderly are deficient in sensory reweighting. One explanation for the apparent discrepancy is that the slow, small-amplitude visual stimuli used in this study probe the asymptotic dynamics of the postural response. If given enough time, even the fall-prone elderly are able to adapt to a new sensory environment appropriately. However, the asymptotic adaptive response may not be functional in terms of preventing falls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of techniques that allowed a sensory input to be selectively diminished through support surface or visual sway-referencing, Nashner et al. introduced the concept of sensory reweighting for postural control over 20 years ago (cf. Nashner et al. 1982; Black et al. 1983, 1988). It is now a generally held view that visual, vestibular and somatosensory inputs are dynamically re-weighted to maintain upright stance as environmental or nervous system conditions change (Horak and Macpherson 1996; Shumway-Cook and Woollacott 2001). Environmental changes such as moving from a light to a dark environment or from a fixed to a moving support surface (e.g., onto a moving walkway at the airport) require an updating of sensory weights to current conditions so that muscular commands are based on the most precise and reliable sensory information available (Teasdale et al. 1991; Wolfson et al. 1985; Woollacott et al. 1986).
Despite its prevailing acceptance, properties of sensory reweighting are not well understood. A commonly used technique to study sensory reweighting properties is sinusoidal driving of postural sway. The technique stimulates postural sway with a single sensory or pseduo-random input such as vision (Peterka and Benolken 1995; Peterka 2002) or dual-driving with vision and somatosensation simultaneously (Oie et al. 2002; Allison and Jeka 2004) to determine the frequency-response function, which describes gain and phase. Without exception, the results have displayed what we refer to as “inverse gain” reweighting. Increasing (decreasing) the amplitude of the sensory drive at a particular frequency has led to a corresponding decrease (increase) in gain, a clear nonlinear effect that can be interpreted as an upweighting (downweighting) of the sensory modality. The reweighting due to stimulus amplitude change occurs regardless of sensory modality, suggesting that it may be a general property of how the nervous system processes sensory information.
Despite the important information that sway-referencing and oscillatory inputs have contributed to our knowledge of the postural control system, it is arguable that these are not the type of sensory inputs commonly encountered during daily life (i.e., poor ecological validity). Far more often, we encounter visual stimuli that translate across the visual field as we locomote or stand in one spot such as at a street corner with traffic flow across our visual field. It is of interest, therefore, to investigate whether the “inverse gain” response holds for other forms of sensory input. Recent findings suggest that it does not.
Ravaioli et al. (2005) analyzed postural sway during quiet stance while subjects stood in front of a visual display consisting of a medial–lateral oscillation (0.2 Hz, 4 mm) plus a constant velocity translation, whose velocity was varied across condition. The visual display translated from left-to-right at varying speeds, depending upon whether the small oscillation enhanced or diminished the translation from cycle-to-cycle. The oscillation served as a probe to provide a measure of coupling to the visual stimulus through gain (amplitude of body sway/amplitude of stimulus, at the stimulus frequency). Because the oscillation was constant across condition, any changes in gain or phase across condition were attributed to the change in translation velocity. The results showed that an increase in translation velocity from 0 to 1 cm/s led to an increase in gain, contrary to the observed decrease in gain with increasing amplitude of sinusoidal sensory inputs (Oie et al. 2002; Peterka and Benolken 1995; Peterka 2002). However, further increases in translation velocity led to decreases in gain, similar to the way that increasing oscillation amplitude typically reduces gain. Such results suggest that the mechanism underlying sensory reweighting is not based on the simple rule “increased amplitude leads to decreased gain” (i.e., inverse gain reweighting).
Here we test this rule rigorously by comparing pure oscillatory and translational stimuli within subjects. Visual stimuli in Oie et al. (2002) and Ravaioli et al. (2005) differed not only in the form of the stimulus (sinusoid vs translation), but also in terms of stimulus velocity, making a direct comparison inconclusive. Here we compared sinusoidal and translational stimuli at similar stimulus velocities to focus on whether stimulus amplitude alone was the key feature behind sensory reweighting with both types of stimuli.
Sensory reweighting in the elderly
A second focus of this study was to compare sensory reweighting in healthy young adults with elderly individuals. Falls in the elderly are attributable to multiple underlying causes (weakness, drug-induced dizziness, stiffness/inflexibility, etc.), one of which is central sensory re-weighting processes (Horak et al. 1989). Sensory reweighting is thought to degrade with increasing age, and is hypothesized to be particularly deficient in fall-prone versus healthy older adults (Horak et al. 1989; Teasdale et al. 1991, 1993; Alexander 1994; Woollacott 2000). Older adults with a history or high risk of falls are more impaired than their healthy, age-matched counterparts on tests of sensory integration. Fallers demonstrate greater instability in conditions where only one sensory input is altered compared to their healthy cohorts (Anacker and Di Fabio 1992; Baloh et al. 1995; Shumway-Cook and Woollacott 2000). They typically fail to adapt to altered sensory conditions, often losing balance repeatedly despite continued exposure to the sensory condition (Horak et al. 1989; Whipple and Wolfson 1989). Fall-prone elders are also hypothesized to be more visually dependent, failing to use reliable somatosensory cues in environments where visual inputs are unstable (Sundermier et al. 1996; Simoneau et al. 1999).
As discussed above, a deficit in sensory reweighting may be one (of many) mechanism underlying poor balance control in the fall-prone elderly. Thus, young healthy individuals were compared with healthy elderly and elderly designated as “fall-prone” with both translatory and sinusoidal stimuli to determine whether the hypothesized basis for poor balance control in the “fall-prone” elderly is related to their ability to reweight sensory inputs appropriately.
Methods
Subjects
Three groups of subjects participated in the experiment. Healthy young adults (“HY”, N = 12) were students from the University of Maryland who ranged in age from 18 to 27 years, with a mean age of 22.0 ± 3.12 years, and had no known musculoskeletal or neurological disorders that might have affected their ability to maintain balance. Healthy older adults (“HO”, N = 7), who ranged in age from 79 to 84 years, with a mean age of 81.1 ± 2.12 years, and fall-prone older adults (“FP”, N = 15), who ranged in age from 68 to 84 years with a mean age of 80.7 ± 5.47 years, were recruited from a large congregate retirement community, University of Maryland alumni, and the local community.
All elderly volunteers completed an eligibility questionnaire and were excluded from the study if they had any of the following: known visual conditions or impairments affecting daily function (e.g. reading, driving, etc.); dizziness, vertigo or known vestibular conditions or impairments; lower extremity numbness or conditions leading to somatosensory loss; neurological disorders; cognitive decline; low endurance or inability to independently assume and maintain the stance position required for experimental testing. Older subjects with chronic and stable orthopedic conditions, e.g. osteoarthritis of the knee, were included while those with recent musculoskeletal changes such as joint surgery within the prior 2 years were excluded. Limited polypharmacy was permitted; HO subjects were excluded if they took more than four prescription medications, FP subjects were excluded if they took more than six prescription medications. Any subject taking medications that are known to affect the central nervous system, e.g, sleep aids, anti-seizure, anti-depressant, anti-anxiety medications, etc. was also excluded.
HO subjects had no history of falls or near falls and no decline in functional status within the past year. Further, eligibility for the HO group was limited to older adults who were functionally completely independent in the community without an assistive gait device, engaged in daily physical activity such as stair-climbing, shopping or gardening, and participated at least three times weekly in moderate exercise such as tennis, swimming, or running.
Fall-prone subjects had a history of one or more unexplained falls within the past year (range 1–5, mean = 2); most also reported numerous near-fall incidents and noticeable decline in their balance requiring adaptations in their daily functional activities. Typical adaptations included the use of a cane when walking outdoors, inability to ascend or descend stairs without a railing, and cessation of balance intensive activities such as dancing or travel. Elderly individuals who were determined to be eligible for the FP group based on questionnaire results subsequently underwent clinical screening by a licensed physical therapist to ensure adequate mental status and vision, intact lower extremity somatosensation, and to rule out bilateral vestibular loss.
All subjects received written and verbal descriptions of and instructions for the test procedures. Written consent was obtained from all subjects according to the guidelines prescribed by the Internal Review Board at the University of Maryland before beginning the experiment.
Experimental apparatus
Visual display
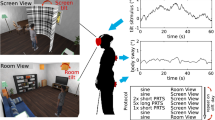
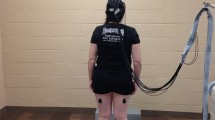
The visual display consisted of 100 white right triangles, 0.8 × 1.6 × 1.79 cm, on each side and rotated by a random angle in the frontal plane on a black background. The display was rear-projected on a translucent 2.5 m × 2 m screen, by a graphics workstation (Intergraph) and CRT video projector (Electrohome ECP4500). The spatial resolution of the visual display system was 1,024 × 768 pixels with a vertical refresh rate of 60 Hz. The displays were generated at a rate of 25 frames/s. The dots were randomly positioned within ± 60° of vertical and ± 70° of horizontal visual eccentricity. No triangles were positioned within a horizontal band of ± 5° in height about the vertical horizon of the subject’s eyes. This black strip in the middle of the stimulus was made to suppress the visibility of aliasing effects, which are most noticeable in the foveal region (Dijkstra et al. 1994a), as the stimulus translated across the screen.
Custom software was written in Visual C + + to generate the visual displays, utilizing the standard OpenGL libraries. We utilized a visual frame rate of 25 Hz that was far below the maximal possible frame rates the computer could generate (> 66 Hz), to maintain a consistent frame rate throughout a trial. The subjects wore a pair of goggles (not shown in Fig. 1) that limited the field of view to approximately ± 60° wide and ± 50° high. This ensured that the edges of the screen were not visible to the subject.
Kinematics
Body kinematics were measured using an OptoTrak (Northern Digital, Inc.) system. The bank of three infrared cameras was placed about 1.5 m behind and 2.5 m to the right of the subject to measure the movements of the markers used to estimate the body CoM in the frontal plane. Each subject had infrared emitting diode markers placed on the right ankle (lateral malleolus), right knee (fibular head), right hip (greater trochanter) and the shoulder (acromion process). All signals were collected at 100 Hz and stored on a personal computer (Gateway E4200) for offline data analysis.
Procedures
All subjects completed eight trials in total, two for each of four conditions. In each condition, the visual display moved in the medial–lateral direction either by (1) oscillating at different amplitudes or (2) simultaneously oscillating at a single amplitude and translating to the right at different speeds. The oscillation amplitude–translation speed conditions were: 4 mm–4 cm/s (A4T4), 4 mm–1 cm/s (A4T1), 4 mm–0 cm/s (A4T0), 8 mm–0 cm/s (A8T0). The frequency of oscillation was 0.2 Hz for all conditions, which is known to typically produce a strong sway response. Table 1 shows the properties of the visual stimulus in each condition. The order of condition presentation was randomized within blocks of four trials. Each trial was 210 s and approximately 120 s of seated rest was given between trials, though the subjects were allowed more time if needed.
The subject stood at a distance of 40 cm from the visual display screen with a shoulder width parallel stance as shown in Fig. 1. A small piece of tape on the platform marked the position of the toes, so that the same foot position was maintained on each trial. CoM motion was analyzed only in the medial–lateral direction. The subject was instructed to maintain his/her gaze directly in front of his/herself, which corresponded to the horizontal black strip positioned at the height of subject’s eyes at the start of the experimental session. The subjects were asked not to hyper-extend their knees and to keep their weight distributed equally between their feet. After a trial was completed, the subject was asked to sit and rest for at least 2 min.
Analysis
CoM estimation
To calculate the trajectories of CoM we used Winter’s method (Winter 1990), which is based on segmental kinematics. A two-dimensional model was used to represent the kinematics of the body in the frontal plane. The anatomical structure was assumed as a set of three rigid segments representing head–arms–trunk (HAT), thighs, and legs, based upon markers placed at the ankle (lateral malleolus), knee (lateral femoral condyle), hip (greater trochanter) and shoulder (acromium) on the right side of the subjects’ bodies. The CoM location of each segment was assumed to lie on the line connecting two adjacent joints based upon anthropometric measures presented in Winter (1990), and the total body CoM was computed as a weighted summation of the segmental CoM estimates. In what follows, CoM refers to the linear displacement of the medial–lateral CoM with the mean for each trial subtracted.
Gain and phase
Linear, spectral analysis was performed for each trial by computing the individual Fourier transforms of the time series of CoM postural displacements and of the oscillatory component of stimulus motion. For each modality, the frequency-response function (FRF) at the stimulus frequency was computed by dividing the transform of the estimated CoM by the transform of the stimulus resulting in a complex-valued FRF. Because stimuli were presented at the same frequency in every condition, we evaluate the FRF only at the stimulus driving frequency. Gain is the absolute value of the FRF at the stimulus frequency. Unity gain indicates that the component’s amplitude at the stimulus frequency exactly matches the amplitude of the sensory stimulus.
Phase, the argument of the FRF, indicates the temporal relationship between the sensory stimulus and body sway, and was defined as the angle of the FRF at the stimulus frequency. A phase lead means that body sway is temporally ahead of the sensory stimulus (and vice versa for a phase lag). In-phase motion with unity gain means that there is no relative motion between the body’s CoM and the sensory environment at the stimulus frequency. Otherwise, the body’s CoM moves relative to the sensory environment.
Sway variability
The variability of the position and velocity of the CoM was computed as the standard deviation of body sway after the deterministic response to the sensory drive was subtracted (cf. Jeka et al. 2000). CoM velocity was computed by downsampling the position trajectory to 10 Hz and taking finite-difference derivatives. (We used downsampling as a simple way to reduce the effect of measurement noise; CoM position has very little power above 5 Hz so downsampling does not produce an aliasing problem.) For the position and velocity trajectories, we computed the Fourier transform, removed the value of the transform at the stimulus frequency (0.2 Hz), and then computed the inverse transform, resulting in “residual” position and velocity trajectories. Position or velocity variability was computed as the standard deviation of the residual position or velocity trajectory, respectively.
Statistical analysis
There were four measures of interest: gain, phase, position variability, and velocity variability. Our analysis was complicated by the fact that some subjects exhibited low gains in some conditions. When gain is low, small errors in estimating the FRF leads to large errors in estimating phase and a positive bias in estimating gain. To address these problems, instead of averaging gain and phase across subjects, for each group and condition we averaged the FRF across subjects. We then used the absolute value and argument of the mean FRF as estimates of “group gain” and “group phase”, respectively, for the given group and condition. Figure 2 illustrates this technique in the Results section. Our analysis of group gain and phase was based on the assumption of multivariate normality for the real and imaginary parts of the FRF.
Individual frequency response functions (FRF) in the complex plane from each trial of each healthy young subject in the A4T0 condition. The mean FRF (black circle) with its elliptical 95% confidence interval are also shown. Gain (= 0.437) and phase (= 3.52°) were extracted as the absolute value and argument, respectively, of the mean FRF
Thus, our analysis was based on four underlying measures: the real part of the FRF, the imaginary part of the FRF, position variability, and velocity variability. Taking into account the repeated factor of condition, there was a total of 16 dependent variables (four measures times four conditions). We assumed multivariate normality for these dependent variables and the same covariance matrix for all groups.
In order to test whether sway was effected by the visual motion oscillation, for each group and condition we tested whether the FRF was significantly different from zero using a F-test. Next, we performed overall tests on the four underlying measures for main group and condition effects and a group-by-condition interaction. We then tested each of our measures of interest. Finally we performed pairwise tests for main and simple effects, with a Bonferroni correction for the number of pairs (three pairs for group comparisons, six pairs for condition comparisons). For tests not involving gain or phase, we used a standard multivariate test based on Wilks’ Λ. Tests of gain or phase required a nonlinear analysis, since the mean real and imaginary parts of the FRF are nonlinear functions of group gain and phase. For example, to test for a condition main effect for gain, we computed maximum-likelihood estimates of group gain and phase for each group and condition with and without the constraint of no condition main effect for gain. The two fits were compared using Wilks’ Λ with the same degrees of freedom as in the corresponding linear case.
Results
The four underlying measures considered together showed a highly significant Condition effect (P < 0.0001), a marginally significant Group effect (P = 0.052), and a marginally significant Group × Condition interaction (P = 0.094). Results for individual measures of interest from tests for Group and Condition main and simple effects are reported below.
CoM gain and phase
CoM sway exhibited a significant response to the visual motion oscillation for each group and condition (P < 0.03). To illustrate how we calculated gain and phase from CoM sway, Fig. 2 shows the individual FRFs plotted in the complex plane for each of two trials in the A4T0 condition for each young healthy subject. The mean FRF is plotted with its elliptical 95% confidence interval. In this example, gain = 0.4 and phase = 3.52°.
Gain
Figure 3a, b show the gain and phase results for each group. Mean gain shows a strong dependence upon both translation velocity and oscillation amplitude, supported by a significant effect for Condition (P < 0.0001). Pairwise comparisons showed that gain was significantly different between all conditions (P < 0.001) except conditions A4T0–A4T1 and A8T0–A4T4. The lack of difference between conditions A4T0 and A4T1 indicates that adding a slow translation to the oscillatory display had no effect on the postural response. No difference between conditions A8T0 and A4T4 indicates that adding a relatively fast translation to the oscillatory display had an equivalent effect as increasing oscillation amplitude, resulting in a decreased gain when compared to the A4T0 and A4T1 conditions. Each group displayed a roughly similar pattern of behavior. There was a significant Group main effect (P < 0.04), with pairwise comparisons showing differences in gain only between healthy elderly adults and young adults in Conditions A4T1 and A4T0.
Phase
Figure 3b shows that phase displayed the same pattern across all groups. The CoM maintained an approximately in-phase relationship in the A4T1, A4T0 and A8T0 conditions, but showed a phase lead of 30–40° in the A4T4 condition, consistent with previous results (Ravaioli et al. 2005). These results led to a significant Condition effect for phase (P < 0.03). Pairwise comparisons indicated that phase differed between the A4T4 condition and all other conditions, which did not differ from each other. No significant effects for Group (P = 0.678) were found.
CoM position and velocity variability
Figure 4a, b shows the CoM position and velocity variability results, which showed a similar pattern across conditions. Position and velocity variability was lowest in the A4T0 condition and increased as translation velocity or oscillation amplitude increased. These results were supported by highly significant Condition main effects on CoM position and velocity variability (Ps < 0.0001). Pairwise comparisons showed that position and velocity variability were higher in condition A4T4 than all other conditions. Comparisons between other conditions showed that position variability did not differ, but velocity variability was significantly higher in the A8T0 condition than in the A4T0 condition. No significant Group differences were observed for position variability (P = 0.320) and velocity variability (P = 0.153).
Discussion
The present results extend those of Ravaioli et al. (2005) by directly comparing the effects of oscillatory and translational visual movement on postural sway. The main condition effect for the three subject groups clearly showed that adding a slow translation to the oscillating visual display had a different effect than merely increasing the amplitude of the oscillation. Consistent with previous studies (Peterka and Benolken 1995; Oie et al. 2002), increasing the amplitude of the oscillation from 4 to 8 mm with no translation led to a pronounced decrease in gain, indicating a downweighting of vision. However, adding a 1 cm/s translation to the 4-mm oscillation led to no significant change in gain, even though adding the translation resulted in a higher RMS velocity (1.06 cm/s) when compared to increased oscillation amplitude (0.71 cm/s).
The fact that gain is more sensitive to increases in oscillation amplitude than to increases in translation velocity is interesting because it questions findings suggesting that the postural response is sensitive primarily to visual stimulus velocity (Dijkstra et al. 1994b; Jeka et al. 2004; Kiemel et al. 2002; Masani et al. 2003; Stoffregen 1986). To explore this issue we consider an adaptive postural model from Carver et al. (2005), which we summarize in the Appendix. In the model, the visual input is the relative velocity between the eyes and the visual scene plus noise. Adaptation is based on the neural controller changing the relative weighting of vision and other sensory inputs to minimize the mean-squared torque produced by the ankle muscles. The model qualitatively reproduces the decrease in gain caused by increasing the oscillation amplitude of the visual scene (circles in Fig. 5b). However, because the model treats visual scene oscillations and translations equally, it predicts large decreases in gain for translation velocities on the order of 1 cm/s or larger (Fig. 5a), contrary to our experimental results.
Simulations of adaptive models. a, c, e Visual motion consists of 4-mm 0.2-Hz oscillation and translation. b, d, f Visual motion consists of 0.2-Hz oscillation with no translation. Error bars indicate 95% confidence intervals based on ten 1,000-s trials. See Appendix for additional information
Therefore, our results suggest that the postural control system has some mechanism that specifically compensates for visual scene translations. A simple example of such a mechanism is a high-pass filter of visual input, because it removes any constant-velocity translation from the visual input. Adding a high-pass filter to the model has little effect on its response to changes in oscillation amplitude (compare squares to circles in Fig. 5b), but completely removes the dependence of gain on translation velocity (Fig. 5a).
The model with translation compensation is qualitatively consistent with our results at low translation velocities, but does not reproduce the decrease in gain at higher translation velocities. This suggests that there is some constraint on the postural control systems that prevents it from fully compensating for a visual-scene translation when its speed is too high. One possibility is that the sensory measurement of relative velocity between the visual scene and the eyes becomes less accurate at high relative speeds (Ravaioli et al. 2005). We added this feature to the model by making the visual sensory noise increase with increasing relative speed, resulting in a model with state-dependent noise (cf. Harris and Wolpert 1998). We adjusted the strength of the state-dependence so that it produced little change in gain at a translation velocity of 1 cm/s, but produced a large decrease in gain at 4 cm/s (compare triangles to squares in Fig. 5a). With no translation, the state-dependence of the visual sensory noise had little effect on gain for our range of oscillation amplitudes (Fig. 5b).
Although adding translation compensation and state-dependent visual noise to the adaptive model greatly improved its qualitative agreement with our data, there are some features of the data in this paper and Ravaioli et al. (2005) that are still not accounted for by the model. In the model, the phase between the body and visual scene shows little or no dependence on the translation velocity (not shown), in contrast to the experimentally observed increase in phase at the 4 cm/s translation velocity (Fig. 3b). Also, in Ravaioli et al. (2005) we detected an increase in gain when translation velocity increase from 0 to 1 cm/s, which cannot be explained by the model.
Sway variability: Our results show that when an increase in the motion of the visual scene produced a decrease in visual gain, it also tended to produce an increase in sway variability (compare Figs. 3, 4). The adaptive model shows the same relationship between gain and variability (Fig. 5). In the model, sensory weights are adjusted to minimize the mean-squared torque produced by the ankle muscles. When the body leans relative to vertical, more muscle torque is needed to counteract the force of gravity. Therefore, minimizing mean-squared muscle torque is roughly the same as minimizing mean-squared lean angle. There are three contributions to mean-squared lean angle: the average lean angle, which is zero in the cases we will consider; power at the oscillation frequency, which is related to gain; and power at other frequencies, which is related to sway variability.
We first consider why variability in the model increases with increasing translation speed (Fig. 5c, e). For the model with translation compensation and state-dependent visual noise, the increase in sway variability is a direct result of the increase in visual noise. Re-weighting partially compensates for the increase in visual noise, but some increase in variability is unavoidable.
The reason for the increase in variability with increasing oscillation amplitude (Fig. 5d, f) is different, since state-dependent noise has little effect for our range of oscillation amplitudes. When the oscillation amplitude is zero, sway variability is the only contribution to mean-squared lean angle. The model thus minimizes sway variability as a consequence of minimizing mean-squared ankle torque. When the oscillation amplitude becomes positive, power appears at the oscillation frequency and the system down-weights the visual input to reduce this power. However, there is a trade-off. Reducing power at the oscillation frequency leads to increased power at other frequencies, so down-weighting vision leads to an increase in sway variability.
Eye position: An alternative mechanism that may account for the present results is suggested by recent findings showing that the gaze signal (eye position) may be involved in control of sway during quiet stance (Glasauer et al. 2005). Glasauer had subjects actively track a sinusoidal stimulus with the eyes alone and with the eyes–head while standing. The evidence did not support the use of retinal slip but instead postural sway was correlated to the gaze signal. It is possible that the ocular motor control (eye position) signal may also be playing a role in the present paradigm.
Effects of aging—transient versus asymptotic responses
Several studies have demonstrated increased postural sway in response to dynamic visual environments in healthy and fall-prone older adults, concluding that older adults are “visually dependent” or “visually sensitive” (Simoneau et al. 1999; Sundermier et al. 1996;Wade et al. 1995). Visual dependence is conceived as an over-reliance on visual cues that may be inaccurate or unreliable in the presence of stable and reliable somatosensory and vestibular cues. It is considered a multisensory re-weighting deficit in that there is a failure to ‘switch’ from inaccurate visual information to accurate somatosensory and vestibular information, i.e., to down-weight vision and up-weight somatosensation and vestibular inputs.
The favored explanation for visual dependence is age- or disease-related peripheral sensory loss in the somatosensory and vestibular systems. If the postural control system cannot rely on somatosensory and/or vestibular inputs, it must over-rely on (i.e., increase the weighting to) vision. For example, Peterka (2002) found that subjects with known bilateral vestibular loss weight vision and proprioceptive cues more highly than do individuals with intact vestibular function. However, several recent studies have found larger responses to dynamic visual stimuli despite the employment of careful screening tests for peripheral somatosensory and vestibular sensory loss and the exclusion of subjects with sensory loss (Borger et al. 1999; Simoneau et al. 1999). Their findings argue against peripheral somatosensory or vestibular loss as the sole or primary causative factors of “visual sensitivity” in healthy and fall-prone older adults.
Contrary to previous studies showing elderly “visual sensitivity”, the present results showed only marginal differences between groups. This is consistent with recent work from our laboratory showing that healthy and fall-prone elderly are able to reweight sensory information similar to healthy young adults (Allison and Jeka 2004; Allison et al. 2006). One explanation for this apparent discrepancy is the timescale of the trials and the type of stimuli, both equally important. Visual stimuli have historically tended to be large in amplitude with relatively short trials (Sundermier et al. 1996; Simoneau et al. 1999) when compared to the stimuli used in our studies (e.g., Oie et al. 2002; Allison and Jeka 2004; Allison et al. 2006). Short trials mean that the response to the stimulus is what we refer to as the “transient” response to the visual perturbation. Each subject begins a trial with a static visual environment. The visual scene then abruptly begins to move. A trial of short length (e.g., 20–30 s) may be measuring primarily the short-term adaptation to the moving visual environment. In contrast, relatively long trials measure the “asymptotic” response, the resulting behavior after the nervous system has fully adapted and equilibrated to the new visual stimulus.
Amplitude of the visual stimulus also plays a role. Adapting one’s response from a static visual environment to a large, moving visual stimulus resembles a perturbation, again stressing the transient response because the subject must quickly downweight vision to diminish the postural response and avoid a fall. The threat of causing a fall does not exist with a small amplitude stimulus. Moreover, because a small amplitude stimulus is more difficult to distinguish from the visual flow created by self-motion, it is less like a perturbation, instead probing the dynamics of the fully-adapted state.
Our interpretation of the minor differences observed between the healthy elderly, fall-prone elderly and young subjects in the present results is that the visual stimuli are primarily probing the asymptotic dynamics of the postural response. If peripheral sensation is intact and if given enough time, even the fall-prone elderly are able to adapt appropriately.
Conclusion
The current results question two current ideas in the postural control literature. First, commonly used sinusoidal stimuli (including many studies in our own lab) may limit the understanding of the control structure underlying postural sway dynamics. These results replicate and add to those from a previous study (Ravaioli et al. 2005), indicating that the postural response is sensitive to more than just stimulus velocity. Translatory motion led to a qualitatively different postural response than oscillatory input, invoking the need for “state-dependent noise” to explain our results. Second, the minor differences found between subject groups questions the commonly held view that the fall-prone elderly cannot reweight sensory information effectively (cf. Horak et al. 1989), also consistent with previous findings (Allison and Jeka 2004; Allison et al. 2006). The present methods investigate adaptation over a longer time scale than most studies, emphasizing the need to distinguish reweighting over short versus longer-term durations. Our evidence suggests that even the fall-prone elderly can reweight sensory information appropriately. They may become fall-prone when the process of adaptation to a new sensory environment slows down and becomes dysfunctional due to the more rapid adaptation required to prevent a fall.
References
Alexander NB (1994) Postural control in older adults. J Am Geriatr Soc 42:93–108
Allison L, Jeka JJ (2004) The role of multisensory integration in balance disorders. In: Calvert G, Spence C, Stein BE (eds) Handbook of multisensory processes. MIT press, Boston
Allison L, Kiemel T, Jeka JJ (2006) Multisensory reweighting is intact in healthy and fall-prone older adults. Exp Brain Res (submitted)
Anacker SL, Di Fabio RP (1992) Influence of sensory inputs on standing balance in community-dwelling elders with a recent history of falling. Phys Ther 72:575–581
Baloh RW, Spain S, Socotch TM, Jacobson KM, Bell T (1995) Posturography and balance problems in older people. J Am Geriatr Soc 43:638–644
Black FO, Wall C III, Nashner LM (1983) Effects of visual and support surface orientation references upon postural control in vestibular deficient subjects. Acta Otolaryngol 95(3–4):199–210
Black FO, Shupert CL, Horak FB, Nashner LM (1988) Abnormal postural control associated with peripheral vestibular disorder. Prog Brain Res 76:263–275
Borger LL, Whitney SL, Redfern MS, Furman JM (1999) The influence of dynamic visual environments on postural sway in the elderly. J Vestib Res 9:197–205
Carver S, Kiemel T, van der Kooij H, Jeka JJ (2005) Comparing internal models of the dynamics of the visual environment. Biol Cybern 92:147–163
Dijkstra TMH, Schöner G, Gielen CCAM (1994a) Temporal stability of the action–perception cycle for postural control in a moving visual observed with human stationary stance. Exp Brain Res 97:477–486
Dijkstra TMH, Schöner G, Giese MA, Gielen CCAM (1994b) Frequency dependence of the action-perception cycle for postural control in a moving visual environment: relative phase dynamics. Biol Cybern 71:489-501
Glasauer S, Schneider E, Jahn K, Strupp M, Brandt T (2005) How the eyes move the body. Neurology 65(8):1291–1293
Harris CM, Wolpert DM (1998) Signal-dependent noise determines motor planning. Nature 394:780–784
Higham DJ (2001) An algorithmic introduction to numerical simulation of stochastic differential equations. SIAM Rev 43:525–546
Horak FB, Macpherson JM (1996) Postural orientation and equilibrium. In: Handbook of physiology. Exercise. Regulation and integration of multiple systems. Am Physiol Soc, Washington, pp 255–292
Horak FB, Shupert CL, Mirka A (1989) Components of postural dyscontrol in the elderly: a review. Neurobiol Aging 10:727–738
Jeka JJ, Oie KS, Kiemel T (2000) Multisensory information for human postural control: integrating touch and vision. Exp Brain Res 134:107–125
Jeka JJ, Kiemel T, Creath R, Horak FB, Peterka R (2004) Controlling human upright stance: velocity information is more accurate than position or acceleration. J Neurophysiol 92:2368–2379
Kiemel T, Oie KS, Jeka JJ (2002) Multisensory fusion and the stochastic structure of postural sway. Biol Cybern 87:262–277
Masani K, Popovic MR, Nakazawa K, Kouzaki M, Nozaki D (2003) Importance of body sway velocity information in controlling ankle extensor activities during quiet stance. J Neurophysiol 90:3774–3782
Nashner LM, Black FO, Wall C (1982) Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci 2(5):536–544
Oie KS, Kiemel T, Jeka JJ (2002) Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cogn Brain Res 14:164–176
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88:1097–1118
Peterka RJ, Benolken MS (1995) Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res 105:101–110
Ravaioli E, Oie K, Kiemel T, Chiari L, Jeka JJ (2005) Nonlinear postural control in response to visual translation. Exp Brain Res 160(4):450–459
Shumway-Cook A, Woollacott M (2000) Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci 55:M10–M16
Shumway-Cook A, Woollacott MH (2001) Motor control: theory and practical applications. Lippincott, Philadelphia
Simoneau M, Teasdale N, Bourdin C, Bard C, Fleury M, Nougier V (1999) Aging and postural control: postural perturbations caused by changing the visual anchor. J Am Geriatr Soc 47:235–240
Stoffregen T (1986) The role of optical velocity in the control of stance. Percept Psychophys 39(5):355–360
Sundermier L, Woollacott MH, Jensen JL, Moore S (1996) Postural sensitivity to visual flow in aging adults with and without balance problems. J Gerontol A Biol Sci Med Sci 51:M45–M52
Teasdale N, Stelmach GE, Breunig A (1991) Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol 46(6):B238–B244
Teasdale N, Bard C, LaRue J, Fleury M (1993) On the cognitive penetrability of posture control. Exp Aging Res 19:1–13
Wade MG, Lindquist R, Taylor JR, Treat-Jacobson D (1995) Optical flow spatial orientation, and the control of posture in the elderly. J Gerontol B Psychol Sci Soc Sci 50:51–58
Whipple R, Wolfson LI (1989) Abnormalities of balance, gait and sensorimotor function in the elderly population. In: Duncan PW (ed) Balance: proceedings of the APTA forum. American Physical Therapy Association, Alexandria, pp 61–68
Winter DA (1990) Biomechanics and motor control of human movement. Wiley, New York
Wolfson LI, Whipple R, Amerman P, Kaplan J, Kleinberg A (1985) Gait and balance in the elderly. Two functional capacities that link sensory and motor ability to falls. Clin Geriatr Med 1(3):649–659
Woollacott MH (2000) Systems contributing to balance disorders in older adults. J Gerontol A Biol Sci Med Sci 55:M424–M428
Woollacott MH, Shumway-Cook A, Nashner LM (1986) Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev 23(2):97–114
Acknowledgements
The support for this research was provided by: NIH grants 2RO1NS35070 and 1RO1NS046065 (John Jeka, PI) as part of the NSF/NIH Collaborative Research in Computational Neuroscience Program. Leslie Allison was supported by a PODS II award from the Foundation for Physical Therapy. Yuanfen Zhang was supported by the David H. Clarke Graduate Fellowship from the Department of Kinesiology at the University of Maryland.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
To interpret our results we consider an adaptive postural model from Carver et al. (2005) and two modifications of the model. Here we briefly summarize the model and then describe our modifications. See Carver et al. (2005) for additional details and motivation.
The body is modeled as bending only at the ankle in the medial–lateral (ML) direction. Three forces affect the body: gravity, passive ankle stiffness, and a torque produced by muscles at the ankle. The muscle torque equals the torque specified by a neural controller plus white noise, with the specified torque based on estimates of the body’s position and velocity. The state vector for the body is x(t) = [x 1(t) x 2(t)]T, where x 1(t) and x 2(t) are the ML position and velocity of the body at eye level. The output of the model is ρx 1(t), where ρ < 1 is the ratio between displacement of the center of mass and displacement at eye level. Using a small angle approximation for deviations from vertical, the body’s dynamics in matrix-vector form are given by
where u(t) is a scalar control signal proportional to specified ankle torque, \( \ifmmode\expandafter\hat\else\expandafter\^\fi{x}(t) \) is an estimate of x(t), ξ p(t) is a vector white-noise process with spectral density matrix Q, and
The parameter γ describes the net effect of gravity and passive ankle stiffness on the body, σ is the noise level, c 1 is the position feedback gain, and c 2 is the velocity feedback gain.
The model assumes that the neural controller has access to noisy sensory measurements. The visual measurement, z 1(t), is equal to the relative velocity between the eyes and the visual scene plus white noise. For simplicity, all nonvisual inputs are lumped together into a measurement z 2(t) that equals the body’s velocity plus white noise. The measurement vector z(t) is given by
where x v(t) is the medial–lateral position of the visual scene, ξ m(t) is vector white-noise process with spectral density matrix R, and
The parameters σ 21 and σ 22 are the noise levels of the visual and nonvisual sensory measurements, respectively.
The state estimate \( \ifmmode\expandafter\hat\else\expandafter\^\fi{x}(t) \) is generated from the noisy sensory measurements using an adaptive Kalman filter:
where θ(t) is the adaptive parameter and K(θ) has the form of a Kalman gain matrix with θ = σ 21:
The adaptive parameter θ(t) moves according to a gradient-descent rule to minimize mean-squared specified ankle torque:
where ε sets the timescale of the adaptation and the vectors x θ (t) and \( \ifmmode\expandafter\hat\else\expandafter\^\fi{x}_{\theta } (t) \) are additional variables. See Carver et al. (2005) for the derivation of Eqs. 4, 5, and 6.
Our two modifications of the model concern the velocity measurement \( z_{1} (t) = \ifmmode\expandafter\dot\else\expandafter\.\fi{x}_{1} (t) - \ifmmode\expandafter\dot\else\expandafter\.\fi{x}_{{\text{v}}} (t) + \xi _{{{\text{m}}1}} (t). \) Our first modification of the model is designed to compensate for a translation of the visual scene. We apply a first-order high-pass filter to the visual measurement before it is inserted into Eq. 2, which removes the effect of a constant-velocity translation. The transfer function of the filter is τ v s/(1 + τ v s), where s is the Laplace variable and τ v is the filter’s time constant.
Our second modification is to make the level of visual measurement noise depend on the relative speed between the eyes and the visual scene. Specifically, we change the visual measurement noise level from σ 21 to \( {\sqrt {\sigma ^{2}_{{210}} + \sigma ^{2}_{{212}} {\left( {\ifmmode\expandafter\dot\else\expandafter\.\fi{x}_{1} - \ifmmode\expandafter\dot\else\expandafter\.\fi{x}_{{\text{v}}} } \right)}^{2} } }. \) When relative speed is high, the amount of measurement noise is proportional to relative speed.
We used the following parameter values for the original model: ρ = 0.6, γ = 1 s−2, c 1 = 7.25 s−2, c 2 = 3 s−1, σ = 0.1 cm s−3/2, σ 21 = σ 22 = 0.25 cm s−1/2, ε = 0.002 s−1. For the model with translation compensation, τ v = 20 s. For the model with state-dependent visual noise, σ 210 = 0.25 cm s−1/2 and σ 212 = 0.10 s1/2. Models were simulated using the Euler–Maruyama method (Higham 2001) with a time step of 0.005 s. Transients at the beginning of the simulations were discarded.
Rights and permissions
About this article
Cite this article
Jeka, J., Allison, L., Saffer, M. et al. Sensory reweighting with translational visual stimuli in young and elderly adults: the role of state-dependent noise. Exp Brain Res 174, 517–527 (2006). https://doi.org/10.1007/s00221-006-0502-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0502-y