Abstract
The relations among spatial memory, Stroop-like colour-word subtests, and errors on antisaccade and memory-guided saccadic eye-movement trials for older and younger adults were tested. Two types of errors in the antisaccade task were identified: short latency prosaccade errors that were immediately corrected and longer latency uncorrected prosaccade errors. The age groups did not differ on percentages of either corrected or uncorrected errors, but the latency and time to correct prosaccade errors were shorter for younger than older adults. Uncorrected prosaccade errors correlated significantly with spatial memory accuracy and errors on the colour-word subtests, but neither of these neuropsychological indices correlated with corrected prosaccade errors. These findings suggest that uncorrected prosaccade errors may be a result of cognitive factors involving a failure to maintain the goal of the antisaccade task in working memory. In contrast, corrected errors may be a consequence of a fixation system involving an initial failure to inhibit a reflexive prosaccade but with active goal maintenance enabling correction to take place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioural research has established that the prefrontal cortex of the brain is associated with executive function, including working memory and attention. Although neuronal systems required to complete various executive function tasks can be quite distributed throughout frontal brain regions, some tasks do lead to far greater activation in some specific prefrontal cortex regions than others. In particular, areas of the prefrontal cortex appear to be associated with memory and inhibitory control (West 1996; Elderkin-Thompson et al. 2008) and the dorsolateral prefrontal cortex (DLPFC) is especially implicated in spatial memory (Raz et al. 1999). Functional neuroimaging studies have consistently indicated that activation in the DLPFC occurs during tasks involving non-verbal visual working memory (Gold et al. 1996; Grady 1999). Lesion studies in non-human primates indicate its role in visual monitoring and strategic search (Petrides 1994). In addition, visual perceptual, visuospatial and recognition memory functions are represented in dorsolateral prefrontal cortex and appear linked to parietal visual association cortex involved in spatial memory (Grady 1999). The DLPFC is also part of the neural circuitry controlling eye movements (Munoz and Everling 2004) and is involved in the inhibition of unwanted reflexive eye movements, as demonstrated in patients with lesions affecting the DLPFC (Guitton et al. 1985; Pierrot-Deseilligny et al. 2005).
Eye movements that take place when the point of gaze is shifted from one location in space to another are known as saccades. Most saccades are reflexive, but voluntary saccades may be made intentionally when participants are directed to move their eyes to a specific location. Voluntary saccades occur in the antisaccade task, in which participants are instructed to look away from a peripheral target to its mirror position (Hallett 1978). To successfully perform an antisaccade, participants are required to suppress the reflexive saccade to the target, before generating the intentional saccade to the alternative position (Everling and Fischer 1998). When a participant is unable to inhibit the reflexive saccade, a prosaccade error is produced. Most prosaccade errors are immediately corrected. However, sometimes the prosaccade errors are not corrected, and there have been some reports of differential effects of ageing and dementing conditions on corrected and uncorrected prosaccade error rates. Klein et al. (2000) found that young adults corrected a greater proportion of directional errors than older adults, and Unsworth et al. (2004) also obtained data that suggested that older adults failed to initiate a corrective saccade on some antisaccade trials. Abel et al. (2001) found that, over all antisaccade trials, patients with Alzheimer’s Disease were likely to make more uncorrected prosaccade errors than healthy age-matched controls. In addition, Guitton et al. (1985) noted that patients with frontal lobe lesions also had difficulty generating a corrective saccade after failing to inhibit a reflexive prosaccade. There are therefore two types of prosaccade errors in the antisaccade task:
Corrected errors
These form the majority of errors and have very short latencies, especially when the antisaccade task involves a gap between the offset of the fixation point and target onset (Everling and Fischer 1998). These errors may be due to a failure to inhibit a reflexive saccade due to a reduction in activity of fixation neurons.
Uncorrected errors
Failure to correct reflexive prosaccade errors may be associated with a reduction in cognitive capacity such as ability to focus attention on task requirements and appear to be more common in individuals with dementing conditions or frontal lobe damage. The proportion of uncorrected errors is negatively correlated with scores on the Mini-Mental State Examination (a test of cognitive impairment) (Folstein et al. 1975), suggesting that the processes underlying a failure to generate an antisaccade may be indicative of cognitive decline. Children also fail to correct a large proportion of their errors (Fischer et al. 1997), indicating that the ability to generate a voluntary saccade improves as the brain, in particular the DLPFC, matures.
An increase in overall prosaccade error rate with increasing age has frequently been reported (Olincy et al. 1997; Butler et al. 1999; Klein et al. 2000; Nieuwenhuis et al. 2000) but no study to date has systematically differentiated between the two types of error in any age group. Increased prosaccade error rates have generally been interpreted as an indication that there is an age-related decline in inhibitory control (e.g. Butler et al. 1999), but more recent research suggests that increases in error rate may be due to changes in cognitive function related to attention (Nieuwenhuis et al. 2004). For example, Klein et al. (2000) found that older adults showed reductions in both eye movement and neuropsychological performance and that errors due to the tendency to repeat a particular response on the Wisconsin Card Sorting test (a test of frontal lobe function which assesses cognitive flexibility) and prosaccade error rates were positively correlated.
Two potential sources of error in antisaccade trials have been identified. Fischer et al. (2000) proposed that both poor fixation control and poor voluntary control of saccades contribute to the errors. Saccadic eye movements are programmed by fixation and saccade neurons in the frontal eye field and superior colliculus. These neurons respond reciprocally, so that when fixation neurons are active, saccade neurons cease to discharge, and correspondingly, fixation neurons do not discharge when saccade neurons are active. The two groups of neurons appear to be mutually inhibitory (Munoz and Everling 2004). Consequently, any reduction in the activity of fixation neurons, such as occurs when a gap between fixation offset and target onset is introduced into the antisaccade task, releases saccade neurons from inhibition. The consequent increase in the activity of these neurons increases the likelihood of a reflexive prosaccade error towards the target during an antisaccade trial. Participants with a weakness in the fixation system, as identified by their tendency to make large numbers of express saccades on an overlap prosaccade task, also make a large number of prosaccade errors in the gap antisaccade task (Fischer et al. 2000). In addition, the correlation between fixation losses and antisaccade error rate observed by Barton et al. (2008) in patients with schizophrenia and healthy participants indicates a link between antisaccade errors and the ability to suppress unwanted saccades during fixation. Corrected saccade errors, with their short latency and rapid correction, therefore appear to be result of a weakness in the fixation system. Participants who have high levels of attention and are focussed on the goal of producing an antisaccade are able to make an immediate correction when a prosaccade error occurs by performing a voluntary saccade to the antisaccade destination.
Not all prosaccade errors are the result of the inhibition of automatic prosaccades, as indicated by the significant correlation between antisaccade error rates and errors in a prosaccade task observed by Barton et al. (2008). Fischer et al. (2000) proposed that some prosaccade errors are the result of a failure within a voluntary, cognitive system. A weakness of this system is indicated by a high prosaccade error rate, slower saccade reaction times, a failure to correct errors, and when errors are corrected, a failure of the corrective saccade to reach the opposite side of the display (Fischer et al. 2000). A considerable body of research showing that factors which affect cognitive capacity also affect antisaccade performance provides evidence for the involvement of cognitive factors in performance on the antisaccade task. Patients with dorsolateral frontal lobe deficit have slower antisaccade latencies than control participants (Guitton et al. 1985), as do patients with schizophrenia (e.g. Barton et al. 2008). In addition, individuals with low working memory capacity tend to make more antisaccade errors and have slower antisaccade latencies than individuals with high working memory capacity (Unsworth et al. 2004). Variations in task characteristics such as instructions to participants also strongly influence antisaccade error rates and latencies (Nieuwenhuis et al. 2004). A decrease in the interval between trials produces an increase in both prosaccade errors and latency. This decrease in performance has been thought to be the result of goal-neglect, the temporary failure to maintain a task goal in working memory (Unsworth et al. 2011). As inter-trial interval becomes shorter, participants have less time available between trials to focus attention on the forthcoming trial, leading to temporary lapses of attention on some trials. This loss of attention causes participants to take longer to respond to the task on these trials, resulting in an increase in the number of trials with long latencies in the latency distribution. Unsworth et al. (2011) demonstrated that, at short inter-trial intervals, there was an increase in the tail of the distribution, rather than a shift in its overall position. This finding was consistent with their suggestion that it takes time to activate a task goal in working memory and that goal-neglect contributes to a loss in performance in the antisaccade task.
Godijn and Kramer (2007) proposed an attentional control model of antisaccade production based on the outcome of their experiments on the effects of target type on performance in an antisaccade search task. They suggest that attentional control is guided by working memory processes related to goals relevant for to a particular task and that to generate a correct antisaccade, participants must actively maintain the task goal in working memory. During an antisaccade task, attention must be disengaged from the target location to the antisaccade destination, and any failure or delay in this transfer of attention would result in either an error or a delayed response. Failure to fully transfer attention to the antisaccade destination would be likely to cause the error to remain uncorrected, or if the error is corrected, the end-point of the resulting antisaccade will not reach the desired destination.
We consequently propose that corrected prosaccade errors on the antisaccade task are most likely to be the outcome of a weakness in the fixation system, whereas uncorrected errors are the outcome of goal-neglect. The aim of the current study was to investigate the characteristics of both corrected and uncorrected errors in the antisaccade task using groups of younger and older participants, and to assess whether these errors are differentially influenced by cognitive factors such as inhibitory control and working memory. We also aimed to investigate the characteristics of premature error rates on the memory-guided saccade task. These errors are correlated with prosaccade error rates in the antisaccade task and apparently include both reflexive errors and premature memory-guided responses (Abel and Douglas 2007). To assess the involvement of cognitive factors in both error rates and antisaccade latencies, we correlated these oculomotor variables with performance on two neuropsychological tests.
If different neural processes are responsible for corrected and uncorrected errors, we would expect these to correlate differentially with performance on the neuropsychological tests. One of the tests was a set of Stroop-like subtests (Delis et al. 2001; Stroop 1935) that required inhibition of the prepotent tendency to read a word. We proposed that the inhibitory processes involved in these tasks would correlate with corrected error rate on the antisaccade task, which also requires inhibition of a reflexive tendency. To assess visuospatial working memory, a spatial memory task (Greenwood et al. 2005) was used to assess capacity to quickly encode and recall the location of dots presented on a monitor. Since spatial working memory capacity and attentional control are closely associated (Godijn and Kramer 2007), we predicted that spatial working memory would correlate with uncorrected errors on the antisaccade task but not with corrected errors.
Overall, older adults were expected to make more errors on the antisaccade trials than younger adults, and it was also predicted that the type of error would be differentially affected by age group. Based on the proposal by Nieuwenhuis et al. (2004) that the performance of older adults is affected by goal-neglect, and if this is responsible for uncorrected saccade errors, we specifically predicted that older adults would make more uncorrected errors than younger adults.
Method
Participants
Twenty-five adults and thirty-one older adults were recruited. The younger group were predominantly undergraduate students from Southern Cross University, but there were 5 non-students. The older group were recruited through local advertisements and all were living independently in the community. Participants took part voluntarily and did not receive any remuneration for their participation. All participants gave their written, informed consent, and the study was approved by the Human Research Ethics Committee of Southern Cross University.
Data for the antisaccade task were collected for 31 older participants. However, two of these participants appeared to have difficulty with the task, and their data were not included in the analyses as fewer than 75% of their trials could be analysed. A further participant in the older group was excluded as he performed a total of only 3 correct antisaccade trials out of 120. Of the remaining 28 participants, 11 were men, with ages for the older adult group ranging from 59 to 71 years (M = 66.25, SD = 3.05). Due to calibration difficulties for positions above and below the horizontal plane for two older participants wearing trifocal or multifocal lenses, accurate memory-guided saccade data could not be collected for those additional two participants. Consequently, data for the memory-guided saccade task were available for only 26 older participants, of whom 11 were men, with ages ranging from 59 to 70 years (M = 65.96, SD = 2.96). Antisaccade and memory-guided saccade data were collected for 25 younger participants, for one of whom less than 75% of the trials could be analysed. Of the remaining 24 younger participants, only 6 were men. The ages of the younger group ranged from 19 to 35 (M = 27.0, SD = 5.97). Neuropsychological measures were completed on all participants but only data for which saccade results were also available as noted above were included in the analyses.
Apparatus
All tasks were presented on a 19-inch CTX computer monitor with a resolution of 1,024 × 768 pixels at 60 Hz to participants seated at a computer desk in a well-lit room. For the eye-movement tasks, a chin and head rest stabilised head position and maintained a 77-cm distance from the computer monitor. Eye movements were recorded with an Eyelink 1000 eye tracking system (SR Research), connected to an eye position camera. Specifically, eye movements were recorded by monitoring pupil position and corneal reflectance with a sampling rate of 1,000 Hz and spatial resolution of 0.05 deg. Eye-movement stimuli were created using SR Research Experiment Builder 1.1.2. Saccades were detected when eye velocity was 30 deg/s, acceleration exceeded 8,000 deg/s/s and position changed by >.15 deg. All variables were extracted from the recordings using SR Research Dataview 1.7.5.
Eye-movement stimuli
Saccade tasks
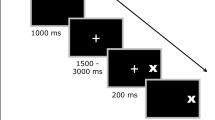
For prosaccades, a 0.5 deg yellow circle was displayed in the centre of a black background on the screen for 800 ms, followed by a 200 ms gap during which the screen was blank. A 0.5 deg, yellow target circle was subsequently presented for 1,000 ms, 8 deg to the left or right of the fixation circle, and participants were required to move their eyes to the target as soon as it appeared. Left versus right target presentation was randomised. After target offset, the central fixation circle reappeared in preparation for the next trial. The antisaccade stimuli were identical to those of prosaccades, except that the stimuli were presented in red, and the participants were instructed not to look at the stimulus, but to move their eyes to the mirror image position on the opposite side of the screen (Fig. 1a). The yellow or red fixation circles cued the participant as to whether a prosaccade or antisaccade was required, respectively (see further detail below).
a The antisaccade task showing eye position following target onset for a correct antisaccade, corrected prosaccade error, and uncorrected prosaccade error. The double arrows labelled a, b and c represent the latency of a correct antisaccade, corrected prosaccade error and uncorrected prosaccade error, respectively. The arrow labelled d represents the time to correct a correct prosaccade error, and e represents antisaccade amplitude. b The memory-guided saccade task showing eye position following target onset for a premature saccade and an accurate memory-guided saccade. The arrow labelled a represents premature saccade latency, and b represents memory-guided saccade latency. The arrow labelled c represents the amplitude of the memory-guided saccade
Memory-guided saccade task
A central yellow cross fixation cue was presented on the black background and remained present until fixation was stable. A subsequent circular 0.5 deg, yellow target was randomly presented at one of 12 peripheral locations 8 deg from the centre, ranging in angular position from 0 to 330 deg in steps of 30 deg. After 250 ms, the peripheral circular target disappeared. The central fixation cross remained for a further 2,000 ms following the offset of the peripheral target. Participants were asked to continue to focus on the central fixation cross until it disappeared, after which they looked at the location of the most recent peripheral target. After 1,000 ms, the central fixation cue reappeared in preparation for the subsequent trial (Fig. 1b).
Neuropsychological tasks
Stroop-like colour-word subtests
The colour-word subtests (Delis et al. 2001) assess basic visual processing speed on colour naming and word reading as well as the interference effect related to naming an ink colour while ignoring the incongruent colour word (i.e. the Stroop effect). In this Stroop-like subtest, participants must inhibit an over-learned response (reading the word) and perform a counter-intuitive task instead (name the ink colour).
A computerised version of the colour-word subtests was used. The Colour Naming subtest required participants to name aloud as quickly as possible the colour of 1 cm × 1 cm coloured squares presented in rows on the computer monitor. Participants were then asked in a second subtest (Word Reading) to quickly read aloud a page of colour words (i.e. GREEN, BLUE, RED) written in black ink. As is assessed in the standard Stroop test (Stroop 1935), participants were then required on the interference subtest to name aloud the colour of the ink used in colour words written in either congruent (e.g. RED written in red ink) or incongruent fonts (e.g. GREEN written in red ink). Total time in seconds for each subtest and the number of uncorrected and self-corrected errors were recorded by the examiner by means of a stopwatch.
Spatial memory
A spatial working memory task (SPWM) devised by Greenwood et al. (2005) was administered via computer. Participants were instructed to keep their eyes on a fixation crosshair that appeared in the centre of the screen for 1 s. After the fixation, one, two or three black dots appeared at randomised locations on the screen for 500 ms. The dots then disappeared and the fixation cross reappeared for 3 s. During this time, participants were required to remember the locations of the previously presented black dots. At the end of the 3 s delay, a red dot appeared on the screen in either one of the same locations as the target dots (match condition) or at a different location (non-match condition). On non-match trials, the distance between the correct location and the test dot was varied over three levels. Participants had 2 s to respond to the red dot by pressing one of two keys on a button box—the left key for non-match trials or the right key for a match trial, using the forefinger on their left and right hands. There were 18 practice trials before the onset of the measured trials. One hundred and twenty trials were presented with 40 trials for each set size (1, 2, or 3 black dot locations). Sixty trials were match trials and 60 non-match trials. Accuracy for each of the 1, 2 and 3-item stimuli were recorded and averaged over match and non-match trials. Reaction time to the three set sizes was similarly recorded.
Procedure
After signing the consent form, participants were allocated to the first of the four tasks, previously determined by counterbalancing the order. The two neuropsychological tests and the two eye-movement tasks were presented together, with the order of presentation of the two sets of tasks counterbalanced. The two eye-movement tasks and two neuropsychological tasks within each set were also counterbalanced and were separated by short rest periods.
Verbal instructions were provided to all participants prior to each task, and detailed instructions were displayed on the computer monitor before the commencement of trials. The responses and eye position of participants were also monitored during the practice trials to confirm compliance with the task instructions.
Eye-movement tasks
For both eye-movement tasks, a 9-point calibration was performed at the beginning of each block of trials, and participants were given the opportunity to have a short break between each block. For the saccade task, a block of 12 practice prosaccade trials was followed by a block of 12 practice antisaccades. Experimental data collection then commenced. A block of 60 prosaccade trials was followed by a block of 60 antisaccade trials (blocked trials). These two blocks were then followed by two blocks of 60 trials each, in which the trials were randomly switched between antisaccade and prosaccade (mixed trials). The type of the subsequent trial was cued by the central fixation circle, which changed colour 500 ms prior to the gap preceding target onset. Prior to the commencement of each block, participants were informed of the trial type to be presented in each block. For the memory-guided saccade task, one practice block of 12 trials was followed by three experimental blocks consisting of 12 trials.
Data analysis
Antisaccade data
The first saccade after target onset was considered the saccadic response. Trials for which participants failed to make a saccade of at least 5 deg, or for which the saccade occurred before 80 ms after target onset (anticipatory eye movements) or more than 800 ms after the target (delayed responses) were excluded from the analysis. Mean percentage of trials that were not analysed was 9.2% for the older group and 7.4% for the younger group. This difference was not significant. For antisaccade trials, incorrect prosaccades were categorised as corrected if there was a subsequent antisaccade with an amplitude greater than 10 deg in the correct direction and as uncorrected if there was no subsequent correction. The latencies of corrected and uncorrected prosaccades, and the time taken to make a subsequent corrective antisaccade, were also derived from the eye-movement recordings. Figure 1a depicts a correct antisaccade along with the two types of prosaccade errors and their respective latencies.
The number of corrected and uncorrected prosaccade errors was totalled for each participant for blocked and mixed antisaccade trials separately and expressed as percentages of the total number of trials available for analysis. Error rate distributions were positively skewed, and percentage errors for each condition were therefore square root transformed to normalise the distributions. The transformed percentage errors were then entered into a mixed between-within ANOVA, with Age Group as the between subjects factor, and Error Type (corrected vs. uncorrected) and Block type as the within subjects factors.
Memory-guided saccade data
Memory-guided saccade trials were included in the analysis if a saccade greater than 2 deg and less than 10 deg occurred subsequent to target offset. The mean percentage of trials that did not fulfil these criteria was 4.8% for older participants and 4.5% for younger participants. This difference between age groups was not significant. Analysed trials were scored as correct if the participant successfully refrained from making a saccade towards the target prior to the offset of the fixation cue, and as premature if a reflexive prosaccade of at least 2 deg was made subsequent to the onset of the target, but prior to the offset of the fixation point. The latency and amplitude of each correct memory-guided saccade was recorded. In addition, the delay between target onset and initiation of premature saccades was recorded. Premature saccades were expressed as a percentage of the trials available for analysis. These variables are depicted in Fig. 1b.
Colour-word subtest data
Completion time in seconds and the number of corrected and uncorrected errors for each subtest of the colour-word subtests (colour naming, word reading, colour-word interference) were included in the analyses. In addition, a within-subject composite score was computed to control for colour naming speed that may have differed across age groups. The composite “interference score” was computed as (Interference subtest time − Colour Naming time)/(Interference time + Colour naming time) × 100 so that the change in completion time during the interference subtest could be understood in terms of per cent difference relative to an estimate of the individual’s overall speed on both subtests.
Results
Antisaccade error analysis
The mean percentage of corrected and uncorrected prosaccade errors for both antisaccade trials for the younger and older age groups for blocked and mixed trials is shown in Fig. 2. Overall, participants corrected more errors than they failed to correct, F(1,50) = 38.84, P < .001, η2 = 43.7%. Although there was an overall tendency for older participants to produce more prosaccade errors than younger participants, the main effect of age was not significant, F(1,50) = 1.18, P > .05, η2 = 2.3%. The main effect of Block Type was significant, with a larger percentage of errors occurring on mixed than on blocked trials, F(1,50) = 31.24, P < .001, η2 = 38.5%. The Age Group × Error Type interaction was not significant, F(1,50) = .043, P > .05, η2 = .1%, indicating that the there were no differences between the two age groups on either corrected or uncorrected error rates. None of the other interactions were significant: Age Group × Block Type F(1,50) = .90, P > .05, η2 = .2%; Block Type × Error Type, F(1,50) = .074, P > .05, η2 = .2%; Age Group × Block Type × Error Type, F(1,50) = .15, P > .05, η2 = .3%.
The temporal nature of corrected prosaccade errors was examined further by comparing the latencies of the erroneous prosaccades with correct saccades produced during prosaccade trials. Prosaccade errors are thought to be a result of the “visual grasp reflex,” the reflexive tendency to look toward a new target, and would be expected on average to be faster than the mean latency of prosaccades (which would include a range of saccade latencies including short latency express saccades). The latency of corrected erroneous prosaccades was significantly faster (M = 153.26, SD = 41.96) than that of correct prosaccades in the prosaccade task (M = 170.87, SD = 41.05), t(51) = 3.95, P < .001, d = 0.4, suggesting that corrected prosaccades are due to an initial reflexive response.
Mean latencies for both uncorrected and corrected prosaccade error trials are shown in Fig. 3a. The mean prosaccade latency for a corrected error trial was significantly longer for the older group than for the younger group, t(50) = 4.12, P < .001, d = 1.17, as was the time taken to correct the error, t(50) = 3.59, P = .001, d = 1.00. There was no difference, however, in mean latency between the two groups for uncorrected prosaccade errors. Overall, the latency for corrected errors (M = 158, SD = 42.46) was significantly, and substantially, shorter than latency for uncorrected errors (M = 348, SD = 183.57), t(43) = 6.95, P < .001, d = 1.71.
a Mean prosaccade latencies for younger and older participants for the antisaccade task (corrected prosaccade, non-corrected prosaccade and time to correct prosaccade errors) and for premature prosaccades in the memory-guided saccade paradigm. b Time taken to complete colour-word subtests (colour naming, word reading and colour-word interference) and computed composite interference score for older and younger participants
Memory-guided saccades
Older participants produced a significantly higher percentage of premature saccades (M = 18.92, SD = 12.71) than younger participants (M = 11.99, SD = 8.72), t(48) = 2.23, P = .031, d = .63. The mean latency of these premature saccades was significantly and substantially longer that the latencies for both corrected, t(46) = 20.46, P < .001, d = 2.99, and uncorrected prosaccade t(45) = 6.72, P < .001, d = .99, errors in the antisaccade paradigm. Although premature saccade latencies tended to be longer for the older adult group, this difference was not significant (Fig. 3a).
Relations among colour-word subtests and saccade variables
Except for word reading, completion times on the colour-word subtests were influenced by age (Fig. 3b). In particular, as shown by the differences on the composite measure of interference, older adults were significantly more affected by colour-word interference than younger adults t(50) = 3.64, P = .001, d = 1.01. Those in the older age group on average demonstrated a greater cost in processing speed due to interference even after controlling for basic colour naming speed. To test whether performance on the eye-movement tasks and inhibition of response were related, correlations were computed between antisaccade error rates and anti- and prosaccade latencies, and performance on the colour-word task. Preliminary examinations of the distributions of the error rates indicated that although the distributions were positively skewed, there were no outliers which substantially influenced the size of the correlations. The two performance measures assessed were the total number of colour-word errors (summed over corrected and uncorrected error types) in the interference subtest and the composite interference score. The correlations are shown in Table 1. The total number of errors on the colour-word task correlated significantly and positively with uncorrected prosaccade errors but did not correlate with corrected errors. The difference between these correlations was close to significance, z = 1.87, P = .061. There were also significant positive correlations between the colour-word variables and antisaccade latency. The significant correlation between the interference score, and both antisaccade and prosaccade latencies, suggests that the greater the cost to a participant due to colour-word interference, the longer it takes to programme an eye movement. There was no significant correlation between any of the colour-word variables and percentage of premature saccades in the memory-guided protocol.
Relations among spatial memory and saccade variables
An estimate of overall spatial memory accuracy and response time was obtained by averaging accuracy scores and button-press speed across the three dot conditions. Reaction time scores for correct match and mismatch trials were averaged together. There was no statistically significant difference across age groups in overall spatial memory accuracy. However, younger adults (M = 752.25 ms, SD = 196.81) responded more quickly to the test dot than older adults (M = 930.75, SD = 165.38), t(50) = 3.59, P = .001, d = 1.00. Correlations between the average accuracy score, and corrected and uncorrected prosaccade error rate, and percentage of premature saccades for the memory-guided saccade task are displayed in Table 2. There was a moderate negative correlation between spatial memory accuracy and uncorrected error rate, indicating that decreased spatial memory capacity is associated with an increase in uncorrected prosaccade errors. However, spatial memory accuracy was not correlated with corrected errors. This difference between correlations was significant, z = 2.63, P = .009. Spatial memory also correlated significantly with antisaccade latency (Table 2). The correlation between spatial memory and percentage of premature saccades was not significant, suggesting that spatial memory as assessed here is not related to premature saccades.
Antisaccade performance and premature memory-guided saccades
The percentage of premature saccades in the memory-guided saccade task was moderately correlated with the percentage of uncorrected errors in the antisaccade task but was not correlated with the percentage of corrected errors (Table 2). This difference in how saccade error types correlated with premature saccades was significant, z = 2.12, P = .034. There was no significant correlation between either colour-word error rate or spatial memory accuracy and premature saccades. There was a significant correlation between antisaccade latency and uncorrected prosaccade errors in the antisaccade task (Table 2), indicating that participants who had long antisaccade latencies also tended to produce more uncorrected errors.
Discussion
In this study, younger and older adults were compared on corrected and uncorrected prosaccade error rates, and on premature saccades in the memory-guided saccade task. The two groups were also compared on two neuropsychological tasks selected because the underlying cognitive processes that the tasks are designed to measure hypothetically overlapped with those of the saccade tasks. In particular, spatial memory and response inhibition characterised components of both the saccade and neuropsychological measures. Our results showed that older adults did not perform as well on the interference score derived from the colour-word subtests, and on the memory-guided saccade task, as younger adults. However, we did not obtain any significant differences between the two age groups in either corrected or uncorrected error rates.
The major finding of this study was that corrected and uncorrected prosaccade errors in antisaccade tasks differ substantially and that they relate in different ways to neuropsychological tests. As predicted, spatial memory accuracy was negatively correlated with uncorrected errors on the antisaccade task, but not with corrected prosaccade errors. The two correlations differed significantly. Colour-word errors on the Stroop task also correlated with uncorrected errors, but not with corrected errors. Our data confirmed previous research (Everling and Fischer 1998) demonstrating that corrected prosaccades were of very short latency, similar to that of express saccades. We also showed that the corrected prosaccade latencies had low variability, and the lack of a correlation of the error rate with either neuropsychological test supports the proposal that the majority of these errors occur due to a failure to inhibit the visual grasp reflex rather than to cognitive factors.
We initially proposed that there would be a correlation between corrected error rate and performance on the Stroop task because inhibition of a prepotent response is involved in both these measures. The failure to obtain such a correlation implies that the inhibitory processes underlying the Stroop test and antisaccade corrected error rate differ. Different inhibitory paradigms are proposed to have varying levels of executive control, not all involving the prefrontal cortex (Nigg 2000). It is therefore likely that the fixation system underlying inhibition of reflexive saccades is more automatic than processes involved in an executive inhibition task such as the Stroop test, which requires the ability to deliberately inhibit a dominant or prepotent response when necessary (Andres et al. 2008).
Uncorrected prosaccade errors were longer in latency than those associated with corrected errors and showed much more inter-individual variability in latency. They correlated significantly with total errors on the colour-word tasks, accuracy on the spatial memory task, and also with percentage of premature saccades on the memory-guided saccade task, suggesting that the failure to correct prosaccade errors is due to cognitive factors, such as working memory and attentional control.
These correlations between error types and neuropsychological tasks can be interpreted in relation to the fixation and voluntary systems involved in antisaccade performance identified by Fischer and colleagues (Fischer et al. 1997; Everling and Fischer 1998; Fischer et al. 2000). Corrected errors were similar in latency to express saccades, indicating that most of these errors occurred as a failure of the fixation system to inhibit a reflexive saccade. In the gap antisaccade task, the 200 ms gap between offset of the fixation point and target onset causes fixation neurons to become disengaged, allowing saccade neurons to become disinhibited. Consequently, when a gap is present, a strong fixation system is required to suppress a reflexive saccade towards a stimulus long enough for a voluntary antisaccade to be programmed. Any weakness in the fixation system would mean that participants are less able to suppress reflexive saccades and make larger numbers of prosaccade errors in the gap antisaccade task (Fischer et al. 2000). After making a prosaccade error, participants who are able to maintain their focus on the antisaccade goal subsequently programme a voluntary saccade and the reflexive error is corrected. This rapid correction of the prosaccade errors may be achieved by the rapid transfer of attention from the target to the antisaccade destination enabling the subsequent corrective saccade to be programmed (Godijn and Kramer 2007).
The lack of any significant correlation between corrected errors and the neuropsychological tests suggests that corrected errors are not strongly influenced by cognitive factors. It is possible, however, that cognitive processes may contribute to a proportion of these errors. For example, a brief period of goal-neglect at the onset of a target may result in a delayed response culminating in a prosaccade. The remainder of the time the target is visible would then be sufficient for a participant to refocus attention and to make a corrective saccade before the end of the trial. A prosaccade error may consequently be the result of either a loss of fixation causing a failure to inhibit the visual grasp reflex or a failure to maintain attention. However, only a failure to maintain and implement the goal of producing an antisaccade would prevent the error from being corrected.
The mean latency of uncorrected errors was approximately twice as long as that of corrected errors, suggesting that the majority of these errors were due to factors other than the failure to inhibit a reflexive saccade. The correlations between uncorrected errors, and spatial working memory and Stroop error rate indicate that these factors are cognitive. There was a significant correlation between spatial memory and antisaccade latency indicating that participants with lower spatial memory accuracy took longer to make an accurate response to an antisaccade trial, and the longer they took to respond, the more likely they were to make an uncorrected error. Since long latency responses appear to be due to a failure to focus attention on a task (Unsworth et al. 2011), the positive correlation between latency and uncorrected error rate also supports the proposal that uncorrected errors are the outcome of a failure to maintain attention.
As shown in Fig. 3a, the average length of time taken to initiate a premature saccade on the memory-guided saccade task (572 ms) was much longer than the latencies of either corrected (151 ms) or uncorrected (352 ms) prosaccade errors. This suggests that processes other than the failure to inhibit a reflexive prosaccade are involved in the production of premature saccades. The average duration of these saccades was longer than the target duration (250 ms), and they are thus premature memory-guided saccades made prior to the offset of the fixation point. The long latencies of premature saccades and the moderate correlation between the percentage of premature saccades and uncorrected errors indicate that premature saccades in the memory-guided task occurred as a failure to maintain the goal of the memory-guided saccade task on some trials. In this task, the fixation point remained present during and after the appearance of the target, enabling fixation neurons to remain engaged, and to continue to inhibit saccade neurons that would otherwise respond to the target. Consequently, very few short latency saccades to the target were made. For both premature saccades and uncorrected errors, participants appear to successfully suppress a reflexive saccade, only to subsequently lose track of the requirements of the task and make an erroneous saccade towards the target later in the trial.
Variations in the attentional demands of tasks and differences between individuals in working memory capacity will lead to variations in the extent to which the task goal is properly maintained. Thus, antisaccade error rate and latency will vary between studies depending on task parameters such as the inter-trial interval. In our study, the interval between the offset of the previous target and the gap was relatively short (800 ms). This short interval may have facilitated the production of uncorrected errors due to the limited time available to focus attention on the subsequent trial (Unsworth et al. 2011).
The interrelations between spatial memory accuracy, percentage of premature saccades and percentage of uncorrected prosaccade errors indicate that a reduction in spatial memory performance affects the capacity to maintain the task goal to programme voluntary saccades when required. Dual-task research, in which a cognitive task is presented at the same time as an antisaccade task, also indicates that an increase in memory load due to the concurrent task reduces performance on the antisaccade task. Under these conditions, an increase in prosaccade errors in healthy participants occurs in the dual-task condition compared to the antisaccade task alone (Roberts et al. 1994; Mitchell et al. 2002). Eenshuistra et al. (2004) specifically compared older and younger participants on a gap antisaccade task in addition to manipulating memory load. As in our study, they obtained no effect of age on prosaccade error rate in the control antisaccade condition with no concurrent memory load. However, in a condition that included a concurrent memory updating task, the number of prosaccade errors was higher for older participants than for younger participants. Further research is necessary to determine which type of antisaccade errors was affected by increasing memory load.
Age group differences
Contrary to expectations, this study did not obtain significant differences between the two age groups in percentages of either corrected or uncorrected prosaccade errors. Although not all age-related antisaccade research has obtained error effects (e.g. Munoz et al. 1998), most previous research has indicated strong effects of age on error rate (Klein et al. 2000; Nieuwenhuis et al. 2000; Olincy et al. 1997). Although Klein et al. (2000) and Nieuwenhuis et al. (2004) found that older adults made more uncorrected antisaccade errors than younger adults, a significant difference between the age groups was not obtained in our study. However, older adults were found to make more premature errors on the memory-guided saccade task than younger adults, supporting previous research (Abel and Douglas 2007; Gottlob et al. 2007). Thus, the prediction that a reduction in the capacity in working memory leading to failures in maintaining task goals reduces performance in oculomotor tasks in older adults was partially supported.
It is possible that any differences in performance between men and women on the antisaccade task may have affected the overall error rates of the two age groups, since there were more men (11) in the older age group, than in the younger group (6). However, a comparison of men and women on both corrected and uncorrected errors indicated that there were no significant differences between the two genders.
Conclusion
This study has shown for the first time that there are clear differences between corrected and uncorrected errors on the gap antisaccade task. Corrected errors appear to be due to a weakness in the fixation system and are unlikely to be the outcome of cognitive factors such as memory limitations or lack of inhibitory control. Uncorrected error rate, on the other hand, was related to performance on both neuropsychological tests, and these errors appear to be due to a failure to maintain the goal of producing an antisaccade in working memory. Any reduction in the capacity of working memory would therefore be more likely to produce an increase in uncorrected errors than in corrected errors. Although our study did not find a significant difference between older and younger age groups in uncorrected errors, a difference did occur in the rate of premature errors in the memory-guided saccade task, another measure that appears to involve goal maintenance.
To date, research that has used the antisaccade task to investigate deficits in psychopathology (for a review, see Hutton and Ettinger 2006) has focussed mainly on the latency and overall error rate of prosaccade errors, without differentiating between the two error types. If, as suggested by this study and that of Fischer et al. (1997), there are two separate systems which give rise to prosaccade errors, it is important that future research involving patient groups distinguishes between the rates of corrected and uncorrected errors in these participants.
References
Abel LA, Douglas J (2007) Effects of age on latency and error generation in internally mediated saccades. Neurobiol Aging 28:627–637
Abel LA, Unverrzagt F, Yee RD (2001) Effects of stimulus predictability and interstimulus gap on saccades in Alzheimer’s disease. Dement Geriatr Cogn Disord 13:235–243
Andres P, Guerinni C, Phillips L, Perfect TJ (2008) Differential effects of aging on executive and automatic inhibition. Dev Neuropsychol 33:101–123
Barton J, Pandita M, Thakkar K, Goff D, Manoach D (2008) The relation between antisaccade errors, fixation stability and prosaccade errors in schizophrenia. Exp Brain Res 186:273–282
Butler KM, Zacks RT, Henderson JM (1999) Suppression of reflexive saccades in younger and older adults: age comparisons on an antisaccade task. Mem Cogn 27(4):584–591
Delis D, Kaplan E, Kramer J (2001) Delis-Kaplan executive function scale. The Psychological Corporation, San Antonio
Eenshuistra RM, Ridderinkhof KR, Molen MW (2004) Age-related changes in antisaccade task performance: inhibitory control or working-memory engagement? Brain Cogn 56(2):177–188
Elderkin-Thompson V, Ballmaier M, Hellemann G, Pham D, Kumar A (2008) Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology 22(5):626–637
Everling S, Fischer B (1998) The antisaccade: a review of basic research and clinical studies. Neuropsychologia 36(9):885–899
Fischer B, Biscaldi M, Gezeck S (1997) On the development of voluntary and reflexive components in human saccade generation. Brain Res 754:285–297. doi:10.1016/s0006-8993(97)00094-2
Fischer B, Gezeck S, Hartnegg K (2000) On the production and correction of involuntary prosaccades in a gap antisaccade task. Vis Res 40:2211–2217
Folstein MF, Folstein SF, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Godijn R, Kramer A (2007) Antisaccade costs with static and dynamic targets. Atten Percept Psychophys 69:802–815. doi:10.3758/bf03193780
Gold JM, Berman KF, Randolph C, Goldberg TE, Weinberger DR (1996) PET validation of a novel prefrontal task: delayed response alternation. Neuropsychology 10:3–10
Gottlob LR, Fillmore MT, Abroms BD (2007) Age-group differences in inhibiting an oculomotor response. Aging Neuropsychol Cogn 14:586–593
Grady C (1999) Neuroimaging and activation of the frontal lobes. In: Miller BL, Cummings JL (eds) The human frontal lobes: functions and disorders. Guilford, New York, pp 196–260
Greenwood PM, Lambert C, Sunderland T, Parasuraman R (2005) Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health’s BIOCARD Study. Neuropsychology 19(2):199–211
Guitton D, Buchtel HA, Douglas RM (1985) Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 58:455–472
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vis Res 18:1279–1296
Hutton SB, Ettinger U (2006) The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43:302–313
Klein C, Fischer B, Hartnegg K, Heiss W, Roth M (2000) Optomotor and neuropsychological performance in old age. Exp Brain Res 135(2):141–154
Mitchell JP, Macrae CN, Gilchrist ID (2002) Working memory and the suppression of reflexive saccades. J Cogn Neurosci 14(1):95–103
Munoz DP, Everling S (2004) Look away: the antisaccade task and the voluntary control of eye movement. Nat Rev Neurosci 5:218–228
Munoz DP, Broughton JR, Goldring JE, Armstrong IT (1998) Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res 121:391–400
Nieuwenhuis S, Ridderinkhof KR, de Jong R, Kok A, van der Molen MW (2000) Inhibitory inefficiency and failures of intention activation: age-related decline in the control of saccadic eye movements. Psychol Aging 15(4):635–647
Nieuwenhuis S, Broerse A, Nielen MMA, Jong Rd (2004) A goal activation approach to the study of executive function: an application to antisaccade tasks. Brain Cogn 56:198–214
Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126:220–246
Olincy A, Ross RG, Young DA, Freedman R (1997) Age diminishes performance on an antisaccade eye movement task. Neurobiol Aging 18(5):483–489
Petrides M (1994) Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J (eds) Handbook of neuropsychology. Elsevier, Amsterdam, pp 59–82
Pierrot-Deseilligny C, Muri RM, Nyffeler T, Milea D (2005) The role of the human dorsolateral prefrontal cortex in ocular motor behaviour. Ann N Y Acad Sci 1039:239–251
Raz N, Briggs SD, Marks W, Acker JD (1999) Age-related deficits in generation and manipulation of mental images: II. The role of dorsolateral prefrontal cortex. Psychol Aging 14(3):436–444
Roberts RJ, Hager LD, Heron C (1994) Prefrontal cognitive processes: working memory and inhibition in the antisaccade task. J Exp Psychol Gen 123(4):374–393
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643–662
Unsworth N, Schrock JC, Engle RW (2004) Working memory capacity and the antisaccade task: individual differences in voluntary saccade control. J Exp Psychol Learn Mem Cogn 30:1302–1321
Unsworth N, Spillers GJ, Brewer GA, McMillan B (2011) Attention control and the antisaccade task: a response time distribution analysis. Acta Psychol 137:90–100
West RL (1996) An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 120(2):272–292
Acknowledgments
This research was supported in part by a grant from the Southern Cross University Associate Dean’s Research Support Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bowling, A.C., Hindman, E.A. & Donnelly, J.F. Prosaccade errors in the antisaccade task: differences between corrected and uncorrected errors and links to neuropsychological tests. Exp Brain Res 216, 169–179 (2012). https://doi.org/10.1007/s00221-011-2921-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2921-7