Abstract
Persons with Mild Cognitive Impairment (MCI) are at high Alzheimer’s Disease (AD) risk but the development of sensitive measures to assess subtle cognitive decline in this population poses a major challenge for clinicians and researchers. Eye movement monitoring is a non-invasive, sensitive way to assess subtle cognitive processes in clinical populations. We conducted a critical review and a meta-analysis of the literature on pro and antisaccade paradigm in AD/MCI. The meta-analysis included 20 studies, all of which used the prosaccade paradigm and 13 of which studied the antisaccade paradigm as well. Our meta-analysis showed that AD but not MCI patients showed longer prosaccade latencies when compared to controls. While antisaccade latencies did not differentiate between patients from controls, antisaccade error rate were significantly increased among patients in comparison to controls in over 87% of the studies. These findings highlight antisaccade error rate as a reliable tool to distinguish inhibition abilities between AD/MCI and healthy older persons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Alzheimer’s Disease (AD) is degenerative brain disease that is the most common expression of dementia, occurring in 50–70% of cases (Alzheimer’s Association 2015). AD is typically characterized by sequence of biological events and clinical manifestations of episodic memory deficits often accompanied by mild cognitive deficits (e.g., attention, executive function) (Silverberg et al. 2011; Sperling et al. 2011). One way to assess cognitive processes and deficits in pre-clinical populations is by eye movement monitoring. Since eye movement monitoring can be used to detect cognitive deficits in early stage AD, its use could promote early intervention to alleviate symptoms (Sperling et al. 2011). Eye movement monitoring is a robust, non-invasive, and sensitive instrument for examining altered patterns of oculomotor behavior that does not pose psychomotor demands (Anderson and MacAskill 2013; Hannula et al. 2010; Pereira et al. 2014; Santana et al. 2015).
Eye movement monitoring is typically conducted by tracking and analyzing participants’ saccades (i.e., rapid eye movement) while performing a visual task. Specifically, participants shift the focus of gaze from one spatial location to another, either towards the stimulus (prosaccade) or away from it (antisaccade) (Molitor et al. 2015). Saccades are an inherent part of the constant cycle of perception, action and cognition (Deubel and Schneider 2003). Because of its association with attention, saccades are likely to be disturbed by cognitive impairments that are associated with neurodegenerative disorder (Deubel and Schneider 2003; Anderson and MacAskill 2013). Therefore, in persons with AD, saccades’ abnormality can serve as a probe to cognitive impairment (Anderson and MacAskill 2013).
One diagnosis that has been associated with a greater risk of developing AD is Mild Cognitive Impairment (MCI) (Petersen 2004; Petersen and Bennett 2005; Petersen et al. 1999; Jak et al. 2016; Sperling et al. 2011; Mitchell and Shiri-Feshki 2009). MCI has been defined as a transitional stage between normal and pathological aging in which one experiences cognitive deficits while activities of daily living are largely intact (Petersen 2004). Although MCI is heterogeneous in its clinical presentation, its most common manifestation is the ‘amnestic form’, when memory is significantly impaired (Petersen et al. 1999). In order to enable early therapeutic intervention in AD, significant research has been conducted on the development of precise MCI diagnostic instruments (Jak et al. 2016). It has been found that the integration of various eye movement measures (i.e., novelty preference, saccade orientation, and fixation duration) enabled differentiating between persons with MCI and controls at a level of 87% accuracy, 97% sensitivity, and 77% specificity (Lagun et al. 2011). Given the psychological burden involved in receiving an MCI diagnosis and the potential therapeutic advantages of earlier AD diagnosis, it is important to identify sensitive and reliable MCI criteria to best identify high-risk cognitive profiles (Jak et al. 2016). Thus, eye movement measures may serve as clinically useful markers of MCI diagnosis (Pereira et al. 2014; Seligman and Giovannetti 2015).
Using Eye Tracking to Assess Cognitive Functions in AD/MCI
In AD, changes in eye movement characteristics may reflect abnormalities in visual scan processes that are necessary for visuospatial memory, which is impaired among persons with AD (Bundesen 1990). Thus, identifying basic visual perception process impairments may serve as a marker for the development of memory difficulties in persons with AD (Pereira et al. 2014). Several studies have found memory impairments using eye monitoring among amnestic (Hannula et al. 2007; Ryan et al. 2000) and MCI/AD patients (Crutcher et al. 2009; Hannula et al. 2010; Nakashima et al. 2010; Yeung et al. 2013). Among the latter, memory impairments have been detected by utilizing eye movement monitoring (for reviews see Hannula et al. 2010; Pereira et al. 2014). For example, impaired novelty preference (i.e., decreased amount of time spent viewing novel items as compared to repeated ones) has been found among persons with probable MCI in comparison to healthy young and old controls (Yeung et al. 2013). Also, eye tracking is often used to evaluate executive functions (Hutton and Ettinger 2006; Leigh and Kennard 2004) including in early-stage AD (Mitchell and Shiri-Feshki 2009). For example, it has been found that persons with MCI showed a decrease in divided attention in comparison to controls (Okonkwo et al. 2008). Others have found that persons with AD had decreased performance on alternating attention measures on the Stroop task in comparison to controls (Bélanger et al. 2010) and had longer fixation times, scan durations, and greater number of fixations on a selective visual attention task in comparison to healthy controls (Rösler et al. 2000). These findings highlight the potential interconnections between the cognitive functions of memory, executive function, and attention (Pereira et al. 2014), and the hippocampal complex may be involved in a range of cognitive processes through its interconnections with temporal and frontal regions (Kent et al. 2016; Moss 2016).
Pro and Antisaccade Tasks
Pro and antisaccade tasks are typically used in eye tracking methods in clinical populations including in persons with AD/MCI (Anderson and MacAskill 2013). These tasks enable the evaluation of memory and executive functions that are adversely affected in AD/MCI. On a prosaccade task, the participants are typically requested to focus on a dot in the center visual field and then to turn the gaze to a target stimulus in the peripheral visual area. The latency indicator is typically measured as the time elapsed from the appearance of the target stimulus to the saccade start time and precision is measured by saccade amplitude. This simple paradigm can appear in one of three variations: gap paradigm (Abel et al. 2002), in which the focal point disappears`~200 ms before the target stimulus appears; step paradigm, in which the centered point disappearance is immediately followed by the appearance of the target stimulus; and overlap paradigm, in which the centered point disappears shortly after the appearance of the target stimulus. In the latter paradigm, latencies are usually longer in comparison to latencies in the gap paradigm (Kalesnykas and Hallett 1994), in what has been termed the ‘gap effect’ (Saslow 1967). In studies of AD/MCI patients, no differences in the gap effect were found in comparison to controls (e.g., Crawford et al. 2015; Abel et al. 2002). Other task variations include changes in the spatial location of the target stimulus (on the horizontal or vertical axis, or both), the distance between stimuli (mostly ranging 3–20°), and the time of appearance of the target stimulus (i.e., fixed or varying). Early studies have shown that these variations affect the reaction time of persons with AD in comparison to healthy controls (Fletcher and Sharpe 1986; Scinto et al. 1994). Nonetheless, findings are limited and inconsistent in terms of the direction of the gap and its significance.
In the antisaccade task, the participant is requested to focus one’s gaze on a dot in the middle of the visual field and then to look at a direction opposite to the appearance of the target stimulus. The indicators are identical to those in the prosaccade task. In addition, the number of errors (i.e., saccades in the direction of the stimulus) and their corrections (i.e., shifting from a prosaccade to antisaccade) are recorded. Healthy individuals initially make frequent errors on this task, but with practice, error rates fall under 15% (Leigh and Kennard 2004). Increased error rate have been documented in persons with ADHD (see O’Driscoll et al. 2005), schizophrenia (Hutton and Ettinger 2006), autism (Minshew et al. 1999) and dyslexia (Biscaldi et al. 2000), all of whom had a documented fronto-striatal pathology (Hutton and Ettinger 2006; Leigh and Kennard 2004). Also, evidence from neuroimaging studies shows Frontal eyes Field (FEF) and Dorso-Lateral Pre-frontal Cortex (DLPC) activity during the antisaccade task (Hutton and Ettinger 2006). In terms of cognitive demand, both inhibition and working memory capacities are inherent in the antisaccade task. It has been found that spatial working memory was highly correlated with frequency of antisaccade error rate and accounted for the majority (68%) of the variance in the errors in AD patients (Crawford et al. 2013). Overall, the evidence suggests that in AD patients, deficits in frontal functions such as working memory and inhibition may contribute to programming of the antisaccade response (Hutton and Ettinger 2006; Crawford et al. 2013).
In order to understand if persons with AD/MCI have increased eye movement abnormalities in comparison to healthy controls, we would conduct a quantitative meta-analysis of the existing findings on eye movement in each of these populations and examine their heterogeneity. Also, because MCI may be construed as a preclinical stage of AD (Petersen 2004), we examined whether there were eye movement characteristics that were shared by the two populations, by assessing the heterogeneity of findings of both groups as a whole. Specifically, we predict that because individuals diagnosed with MCI have relatively preserved cognitive function in comparison to patients with AD, they would show comparable prosaccade paradigm performance to a control group of healthy older persons. Because the antisaccade paradigm is more cognitively demanding, we predict persons with MCIs would have comparable antisaccade performance to AD patients. Finally, we would conduct a quality analysis of the literature to examine if there are pro and antisaccade differences between persons with AD/ MCI and healthy older persons and if these differences are related to age or severity of disease.
Methods
Data Sources
Review material was drawn from the databases PsycINFO, Medline, and Google Scholar for the years 1980–2016. Key search terms were: *AD* OR “Alzheimer’s disease” or “dementia” *MCI* OR “mild cognitive impairment” AND “eye movement” OR “eye tracking” OR “saccade*” OR “ocular motor” OR “ocular movement” OR “oculomotor” OR “sensorimotor” OR “visual movement” OR “visual behavior” OR “visual behavior” OR “orienting” OR “overt attention” OR “covert attention” OR “spatial attention” OR “visual attention” OR “selective attention”.
Inclusion Criteria
In line with PRISMA systematic review guidelines (Moher et al. 2015), inclusion criteria were: 1. Full-length, English language studies published between 1980 and January 2016; 2. The study included an AD/MCI patient group without comorbidities or other neurodegenerative diseases and a healthy matched control group of older persons; 3. Use of visually guided saccade paradigm and/or denoted antisaccade by eye tracking techniques; and 4. Reported statistics for the comparison of saccade data between AD/MCI patients and controls or when not available, included images that enable data extraction.
Data Extraction
The extracted statistical data included mean saccadic Reaction Time (RT) per group, and when available, mean RT’s Standard Deviation (SD) or Standard Error (SE). When means and SD were missing, t-test/FANOVA/p value and number of participants/df were extracted. Several studies reported more than one result for the same group of participants (e.g. multiple saccades results or multiple paradigms). However, since inclusion of non-independent observations risks underestimating the error variance associated with each effect size (Borenstein et al. 2009; Mewborn et al. 2017) in case of multiple results, we used the results of the gap paradigm, which was the most common (67% of all studies, 84.6% of antisaccade paradigm studies) and known to be unaffected by healthy aging relatively to the overlap condition (Pratt et al. 1997). When multiple saccadic indicators were reported we used RT, which was most prevalent (90%). Additionally, when studies compared multiple independent experimental groups with a single control group (Heuer et al. 2013; Peltsch et al. 2014; Yang et al. 2011; Yang et al. 2013) or when results were measured over different time periods within the same sample (Bylsma et al. 1995), calculating an average effect size that collapses over the observations would result in the omission of important moderator data and therefore is not appropriate (see Higgins and Green 2011). Accordingly, effect sizes for each of these non-independent comparisons were included. To avoid underestimating the error variance associated with each effect size, the sample sizes used to calculate the standard errors for each group were divided by the number of their inclusions (see Higgins and Green 2011; Michie et al. 2009; Webb et al. 2012).
Results
Studies Retrieval
The literature search yielded 470 references of which 418 (89%) were duplicates and 29 (6%) did not meet inclusion criteria. After their removal, 23 studies met the inclusion criteria. A flow chart of the systematic review phases is presented in Fig. 1 . The included studies and their characteristics are presented in Table 1. Of 23 studies, 19 had a single patient group of either persons with AD (n = 18) or MCI (n = 1) and four had more than one patient group (n = 4). Two studies were of the same research group had identical numerical outcomes (Crawford et al. 2005; Crawford et al. 2013) and therefore only the later study (Crawford et al. 2013) was included. Two studies did not meet data availability inclusion criteria and were excluded from analysis (Mosimann et al. 2005; Currie et al. 1991). Thus, the 20 remaining studies were included in the analysis, all of which used the prosaccade paradigm, and 13 of which studied the antisaccade paradigm as well. Of the latter 13 studies, three (Fletcher and Sharpe 1986; Abel et al. 2002; Verheij et al. 2012) met the inclusion criteria for prosaccade but not for antisaccade data availability. Thus,10 studies were included in the final analysis of the antisaccade paradigm.
A four-phase flow diagram of the systematic review (adapted from Moher et al. 2009)
Quality Analysis
Quality assessment
We used the following criteria to assess studies’ quality: (1) randomization, (2) double blinding, and (3) proper dealing with withdrawals or dropouts (Jadad et al. 1996). The two former criteria were not relevant because groups were divided by disease status and had apparent behavioral differences. The dropout criterion was not reported in any of the studies. Nevertheless, patients’ withdrawal was reported in 5 studies: due to inability to complete the saccade tasks ((Bylsma et al. 1995; Peltsch et al. 2014; Shakespeare et al. 2014); 4, 1, and 5 patient, respectively), the diagnosis procedure ((Crawford et al. 2015); 3 patients) or technical reasons ((Bylsma et al. 1995); 7 patients).
Prosaccade group differences
Twenty-two studies compared saccade latencies of AD/MCI and healthy, older persons, age-matched controls using prosaccade paradigm (Table 1). Thirteen of 24 comparisons (54%) showed a significant longer latencies among AD/MCI patients in comparison to controls (Boxer et al. 2006; Bylsma et al. 1995; Crawford et al. 2015; Fletcher and Sharpe 1986; Garbutt et al. 2008; Hershey et al. 1983; Heuer et al. 2013; Scinto et al. 1994; Shafiq-Antonacci et al. 2003; Yang et al. 2011; Yang et al. 2013; Boxer et al. 2012). No study found significantly shorter latencies among AD/MCI patients in comparison to controls. Of the 16 studies that used an amplitude, gain, or velocity measure, three reported on increased saccadic amplitude or velocity in the AD group (Bylsma et al. 1995; Fletcher and Sharpe 1986; Shakespeare et al. 2014).
When comparing between studies that found significant prosaccade latencies differences between patients and controls and studies that did not, we found no significant differences in the likelihood to obtain group differences and no significant age differences. Nonetheless, the mean age difference between patients and controls was significantly smaller in studies that showed a group effect in comparison to studies that did not, t(22) = 2.34, p = 0.03. Additionally, cognitive impairment (measured by the MMSE) was significantly lower in studies that showed a group effect in comparison to studies that did not, t(22) = 3.05, p = 0.007. In line with this, a significant negative correlation was found between prosaccade latencies’ group differences and MMSE scores across studies (r = −0.61, p < 0.001). Age and cognitive impairment level differences by prosaccade latencies’ effect size for all groups of patients (AD and MCI) are presented in Table 2.
Antisaccade group differences
Fourteen studies compared AD/MCI/both patients to older persons controls on antisaccade paradigm (Table 1) (Abel et al. 2002; Alichniewicz et al. 2013; Boxer et al. 2006; Boxer et al. 2012; Crawford et al. 2013; Currie et al. 1991; Fletcher and Sharpe 1986; Garbutt et al. 2008; Heuer et al. 2013; Kaufman et al. 2012; Mosimann et al. 2005; Peltsch et al. 2014; Shafiq-Antonacci et al. 2003; Verheij et al. 2012). Of the 14 studies, two compared both MCI and AD to control (Peltsch et al. 2014; Heuer et al. 2013), one investigated only MCI patients (Alichniewicz et al. 2013), and seven only AD patients. Eight (57%) reported saccadic data (i.e., latency, velocity, amplitude, or gain). Of the 10 comparisons that included both MCI and AD or both groups, six (60%) demonstrated significantly longer latencies among patients in comparison to controls. When comparing between studies that found significant antisaccade latencies differences between patients and controls and studies that did not, we found no significant differences in the likelihood to obtain group differences. Additionally, no age or cognitive impairment differences were found between studies that showed a group effect in comparison to studies that did not. Regarding other saccadic indicators, the amplitude/velocity measure was used in a single study with no significant outcomes (Crawford et al. 2013). Direction error rate increased in 14 of the 16 comparisons to controls (87.5%), χ 2(1) = 9, p = 0.003.
Overall, significant differences between AD/MCI patients and controls were more prevalent in the antisaccade error rate rather than prosaccade/antisaccade latencies. The extant latencies findings suggest that there is a chance level likelihood to find a group effect in studies that investigated the differences between AD/MCI patients and controls, and that disease severity (i.e., increased cognitive impairment) and better control over age differences between patients and control are each associated with increased prosaccade latencies (but not antisaccade latencies) among patients in comparison to controls. The analysis of antisaccade findings showed that significant differences in error rate, but not in other indicators, were found between the two groups of patients and controls. Accordingly, it appears that the antisaccade error rate is a sensitive measure that can distinguish between AD/MCI patients and older persons controls and that prosaccade latencies may be able to do so but only at the later stages of the disease, since we found a higher likelihood for a group effect as cognitive impairment increased. In contrast, antisaccade latencies were not associated with either age or cognitive impairment level.
Quantitative Analysis (Meta-Analysis)
Random effect sizes were calculated using mean RT and/or errors rate (Rosenthal 1991). The first stage of the meta-analysis included 42 effect sizes that were derived from the pro and antisaccade paradigms for AD/MCI groups altogether, Q = 53.8, df = 41, p < .01, tau 2 = 0.04, I 2 = 23%. The Q value indicated heterogeneity and therefore the presence of potential moderator(s) (Sánchez-Meca and Marín-Martínez 1997). Accordingly, in the second stage of analysis, we used the paradigm type as a moderator: prosaccade, n = 25, Q = 37.34, df = 24, p < 0.05, tau 2 = 0.04, I 2 = 35%; antisaccade, n = 17, Q = 39.60, df = 16, p < .01, tau 2 = 0.14, I 2 = 59%. For both groups, Q values indicated effect size heterogeneity and therefore the presence of additional moderator(s).
In the analysis of the prosaccade paradigm, we used AD diagnosis of the clinical group (yes, no) as a moderator: AD group, n = 20, Q = 23.92, df = 19, p > .05, tau 2 = 0.03, I 2 = 21%.; MCI group, n = 5, Q = 6.42, df = 4, p > 0.05, tau 2 = 0.02, I 2 = 37.78%. The Q value indicated homogeneity and therefore the mean effect size was considered as the best estimation for the data. In prosaccade studies, overall weighted mean effect size in AD/MCI studies was moderate, 0.52, SE: 0.10, CI: 0.45–0.68 (Figure 2a) and in AD studies only it increased to 0.64, SE: 0.09, CI: 0.37–0.92. In MCI studies it decreased to 0.27, SE: 0.12 CI: 0.01–0.50.
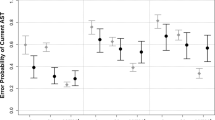
In the antisaccade studies, the mean overall effect size was 0.60, SE: 0.15, CI: 0.29–0.91. When using the same second moderator (AD diagnosis of the clinical group), AD group: n = 9, Q = 25.54, df = 8, p < .01, tau 2 = 0.15, I 2 = 68%; MCI group: n = 3, Q = 28.08, df = 2, p < 0.01, tau 2 = 0.25, I 2 = 92.87%. The mean antisaccade effect size in AD studies was 0.84, SE: 0.19, CI: 0.46–1.23, and in MCI studies 0.61, SE: 0.54, CI: -0.44 – 1.67. However, Q in both patients’ groups indicated heterogeneity, therefore suggesting the presence of additional moderator(s). Therefore, we used the outcome (error rate vs. latencies) in both AD and MCI studies. We then obtained homogenous results: latency: n = 9, Q = 6.60, df = 8, p > 0.05, tau 2 = 0.00, I 2 = 0%; error rate: n = 8, Q = 5.68, df = 7, p > 0.05, tau 2 = 0.00, I 2 = 0.00%. In antisaccade studies the weighted mean effect size for latencies was 0.32, SE: 0.10, CI: 0.14–0.50 (Figure 2b). In the antisaccade error rate studies it increased to 1.13, SE: 0.16, CI: 0.93–1.34 (Figure 2c).
In conclusion, the results suggest that AD disease status (preclinical vs. diagnosed patients) may serve as a moderator of the above effect when using a prosaccade latencies measure. The above analyses revealed a high effect size for distinguishing AD patients and control based on prosaccade latencies, and a moderate effect size for distinguishing MCI patients from controls. Additionally, in the antisaccade paradigm, using latencies separately from error rate measure resulted in homogeneous results, for both AD/MCI patients. Finally, in line with the quality analyses, antisacaade latencies had a lower effect size than prosaccades latencies and antisaccade error rate.
Discussion
This paper examined the differences in eye movement pattern in the pro and antisaccade paradigms between persons with AD/MCI to healthy older persons. We conducted both a meta-analysis and quality analysis of the literature that compared prosaccade and antisaccade performance among AD/MCI patients and controls. Our meta-analysis showed that cognitive impairment is related to prosaccade latencies’ differences between patients and controls. Specifically, AD but not MCI patients showed longer prosaccade latencies when compared to controls, and both patients’ groups did not differ from controls on antisaccade latencies. In line with this, in a qualitative analysis of the literature, we did not find evidence for significantly increased latency among both AD and MCI patients when compared to controls in antisaccade (60%) studies. Also, antisaccade error rate showed significantly increased rates among patients in comparison to controls in over 87% of the studies. Altogether, those analyses suggest that group differences between AD/MCI patients and controls in pro and antisaccade latencies are not readily captured by increased processing time. At the same time, antisaccade error rate performance seems to successfully differentiate between both AD and MCI patients and age-matched controls,
Differences Between Pro and Antisaccade Paradigms by Disease Progression
We found that the antisaccade error rate distinguished between both clinical groups and controls and that prosaccade latencies, but not antisaccade latencies, distinguished only between AD patients and controls. The differences between the paradigms may reflect tasks’ differences in level of difficulty, cognitive processes involved, and amount of learning required to perform each of them (Crawford et al. 2013; Kaufman et al. 2010; Leigh and Kennard 2004). The antisaccade paradigm involves an increased level of cognitive demand because it requires inhibition of the reflexive saccade to a target followed by the working memory guided voluntary saccade to the opposite location (Crawford et al. 2013; Kaufman et al. 2010; Leigh and Kennard 2004). Indeed, it has been found that cognitive impairment among AD patients is positively correlated with antisaccade correct response rate (Abel et al. 2002; Boxer et al. 2006) and negatively correlated with antisaccade error rate (Peltsch et al. 2014; Shafiq-Antonacci et al. 2003). In contrast, prosaccade tasks involve a rapid, automatic oculomotor response that does not require higher-order executive processing (Peltsch et al. 2014). Autopsy studies have demonstrated that oculomotor nuclei are affected by the pathological processes associated with AD (Rüb et al. 2001; Tzekov and Mullan 2014). Accordingly, it may be that the oculomotor function that is assessed in prosaccade tasks becomes impaired at the later AD stages and can be attributed to lesions in oculomotor brain nuclei (Mielke et al. 1995; Thulborn et al. 2000; Tzekov and Mullan 2014). Indeed, some have found a neuroanatomical association between vertical prosaccade velocity and medial longitudinal fasciculus (riMLF) volume (Boxer et al. 2012). These findings support the use of the prosaccade parameter in measuring oculomotor functions that can be attributed to the brain steam oculomotor area in clinical populations.
Antisaccade latencies did not differentiate patients from controls. One possible explanation is that the antisaccade task requires increased cognitive demands and therefore delays RTs in both groups. In line with this, longer antisaccade latencies were previously found to be age-related (e.g., Peltsch et al. 2011; Eenshuistra et al. 2004) while prosaccade latencies were found to be impervious to the effects of aging (Pratt et al. 2006; Peltsch et al. 2011). Taken together, these findings suggest that the processing time of a voluntary movement (required by antisacccade paradigm) lengthens with age while automatic processing (required by prosaccade paradigm) is less so (Peltsch et al. 2011). Nevertheless, antisaccade error rate distinguished between AD/MCI patients and controls. This suggests that inhibition and working memory, which are mediated by the frontal lobes (Miller and Cohen 2001; Smith and Jonides 1999) are among the first functions to become negatively affected by AD and that these impairments may manifest prior to the detectability of memory decline in clinical examinations (Alichniewicz et al. 2013). Both lesion and functional imaging evidence support a critical role of the DLPC and FEF in the antisaccade task (Alichniewicz et al. 2013; Boxer et al. 2006; Kaufman et al. 2010). Indeed, impairments in frontal functions have been reported in early-stage AD (Yun et al. 2011; Bélanger et al. 2010) and attributed to disruptions in distributed neural networks that support memory function (Sperling et al. 2010). Thus, our findings suggest that antisaccade accuracy impairment may reflect analogous effects of frontal impairment in persons with MCI and in persons with AD.
An absence of verbal or manual responses enables the antisaccade task to be used in movements-sensitive neuroimaging environments (e.g., magnetoencephelography (MEG), functional magnetic resonance imaging (fMRI)). Because of its relative simplicity, the antisaccade task is suitable for use as a bedside clinical mental examination in persons of all ages. In line with this, the use of eye-tracking methods to assess early cognitive difficulties has been advocated (Pereira et al. 2014; Seligman and Giovannetti 2015). Considering the scarcity of adequate methods to identify MCI, the current meta-analysis highlights the utility of antisaccade error rate measure as sensitive markers of early and subtle cognitive disruption.
Limitations and Future Directions
This meta-analysis supports the notion that MCI can be distinguished from AD and controls via their performance on the anti and prosaccade paradigm, as MCI patients demonstrated oculomotor deficits on cognitively challenging tasks (antisaccade) while AD patients showed impairment on an oculomotor task (prosaccade), and controls showed a preserved performance on both tasks. Nevertheless, MCI can be subdivided by amnestic symptoms’ manifestation (Jak et al. 2016) rather than considered as a single entity. In line with this, MCI is not always a pre-dementia form of AD but can be a precursor of any type of dementia or in some cases even a transient and reversible state (Vos et al. 2015). To our knowledge, no study has yet investigated MCI subtypes in relation to eye movement performance. It is unclear whether different MCI subtypes are associated with different eyes movement deficits. Also, it is unclear whether a specific eye movement deficit can promote the ability to predict the transition from a specific MCI subtype to AD. These questions warrant further research through longitudinal studies (Peltsch et al. 2014; Crutcher et al. 2009), larger patients’ samples, and by including additional patients’ groups. The choice of method for handling non-independent effect sizes is a matter of ongoing debate (Gurnani and Gavett 2017; Borenstein et al. 2009) and the applicability of newly developed methods (Mewborn et al. 2017) should be considered in future meta-analyses. Finally, we used saccades latencies in the gap paradigm for calculating the effects sizes because it was the most common measure in the AD/MCI investigations. Future studies that extend the currently limited use of various eye-movement measures (i.e., saccadic amplitude and velocity, fixation duration, number of fixation) in additional paradigm conditions (overlap, step) could enable establishing a profile of eye movements’ abnormalities based on a range of measures, both within AD/MCI and across neurodegenerative diseases.
Conclusions
Cognitive deterioration criteria serve as a moderator for the prosaccade latencies groups effects. We found that among diagnosed AD patients, but not persons with MCI, prosaccade latencies are consistently longer when compared to controls. Also, with regards to the antisaccade paradigm, latencies did not differentiate between patients and controls while the error rate measure was pronounced in both AD and MCI patients and therefore is a promising marker for pre-pathologic stages in older persons.
Funding
This work was supported by the Farber Alzheimer’s center foundation. This sponsor had no role in writing of the review and in the decision to submit the article for publication.
References
Abel, L. A., Unverzagt, F., & Yee, R. D. (2002). Effects of stimulus predictability and interstimulus gap on saccades in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 13(4), 235–243.
Alichniewicz, K. K., Brunner, F., Klünemann, H. H., & Greenlee, M. W. (2013). Neural correlates of saccadic inhibition in healthy elderly and patients with amnestic mild cognitive impairment. Frontiers in Psychology, 4, 467.
Alzheimer’s Association. (2015). 2015 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 11(3), 332–384.
Anderson, T. J., & MacAskill, M. R. (2013). Eye movements in patients with neurodegenerative disorders. Nature Reviews Neurology, 9(2), 74–85.
Bélanger, S., Belleville, S., & Gauthier, S. (2010). Inhibition impairments in Alzheimer's disease, mild cognitive impairment and healthy aging: Effect of congruency proportion in a Stroop task. Neuropsychologia, 48(2), 581–590.
Biscaldi, M., Fischer, B., & Hartnegg, K. (2000). Voluntary saccadic control in dyslexia. Perception, 29(5), 509–521.
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to meta-analysis. Chichester, UK: Wiley.
Boxer, A. L., Garbutt, S., Rankin, K. P., Hellmuth, J., Neuhaus, J., Miller, B. L., et al. (2006). Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. Journal of Neuroscience, 26(23), 6354–6363.
Boxer, A. L., Garbutt, S., Seeley, W. W., Jafari, A., Heuer, H. W., Mirsky, J., et al. (2012). Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer disease. Archives of Neurology, 69(4), 509–517.
Bundesen, C. (1990). A theory of visual attention. Psychological Review, 97(4), 523.
Bylsma, F. W., Rasmusson, D. X., Rebok, G. W., Keyl, P. M., Tune, L., & Brandt, J. (1995). Changes in visual fixation and saccadic eye movements in Alzheimer's disease. International Journal of Psychophysiology, 19(1), 33–40.
Crawford, T. J., Devereaux, A., Higham, S., & Kelly, C. (2015). The disengagement of visual attention in Alzheimer's disease: A longitudinal eye-tracking study. Frontiers in Aging Neuroscience, 7, 118. https://doi.org/10.3389/fnagi.2015.00118.
Crawford, T. J., Higham, S., Mayes, J., Dale, M., Shaunak, S., & Lekwuwa, G. (2013). The role of working memory and attentional disengagement on inhibitory control: effects of aging and Alzheimer's disease. Age, 35(5), 1637–1650.
Crawford, T. J., Higham, S., Renvoize, T., Patel, J., Dale, M., Suriya, A., et al. (2005). Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer’s disease. Biological Psychiatry, 57(9), 1052–1060.
Crutcher, M. D., Calhoun-Haney, R., Manzanares, C. M., Lah, J. J., Levey, A. I., & Zola, S. M. (2009). Eye tracking during a visual paired comparison task as a predictor of early dementia. American Journal of Alzheimer's Disease and Other Dementias, 24(3), 258–266.
Currie, J., Ramsden, B., McArthur, C., & Maruff, P. (1991). Validation of a clinical antisaccadic eye movement test in the assessment of dementia. Archives of Neurology, 48(6), 644–648.
Deubel, H., & Schneider, W. X. (2003). Delayed saccades, but not delayed manual aiming movements, require visual attention shifts. Annals of the New York Academy of Sciences, 1004(1), 289–296.
Eenshuistra, R. M., Ridderinkhof, K. R., & van der Molen, M. W. (2004). Age-related changes in antisaccade task performance: Inhibitory control or working-memory engagement? Brain and Cognition, 56(2), 177–188.
Fletcher, W. A., & Sharpe, J. A. (1986). Saccadic eye movement dysfunction in Alzheimer's disease. Annals of Neurology, 20(4), 464–471.
Garbutt, S., Matlin, A., Hellmuth, J., Schenk, A. K., Johnson, J. K., Rosen, H., et al. (2008). Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain, 131(5), 1268–1281.
Gurnani, A. S., & Gavett, B. E. (2017). The differential effects of Alzheimer's Disease and lewy body pathology on cognitive performance: A meta-analysis. [journal article]. Neuropsychology Review, 27(1), 1–17. https://doi.org/10.1007/s11065-016-9334-0.
Hannula, D. E., Althoff, R. R., Warren, D. E., Riggs, L., Cohen, N. J., & Ryan, J. D. (2010). Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Frontiers in Human Neuroscience, 4(166), 52–67.
Hannula, D. E., Ryan, J. D., Tranel, D., & Cohen, N. J. (2007). Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. Journal of Cognitive Neuroscience, 19(10), 1690–1705.
Hershey, L. A., Whicker, L., Abel, L. A., Dell'Osso, L., Traccis, S., & Grossniklaus, D. (1983). Saccadic latency measurements in dementia. Archives of Neurology, 40(9), 592–593.
Heuer, H. W., Mirsky, J. B., Kong, E. L., Dickerson, B. C., Miller, B. L., Kramer, J. H., et al. (2013). Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology, 81(14), 1235–1243.
Higgins, J. P., & Green, S. (2011). Cochrane handbook for systematic reviews of interventions (Vol. 4). UK: John Wiley & Sons.
Hutton, S. B., & Ettinger, U. (2006). The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology, 43(3), 302–313.
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials, 17(1), 1–12.
Jak, A. J., Preis, S. R., Beiser, A. S., Seshadri, S., Wolf, P. A., Bondi, M. W., et al. (2016). Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. Journal of the International Neuropsychological Society, 1-7.
Kalesnykas, R., & Hallett, P. (1994). Retinal eccentricity and the latency of eye saccades. Vision Research, 34(4), 517–531.
Kaufman, L. D., Pratt, J., Levine, B., & Black, S. E. (2010). Antisaccades: A probe into the dorsolateral prefrontal cortex in Alzheimer's disease. A critical review. Journal of Alzheimer's Disease, 19(3), 781–793.
Kaufman, L. D., Pratt, J., Levine, B., & Black, S. E. (2012). Executive deficits detected in mild Alzheimer's disease using the antisaccade task. Brain and Behavior, 2(1), 15–21.
Kent, B., Hvoslef-Eide, M., Saksida, L., & Bussey, T. (2016). The representational–hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiology of Learning and Memory, 129, 99–106.
Lagun, D., Manzanares, C., Zola, S. M., Buffalo, E. A., & Agichtein, E. (2011). Detecting cognitive impairment by eye movement analysis using automatic classification algorithms. Journal of Neuroscience Methods, 201(1), 196–203.
Leigh, R., & Kennard, C. (2004). Using saccades as a research tool in the clinical neurosciences. Brain, 127(3), 460–477.
Mewborn, C. M., Lindbergh, C. A., & Stephen Miller, L. (2017). Cognitive Interventions for cognitively healthy, mildly impaired, and mixed samples of older adults: A systematic review and meta-analysis of randomized-controlled trials. Neuropsychology Review. https://doi.org/10.1007/s11065-11017-19350-11068.
Michie, S., Abraham, C., Whittington, C., McAteer, J., & Gupta, S. (2009). Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology, 28(6), 690–701.
Mielke, R., Kessler, J., Fink, G., Herholz, K., & Heiss, W.-D. (1995). Dysfunction of visual cortex contributes to disturbed processing of visual information in Alzheimer's disease. International Journal of Neuroscience, 82(1–2), 1–9.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202.
Minshew, N. J., Luna, B., & Sweeney, J. A. (1999). Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology, 52(5), 917–917.
Mitchell, A. J., & Shiri-Feshki, M. (2009). Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica, 119(4), 252–265.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4(1), 1.
Molitor, R. J., Ko, P. C., & Ally, B. A. (2015). Eye movements in Alzheimer's disease. Journal of Alzheimer's Disease, 44(1), 1–12.
Mosimann, U. P., Müri, R. M., Burn, D. J., Felblinger, J., O'Brien, J. T., & McKeith, I. G. (2005). Saccadic eye movement changes in Parkinson's disease dementia and dementia with Lewy bodies. Brain, 128(6), 1267–1276.
Moss, R. A. (2016). A theory on the singular function of the hippocampus: Facilitating the binding of new circuits of cortical columns. AIMS Neuroscience, 3(3), 264–305.
Nakashima, Y., Morita, K., Ishii, Y., Shouji, Y., & Uchimura, N. (2010). Characteristics of exploratory eye movements in elderly people: possibility of early diagnosis of dementia. Psychogeriatrics, 10(3), 124–130.
O’Driscoll, G. A., Dépatie, L., Holahan, A.-L. V., Savion-Lemieux, T., Barr, R. G., Jolicoeur, C., et al. (2005). Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biological Psychiatry, 57(11), 1452–1460.
Okonkwo, O. C., Wadley, V. G., Ball, K., Vance, D. E., & Crowe, M. (2008). Dissociations in visual attention deficits among persons with mild cognitive impairment. Aging Neuropsychol C, 15(4), 492–505.
Peltsch, A., Hemraj, A., Garcia, A., & Munoz, D. (2011). Age-related trends in saccade characteristics among the elderly. Neurobiology of Aging, 32(4), 669–679.
Peltsch, A., Hemraj, A., Garcia, A., & Munoz, D. P. (2014). Saccade deficits in amnestic mild cognitive impairment resemble mild Alzheimer's disease. European Journal of Neuroscience, 39(11), 2000–2013.
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194.
Petersen, R. C., & Bennett, D. (2005). Mild cognitive impairment: is it Alzheimer's disease or not? Journal of Alzheimer's Disease, 7(3), 241–245.
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308.
Pratt, J., Abrams, R. A., & Chasteen, A. L. (1997). Initiation and inhibition of saccadic eye movements in younger and older adults: An analysis of the gap effect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 52(2), P103-P107.
Pratt, J., Dodd, M., & Welsh, T. (2006). Growing older does not always mean moving slower: examining aging and the saccadic motor system. Journal of Motor Behavior, 38(5), 373–382.
Rosenthal, R. (1991). Meta-analytic procedures for social research (Vol. 6): Sage.
Rösler, A., Mapstone, M. E., Hays, A. K., Mesulam, M., Rademaker, A., Gitelman, D. R., et al. (2000). Alterations of visual search strategy in Alzheimer's disease and aging. Neuropsychology, 14(3), 398–408.
Rüb, U., Del Tredici, K., Schultz, C., Büttner-Ennever, J., & Braak, H. (2001). The premotor region essential for rapid vertical eye movements shows early involvement in Alzheimer's disease-related cytoskeletal pathology. Vision Research, 41(16), 2149–2156.
Ryan, J. D., Althoff, R. R., Whitlow, S., & Cohen, N. J. (2000). Amnesia is a deficit in relational memory. Psychological Science, 11(6), 454–461.
Sánchez-Meca, J., & Marín-Martínez, F. (1997). Homogeneity tests in meta-analysis: A Monte Carlo comparison of statistical power and Type I error. Quality and Quantity, 31(4), 385–399.
Santana, R., Mendiburu, A., & Lozano, J. A. (2015). Multi-view classification of psychiatric conditions based on saccades. Applied Soft Computing, 31, 308–316.
Saslow, M. (1967). Effects of components of displacement-step stimuli upon latency for saccadic eye movement. JOSA, 57(8), 1024–1029.
Scinto, L. F., Daffner, K. R., Castro, L., Weintraub, S., Vavrik, M., & Mesulam, M. M. (1994). Impairment of spatially directed attention in patients with probable Alzheimer's disease as measured by eye movements. Archives of Neurology, 51(7), 682–688.
Seligman, S. C., & Giovannetti, T. (2015). The potential utility of eye movements in the detection and characterization of everyday functional difficulties in mild cognitive impairment. Neuropsychology Review, 25(2), 199–215.
Shafiq-Antonacci, R., Maruff, P., Masters, C., & Currie, J. (2003). Spectrum of saccade system function in Alzheimer disease. Archives of Neurology, 60(9), 1272–1278.
Shakespeare, T., Yong, K., Kaski, D., Schott, J. M., & Crutch, S. (2014). Abnormalities of fixation, saccade, and pursuit in posterior cortical atrophy compared to typical AD. Alzheimer's & Dementia, 10(4), P199.
Silverberg, N. B., Ryan, L. M., Carrillo, M. C., Sperling, R., Petersen, R. C., Posner, H. B., et al. (2011). Assessment of cognition in early dementia. Alzheimer's & Dementia, 7(3), e60–e76.
Smith, E. E., & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657–1661.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 280–292.
Sperling, R. A., Dickerson, B. C., Pihlajamaki, M., Vannini, P., LaViolette, P. S., Vitolo, O. V., et al. (2010). Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Medicine, 12(1), 27–43.
Thulborn, K. R., Martin, C., & Voyvodic, J. T. (2000). Functional MR imaging using a visually guided saccade paradigm for comparing activation patterns in patients with probable Alzheimer's disease and in cognitively able elderly volunteers. AJNR, 21(3), 524–531.
Tzekov, R., & Mullan, M. (2014). Vision function abnormalities in Alzheimer disease. Survey of Ophthalmology, 59(4), 414–433.
Verheij, S., Muilwijk, D., Pel, J. J., van der Cammen, T. J., Mattace-Raso, F. U., & van der Steen, J. (2012). Visuomotor impairment in early-stage Alzheimer's disease: changes in relative timing of eye and hand movements. Journal of Alzheimer's Disease, 30(1), 131–143.
Vos, S. J., Verhey, F., Frölich, L., Kornhuber, J., Wiltfang, J., Maier, W., et al. (2015). Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain, 138, 1327–1338.
Webb, T. L., Miles, E., & Sheeran, P. (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138(4), 775–808.
Yang, Q., Wang, T., Su, N., Liu, Y., Xiao, S., & Kapoula, Z. (2011). Long latency and high variability in accuracy-speed of prosaccades in Alzheimer’s disease at mild to moderate stage. Dementia and Geriatric Cognitive Disorders Extra, 1(1), 318–329.
Yang, Q., Wang, T., Su, N., Xiao, S., & Kapoula, Z. (2013). Specific saccade deficits in patients with Alzheimer’s disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age, 35(4), 1287–1298.
Yeung, L.-K., Ryan, J. D., Cowell, R. A., & Barense, M. D. (2013). Recognition memory impairments caused by false recognition of novel objects. Journal of Experimental Psychology: General, 142(4), 1384–1397.
Yun, J.-Y., Lee, D. Y., Seo, E. H., Choo, I. H., Park, S. Y., Kim, S. G., et al. (2011). Neural correlates of stroop performance in Alzheimer’s disease: a FDG-PET study. Dementia and Geriatric Cognitive Disorders Extra, 1(1), 190–201.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors report no disclosures or conflict of interest.
Rights and permissions
About this article
Cite this article
Kahana Levy, N., Lavidor, M. & Vakil, E. Prosaccade and Antisaccade Paradigms in Persons with Alzheimer’s Disease: A Meta-Analytic Review. Neuropsychol Rev 28, 16–31 (2018). https://doi.org/10.1007/s11065-017-9362-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-017-9362-4