Abstract
Anticipatory postural adjustments (APAs) play an important role in the performance of many activities requiring the maintenance of vertical posture. However, little is known about how children utilize APAs during self-induced postural perturbations. A group of children, aged 7–16 years, with typical motor development, performed various arm movements while standing on a force platform. APAs were measured by recording the electromyographic activity of six trunk and leg muscles on both sides of the body and displacement of center of pressure (COP). Anticipatory bursts of activity in the dorsal muscle groups of the trunk and legs and suppression in the ventral muscle groups as well as posterior COP displacement were found during the performance of bilateral shoulder flexion. Conversely, during bilateral shoulder extension, the COP displacement was anterior, and APAs were reversed showing bursts of activity in the ventral muscle groups and suppression in the dorsal muscles. During right and left reciprocal arm movements, COP displacement was minimal and APAs were generated in the dorsal muscle groups on the side of the forward moving arm and in the ventral muscle groups on the side of the arm moving into extension. However this pattern reversed for lower leg muscles, where APAs were generated in the ventral muscles on the side of forward moving arm and in the dorsal muscle on the side of the arm moving into extension. The results of this study indicate that children with typical motor development are able to generate APAs, produce task-specific sequencing of muscle activity and differentiate between perturbations in the sagittal and transverse planes. The results of this study indicate that by at least age 7, children who are typically developing demonstrate the ability to generate patterns of anticipatory muscle activation and suppression, along with center of pressure changes, similar to those reported in healthy adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Any perturbation in the body such as a fast arm or leg movement or sudden translation of the support surface endangers the body equilibrium. To minimize the loss of balance, the central nervous system (CNS) activates the trunk and leg muscles prior to the forthcoming perturbation using anticipatory postural adjustments (APAs). Anticipatory postural adjustments were first described in a groundbreaking work by Russian scientists Belen’kii et al. (1967) who reported the existence of the electrical activity in the sacrolumbar muscles of the trunk and upper leg prior to fast shoulder flexion in standing. It was suggested that APAs are used to minimize the negative consequences of a predicted postural perturbation (Bouisset and Zattara 1987; Massion 1992). The second type of adjustment in the activity of postural muscles is observed after the body perturbation has already occurred. These alterations in the muscle activity are termed compensatory postural adjustments (CPAs) and are initiated by sensory feedback signals (Park et al. 2004; Alexandrov et al. 2005). CPAs serve to restore the body’s position after a perturbation has occurred (Macpherson et al. 1989; Maki and McIlroy 1996; Henry et al. 1998).
The efficiency of postural control in standing is affected by the ability to utilize both anticipatory and compensatory adjustments. It was demonstrated recently that predicable perturbations resulted in the generation of strong APAs and significantly smaller compensatory activity of muscles and COP displacements (Santos et al. 2010). This suggests that control of posture could be enhanced by better utilization of APAs.
To date, APAs have been primarily studied in healthy adults and adults with neurological and musculoskeletal conditions which impair posture and balance including Parkinson’s disease, stroke, and amputation (Latash et al. 1995; Aruin et al. 1997; Dickstein et al. 2004; Horak et al. 2005). Thus, the organization of APAs has been studied with regard to arm movements performed in sitting and standing (Dufosse et al. 1985; Bouisset and Zattara 1987) during situations which impose unstable conditions (Gantchev and Dimitrova 1996; Aruin et al. 1998) or increased levels of fear (Adkin et al. 2002) and with regard to both internally, subject initiated (Aruin and Latash 1995a, b), and externally, experimenter triggered, perturbations (Aruin and Latash 1995b; Aruin et al. 2001b; Shiratori and Latash 2001; Santos and Aruin 2008). In addition, the effect of the tasks inducing body rotation has been studied during unilateral and reciprocal arm movements (Aruin et al. 2001a; Shiratori and Aruin 2004). The findings of these studies indicate that APAs in adults are direction specific and scale with magnitude of the perturbation and the level of body instability.
APAs in sitting and standing, in children who are typically developing, have not been extensively studied. However, research describing the ontogeny of APAs in children is steadily growing. There are studies in infants (0–6 months) (Hadders-Algra et al. 1998; van der Fits et al. 1999a; Hedberg et al. 2005) and in older infants and toddlers (6–18 months) (Barela et al. 1999; van der Fits et al. 1999b; Roncesvalles et al. 2001; Witherington et al. 2002; Hedberg et al. 2007), which are beginning to illuminate our understanding of the development of APAs in children. In particular, anticipatory activity in sitting has been observed in muscles of the neck or trunk extensors as early as 6 months with activation in multiple dorsal muscle groups between 9 and 15 months (Witherington et al. 2002). Moreover, it was reported that prior to independent walking, infants reached out for support after balance was compromised, but by 13.5 months infants reached out for support in anticipation of a presumed perturbation (Barela et al. 1999). It has also been shown that APAs in standing infants develops between 10 and 17 months and that by 15 months, with opportunities for practice, infants demonstrate the ability to scale anticipatory muscle activity with increasing loads (Witherington et al. 2002).
Studies of APAs in older children (between 3 and 14 years) used functional tasks that could not be performed by infants including load lifting and release in sitting (Schmitz et al. 1999, 2002, 2003; Jucaite et al. 2003; Martineau et al. 2004; Jover et al. 2006), upper extremity arm pointing and reaching in standing (Haas et al. 1989; Riach and Hayes 1990; Hay and Redon 1999, 2001; Liu et al. 2007), and leg lifting, stepping and initiation of gait (Ledebt et al. 1998; Assaiante et al. 2000; Malouin and Richards 2000; Hahn et al. 2005; Vallis and McFadyen 2005; Wu et al. 2008).
Many of these studies have shown that typically developing children are able to generate anticipatory changes in EMG activity and COP position prior to arm and leg movements which induce postural perturbations. However, studies of APAs during arm movements in standing (Riach and Hayes 1990; Hay and Redon 1999, 2001; Liu et al. 2007) have reported only on center of pressure changes. These studies, involving children between the ages of 4–14 years, have shown that even the youngest children could produce posterior and lateral COP displacements before and during a unilateral shoulder flexion (Riach and Hayes 1990; Hay and Redon 1999). However, the youngest children (4–6 years) also demonstrated increased latency of reaction time and less consistent anterior/posterior anticipatory COP displacements than did older children (7–14 years) and adult subjects tested in an earlier study (Riach and Lucy 1987).
To date, there have been no published papers which incorporate both electromyography and force platform data in the study of APAs in children with typical motor development during functional upper extremity tasks performed in standing. Such information would expand our knowledge of APAs in children and serve to build a body of information, which may eventually lead to the development of interventions to improve APAs in children with neurological and musculoskeletal conditions that impair posture and balance. For example, studies documenting APA patterns prior to gait initiation in older children (Ledebt et al. 1998; Assaiante et al. 2000; Malouin and Richards 2000; Vallis and McFadyen 2005) have served as the foundation for the design of intervention studies to improve APAs prior to gait initiation in children with Down syndrome (Wu et al. 2007, 2008; Angulo-Barroso et al. 2008; Ulrich et al. 2008). This model of using descriptive research to develop interventions has also been used in the area of compensatory postural adjustments (Sveistrup and Woollacott 1997; Burtner et al. 1999; Shumway-Cook et al. 2003; Woollacott et al. 2005).
In summary, there is research describing the ability of children to produce anticipatory changes in center of pressure during the performance arm movements in standing and the organization of APAs prior to the initiation of gait. In addition, knowledge of how healthy children organize APAs in preparation for gait initiation and how they organize CPAs as a means of sustaining posture following a perturbation has been shown to be beneficial for the development of interventions to improve posture and balance in children developmental and neurological conditions. However, there are no studies describing how children organize APAs and sequence muscle activity during the performance of upper extremity tasks in standing.
Therefore, the aim of this study was to investigate anticipatory changes in the activity of dorsal and ventral trunk and lower extremity muscles, as well as changes in center of pressure (COP) position and the sequencing of anticipatory muscle activity in children with typical motor development, during the performance of functional tasks involving bilateral, unilateral and reciprocal arm movements.
These tasks collectively incorporate the most common upper extremity arm movements used by children during the performance of daily activities. Moreover, they were selected because they trigger perturbations in the sagittal and transverse planes, which allowed us to determine whether APA patterns are task specific.
We hypothesized that children aged 7–16 years, who are typically developing, can produce directionally specific anticipatory EMG patterns and changes in center of pressure position following self-induced sagittal and transverse plane perturbations. We also hypothesized that children with typical motor development will demonstrate task-specific sequencing of muscle activity.
Methods
Subjects
Ten subjects, 6 girls and 4 boys, were recruited for this study (see Table 1). All of the subjects, had motor development typical for their age and no known neurological, musculoskeletal or vision impairments. Subjects were a sample of convenience recruited from children of family members, friends and colleagues. Informed consent was obtained from the parent, and each child was required to give both a written and a verbal assent to participate, according to procedures approved by the University of Illinois at Chicago Institutional Review Board (IRB).
Instrumentation
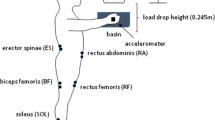
The subjects performed the experimental tasks standing on a force platform (OR-6, AMTI, Inc., USA). Signals from the platform were amplified and data relative to reaction forces were collected in 3 orthogonal planes (F Z —along the force of gravity, F X —parallel to the sagittal plane, and F y —parallel to the frontal plane of the body). Moments of force, MY were collected as well. A miniature unidirectional accelerometer (Model 208CO3, PCB Piezotronics, Inc, USA) was taped to the dorsal surface of the dominant hand with the axis of sensitivity oriented in the plane parallel to the movement associated with each experimental task.
Pairs of disposable pediatric electrodes (3M, Red Dot™) attached over the muscle bellies of the following postural muscles on both sides of the body: Rectus Abdominus (RA), Erector Spinae (ES), Rectus Femoris (RF), Biceps Femoris (Bf), Tibalis Anterior (TA), and Medial Soleus (SOL) (Basmajian 1980) were used to record surface electromyographic activity. During the data collection process, the EMG signals were amplified (gain 2000) and filtered (10–500 Hz, analog filter) by means of a differential amplifier (RUN Technologies, USA). The EMG, force platform, and accelerometer signals were digitized with a 16-bit resolution at 1,000 Hz and customized LabView software (National Instruments, Austin, TX) was used to collect the data and to perform the offline data analysis.
Procedure
Six experimental tasks, involving self-initiated shoulder flexion and extension performed under bilateral, reciprocal, and unilateral conditions, were studied (Fig. 1). The subjects were instructed to stand quietly on the force platform with feet placed shoulder width apart; chalk was used to outline the position of each foot to ensure consistent placement during all experimental tasks. To standardize the arm movement across all tasks, the subjects were asked to hold a lightweight plastic tube (weighting 42.5 g) in each hand.
Schematic illustrations of the experimental tasks. a Bilateral Shoulder Flexion (BLSF: Right and left arms move simultaneously into shoulder flexion). b Bilateral Shoulder Extension (BLSE: Right and left arms move simultaneously into shoulder extension). c Right Reciprocal Shoulder Flexion (RRSF: Right arm moves into shoulder flexion, left into shoulder extension). Left Reciprocal Shoulder Flexion (LRSF: Left arm moves into shoulder flexion, right into shoulder extension). d Right Unilateral Shoulder Flexion (RUSF: Right arm moves into shoulder flexion). Left Unilateral Shoulder Flexion (LUSF: Left arm moves into shoulder flexion). Force platform, as well as the locations of electrodes and an accelerometer are depicted in a
The experimental tasks included Bilateral Shoulder Flexion (BLSF), performed to 90° of flexion (Fig. 1a), and Bilateral Shoulder Extension (BLSE), performed to 45 degrees of extension (Fig. 1b). Reciprocal tasks, which induce rotational perturbations were also studied. During Right Reciprocal Shoulder Flexion (RRSF), the subjects were asked to move their arms, simultaneously performing right shoulder flexion to 90° and left shoulder extension to 45° (Fig. 1c). During Left Reciprocal Shoulder Flexion (LRSF) the movement was reversed, combining left shoulder flexion with right shoulder extension.
In the final two tasks, Right and Left Unilateral Shoulder Flexion (RUSF and LUSF), which also induce rotational perturbations, the subjects were asked to unilaterally perform either right or left shoulder flexion to 90° while maintaining the opposite arm at their side (Fig.1d).
The subjects were instructed to look directly forward and to perform each task “as fast as possible” in a self-paced mode after the computer tone and to execute ‘sharp, clear movements’, sustaining the final position of the arms for 2–3 s. In each experimental task, the subjects were instructed in the set-up and performed 2–3 practice trials before data collection was initiated. Eight trials with a 5 s interval between each trial were performed in each experimental task. To reduce the possibility of muscle fatigue, the subjects were given a 2-min rest between each experimental task. In addition, they were invited to sit, drink water or juice and/or eat a snack between each experimental task. Only one subject asked to sit down; however, several subjects requested juice or water breaks, taken in standing, between experimental tasks. The order of presentation of tasks was randomized across subjects.
Data processing
Data processing was performed offline using customized LabView software (National Instruments, Austin, TX). The process involved rectification and filtering of the EMG signals using a 100 Hz low pass, second-order Butterworth filter and filtering of the platform and accelerometer signals using a second-order Butterworth low pass filter set at 20 Hz. Every trial, for each of the six tasks, was viewed on a monitor and aligned, by an experienced researcher, based on the first visible deflection of the accelerometer trace. This indicated the initiation of arm movement and was labeled T0. The aligned trails in each experimental task were averaged for each subject.
Anticipatory EMG activity was quantified as the integral of EMGs (∫EMG100) occurring during the 100 ms before (T0). The 100 ms interval of integration is consistent with the literature (Shiratori and Aruin 2004; Li and Aruin 2008). To account for baseline activity, the EMG integral of −500 to −450 ms before T0(∫EMG50) was calculated and applied to correct ∫EMG100 using the following formula: ∫EMG = ∫EMG100 − 2∫EMG50.
To compare EMG activity across subjects, the ∫EMG values were normalized by the absolute maximal value identified over all experimental tasks for each muscle (∫EMGAPA). This process was applied individually to each subject. The result is a range of ∫EMGAPA between −1 and 1 with positive values being associated with muscle activation and negative values indicating suppression of background muscle activity. This normalization process has been used in diverse APA studies (Shiratori and Latash 2000; Slijper and Latash 2000).
COP displacements were calculated using the following approximation: \( COP = - {\frac{{M_{y} + F_{x} *d}}{{F_{z} }}} \) where M Y is the moment in sagittal plane, F Z and F X are the vertical and anterior–posterior components of the ground reaction force, and d is the distance from the origin of the force platform to its surface (Winter et al. 1996). Analysis of the center of pressure data (COP) was completed by averaging the horizontal displacements of the COP in the anterior/posterior direction for each subject and for each experimental condition. Then the anticipatory shift in COP was quantified by subtracting the baseline COP value (500–450 ms before T0) from the COP value at T0.
Peak acceleration of the arm for each subject during each task was extracted from the averaged acceleration data for each series.
Repeated measures analysis of variance (ANOVA) was used to compare peak acceleration and COP data across tasks. Post hoc analysis was used to further analyze statistically significant results using Bonferroni correction. To quantify whether anticipatory COP changes were significantly different from baseline for each condition, a one sample t test was used. Single factor ANOVAs were used to analyze the effect of movement direction for each movement type (bilateral, reciprocal and unilateral) using dependent measure of ∫EMGAPA for each muscle. For all tests, statistical significance was set at P < .05.
Results
All subjects were able to perform the experimental tasks. It is important to comment first on the consistency of the peak acceleration during each of the tasks. The subjects performed BLSF with a peak acceleration of 30.17 ± 11.21 m/s2; BLSE with acceleration of 28.42 ± 11.05 m/s2; RRSF and LRSF with an acceleration of 32.49 ± 12.92 and 26.60 ± 10.66 m/s2, respectively; and peak acceleration for RUSF and LUSF were 32.28 ± 8.50 and 27.88 ± 8.35 m/s2, respectively. There was no significant statistical difference between the peaks of acceleration among these tasks.
EMG activity
Bilateral Shoulder Flexion and Bilateral Shoulder Extension
Figure 2 shows EMG traces for the right and left trunk and leg muscles recorded during Bilateral Shoulder Flexion (BLSF) and Bilateral Shoulder Extension (BLSE) for a representative subject. During BLSF anticipatory activation of right and left ES and BF muscles can be seen approximately 100 ms before the initiation of arm movement (T0). When the direction of the bilateral arm movement changed to extension (BLSE), anticipatory muscle activity is seen in right and left RA and RF along with anticipatory inhibition in right and left ES and BF.
EMG patterns for Bilateral Shoulder Flexion (left panels) and Bilateral Shoulder Extension (right panels) for a representative subject (average of 8 trials). The vertical line (T0) represents the onset of arm movement and the point of alignment. Both right and left muscle groups are plotted and EMG scales are in arbitrary units. Dorsal muscle groups (ES, BF) are shown inverted for ease of comparison and their scales are on the right. Since muscle activity in the TA and SOL muscle groups is unremarkable, it is not shown. Note the directional specificity of muscle activity during APAs, with dorsal muscles active during shoulder flexion and ventral muscle activity during shoulder extension
Anticipatory integrals of all muscles studied during performance of the bilateral shoulder flexion and extension movements are shown in Fig. 3. The reversal of the APA integrals, seen as change from activation to inhibition with the reversal of the movement direction, is clearly observed in muscles of the upper leg and trunk. The effect of the direction of the arm movement was statistically significant in both right and left muscle groups: LBF (F (1,9) = 9.97, P < .05), RBF (F (1,9) = 13.02, P < .01), LRA (F (1,9) = 47.04, P < .001), RRA (F (1,9) = 47.30, P < .001), LES (F (1,9) = 26.37, P < .001), and RES (F (1,9) = 33.45, P < .001). No significant difference in ∫EMGAPA was observed in right or left TA or SOL muscles.
Normalized integrals of anticipatory changes in muscle activity averaged across 10 subjects for BLSF and BLSE. Dorsal muscle groups are on the left and ventral muscle groups on the right. Mean values and standard deviation bars are shown. Significant differences in muscle activity for BLSF and BLSE are indicated by an asterisk (P < .05)
Right Reciprocal Shoulder Flexion and Left Reciprocal Shoulder Flexion
During RRSF, when the right arm is moving into shoulder flexion and the left into extension, the most consistent muscle activity was observed in the muscles of the upper leg. The RBF and the LRF were activated in anticipation of the reciprocal arm movement (Fig. 4). This was accompanied by suppression of the RSOL and activation of the LSOL. When the direction of the reciprocal arm movement was reversed (LRSF), the pattern described above was also reversed. That is, the trunk and upper leg muscle groups on the side of the forward moving arm reproduce the activity observed during bilateral shoulder flexion, while the muscle groups on the side of backward moving arm mimic the muscle activity observed during bilateral shoulder extension. This pattern of simultaneously recruiting opposite muscle groups on either side of the body will be identified as reciprocal muscle activity in this paper.
EMG traces for Right and Left Reciprocal Shoulder Flexion for a representative subject (average of 8 trials). For description of the figure arrangements, refer to Fig. 2. Note the effect of the direction of arm movement shown by reversal of anticipatory muscle activity in the RA, BF, RF, and SOL muscle groups
The normalized integrals of EMG activity for the trunk and lower extremity muscles during RRSF and LRSF tasks are shown in Fig. 5. The effect of arm movement direction is demonstrated between these two tasks. The body side which is performing the shoulder flexion shows increased APA activity in the dorsal trunk and upper leg muscles (ES, BF) while the SOL muscles show inhibition of background activity (negative ∫EMGAPA value). At the same time, the body side which is performing the shoulder extension movement shows increased APA activity in the ventral muscles of the trunk and upper leg muscles (RA, RF). This effect of movement direction is significant in the LES (F (1,9) = 5.95, P = .05), LRA (F (1,9) = 7.31, P < .05), RRA (F (1,9) = 5.99, P < .05), RBF (F (1,9) = 7.7.31, P < .05), LRF (F (1,9) = 125.75, P < .001), RRF (F (1,9) = 21.54, P < .005), and LSOL (F (1,9) = 12.34, P < .01).
Normalized anticipatory EMG integrals averaged across 10 subjects for the Right and Left Reciprocal Shoulder Flexion/Extension tasks. Dorsal muscle groups are on the left and ventral muscle groups on the right. Mean values and standard deviation bars are shown. *Significance (P < .05) between muscle groups across tasks
Right Unilateral Shoulder Flexion and Left Unilateral Shoulder Flexion
Figure 6 shows the EMG traces for the right and left trunk and leg muscles for a representative subject. During RUSF and LUSF, there was simultaneous anticipatory activity in the ES muscle groups, but the amplitude of muscle activity was greater on the side opposite of the moving arm (contralateral). However, the anticipatory onset of the ipsilateral BF preceded that of the contralateral BF for both RUSF and LUSF. With respect to the TA and SOL muscle groups, there was anticipatory muscle activity in the LSOL and inhibition in the RSOL during RUSF, with a reversal of this pattern during LUSF. For both RUSF and LUSF, the TA was quiet.
EMG traces for Right and Left Unilateral Shoulder Flexion for a representative subject (average of 8 trials). For description of the figure arrangements, refer to Fig. 2. Note, too, that the RSOL is inhibited, while the LSOL shows anticipatory activity during RUSF. This pattern of muscle activity is reversed during LUSF
Figure 7 shows anticipatory ∫EMGAPA for the right and left unilateral shoulder flexion series averaged across subjects. The effect of movement direction is significant in the LRA (F (1,9) = 7.19, P < .05), LRF (F (1,9) = 12.50, P < .01), RRF (F (1,9) = 7.66, P < .05), RBF (F (1,9) = 7.73, P < .05), LTA (F (1,9) = 4.93, P < .05), RTA (F (1,9) = 9.37, P < .05), and LSOL (F (1,9) = 9.52, P < .05).
Normalized integrals of anticipatory EMG averaged across all subjects for Right and Left Unilateral Shoulder Flexion. Dorsal muscle groups are on the left and ventral muscle groups are on the right. Mean values and standard deviation bars are shown. *Significance (P < .05) between muscle groups across tasks. Note the effect of side with inhibition of the RSOL during RUSF and inhibition of LSOL during LUSF
Onsets of EMG activity
The onsets of EMG activity of all studied muscles and for each of the six experimental tasks are presented in Fig. 8. During BLSF, the anticipatory activity was first observed in the RBF (−52 ms) followed by activity in the RES, LES and finally, the LBF (−43 ms); these four muscles were activated within a range of 9 ms. All other muscle groups were activated after the focal event (T0) in a distal to proximal order as follows: SOL, TA, RF, RA. During BLSE, the ventral muscle groups were activated prior to the focal event (T0). The RRA and LRA were initiated first, followed approximately 50 ms later, and just preceding arm movement, by the RRF and LRF muscle groups. The remaining muscle groups were activated in a proximal-to-distal order as follows: ES, BF, SOL, and TA.
With regard to RRSF and LRSF, the first muscles to be activated were the ventral muscles (RA and RF) on the side of the arm moving into shoulder extension, that is, the LRA and LRF were activated during RRSF and the RRF and RRA during LRSF. This was followed by activity in the dorsal muscle groups (ES and BF) on the side of the forward flexing arm. For RRSF the RBF preceded the RES, but during LRSF the LES preceded the LBF. Activity in the TA followed: RTA during RRSF and LTA during LRSF. The last muscle group activated before the focal movement was the ES on the side of shoulder extension: LES during RRSF and RES during LRSF.
For the unilateral shoulder flexion tasks, the dorsal muscles of the trunk (ES) and upper leg (BF) were activated before the focal movement. For RUSF, the RES and RBF we activated almost simultaneously followed by the LES. The LBF turned on just before the arm movement was initiated. The remaining muscles (TA, SOL, RF and RA) followed a distal to proximal order of activation after the focal movement. For LUSF, the LBF preceded the LES and RES and the RBF was activated just before the arm movement began. The remaining muscles (TA, SOL, RF and RA) followed a distal to proximal pattern of activation.
Center of pressure displacement
In each of the experiments, with the exception of right reciprocal arm movement, the change in COP was in the direction opposite of the arm movement (i.e., shoulder flexion resulted in backward COP displacement during APAs, Fig. 9). Statistically significant differences were found in the displacement of COP between BLSE and all other tasks (F (5,45) = 3.36, P < .05). Post hoc tests revealed significant differences between BLSE and BLSF (P < .001), RUSF (P < .05) and LUSF (P < .005).
COP data were also analyzed, using a one sample t test, to determine if there was a significant shift in the COP during APAs when compared to baseline COP values. The bilateral flexion task, which showed a posterior COP shift, was significantly different from zero (t (9) = −2.8, P < .05, confidence interval (−.0065, −.0069)). Bilateral shoulder extension, which was associated with an anterior COP shift during APA, was also significantly different from zero [t (9) = 7.621, P < .0001 with confidence interval (.0017, .0031)]. All other task conditions showed no significant difference from zero in center of pressure changes prior to initiation of arm movement. For reciprocal and unilateral shoulder movement tasks, we did not expect to see significant differences from zero in anterior/posterior center of pressure displacements due to the rotational nature of the perturbation caused by the arm movement.
Discussion
The main findings of this study indicate that children with typical motor development, between 7 and 16 years of age, are able to produce directionally specific APAs during bilateral shoulder flexion and extension, right and left reciprocal arm movements and right and left unilateral shoulder flexion performed in standing. Changes in center of pressure (COP) were consistent with the direction of displacements reported in adults and children during bilateral shoulder flexion and extension tasks (Riach and Hayes 1990; Liu et al. 2007). Thus, the hypothesis that the children with typical motor development can produce directionally specific anticipatory EMG patterns and changes in center of pressure position whether the perturbation is induced in sagittal or transversal planes was supported. The sequencing of anticipatory muscle activity was altered based on direction of arm movement. This supports our second hypothesis that children with typical motor development can produce task-specific sequencing of muscle activity.
It is important to mention that the subjects in our study ranged in age from 7 to 16 years, and therefore, it is prudent to consider the possible impact that age may have on acquisition of mature anticipatory postural adjustments. For example, some of the younger children (7–9 years) in this study had less consistency in their anticipatory EMG activity (timing and refinement of muscle traces) when compared to the older children (12–16 years).
Age has been found to play a role in the development of mature reactive balance and anticipatory locomotor strategies in typically developing children (Shumway-Cook and Woollacott 1985; Sveistrup and Woollacott 1996; Grasso et al. 1998; Vallis and McFadyen 2005). The variability observed in the youngest subjects in our study is consistent with results reported by researchers who studied reactive balance (Shumway-Cook and Woollacott 1985; Sveistrup and Woollacott 1996), anticipatory locomotor control (Grasso et al. 1998), and strategies for obstacle avoidance during locomotion (McFadyen et al. 2001; Vallis and McFadyen 2005) in typically developing children. For example, in a study of reactive balance following translational platform perturbations, Shumway-Cook and Woollacott (1985) reported that the oldest subjects (7–10 years of age) showed mature reactive balance when compared to the adults in the study. However, the younger subjects (4–6 years of age), who showed EMG patterns similar to those of the older children (7–10 years) and adults, exhibited more variability in muscle activity including earlier or later onset of EMGs and an increased number of EMG bursts.
The results of earlier research on postural control in typically developing children (Shumway-Cook and Woollacott 1985; Grasso et al. 1998; McFadyen et al. 2001; Vallis and McFadyen 2005) taken together with the outcome of the current study suggest that age plays a role in the development of mature postural control. Moreover, research in infants shows that compensatory or reactive postural adjustments (CPAs) in standing develop between 10 and 12 months of age (Sveistrup and Woollacott 1996; Westcott and Burtner 2004), while APAs are refined between 13 and 17 months of age (Witherington et al. 2002). Further, these infant studies indicate that the development of CPAs precedes the development of APAs in standing. Therefore, we find it reasonable to suggest that APAs in children will mature later than the 7- to 10-year-old window reported for CPAs (Shumway-Cook and Woollacott 1985).
APAs prior to bilateral arm movements
The children in this study were able to generate APAs based on the direction of their arm movement. During bilateral shoulder flexion, the dorsal muscle groups (ES and BF) were active; restricting forward movement of the body and sustaining postural alignment. When the children were asked to perform bilateral shoulder extension, the ventral muscle groups (RA and RF) were activated restricting backward movement of the body and sustaining an optimal posture to prevent a backward fall. This would indicate that directionally specific anticipatory postural adjustments, associated with self-initiated, shoulder flexion and extension can be organized by the central nervous system of children who are typically developing. The results of this study indicate that by age seven, children have developed the ability to generate task-dependent APAs in the leg and trunk muscles.
The outcome of this study is also consistent with results of studies in infants and toddlers who are typically developing. As early as 9 months, during sitting reaching tasks, APAs are seen in a single dorsal muscle group and by 15 months, in multiple dorsal muscle groups (van der Fits et al. 1999a, b). Further, the directional specificity of APAs has also been documented in standing reach tasks in 13- to 14-month-old infants (Witherington et al. 2002). The results of the present study would also suggest that directionally specific APAs continue to development as children mature, thus creating the foundation for the consistent, reliable feedforward postural control documented in adults.
APAs prior to reciprocal arm movements
Studies have shown that reciprocal arm movements induce predominantly transverse plane perturbations, and in healthy adults, the CNS selects a pattern of reciprocal anticipatory muscle activity to accommodate these perturbations (Aruin et al. 2001a; Shiratori and Aruin 2004; Bleuse et al. 2005; Lee et al. 2009). The children in this study also demonstrated a similar ability to counteract rotational body perturbations through the use of reciprocal anticipatory muscle activity. During RRSF, which caused a counterclockwise rotational perturbation, anticipatory muscle activity was present in the RES, RBF, and RTA and inhibition of the RSOL, while on the left side of the body, anticipatory activity was seen in the LRA, LRF and LSOL muscles. This pattern was reversed during LRSF.
Previous studies also documented reversals in anticipatory muscle activity when reciprocal flexion/extension arm movements were performed by healthy adults (Aruin et al. 2001a; Shiratori and Aruin 2004). These authors concluded that the activation of reciprocal muscle groups on each side of the body and at each segmental level would minimize the counter-rotation in the transverse plane and assist in maintenance of balance with minimal change in COP. The reciprocal anticipatory muscle activity and minor changes in COP (see Fig. 8) in our study of reciprocal arm movements suggest that similar to adults, the CNS in children is able to differentiate between symmetrical and rotational perturbations and to select directionally specific solutions to maintain balance.
APAs prior to unilateral arm movements
Upper extremity movement associated with Right and Left Unilateral Shoulder Flexion not only causes a sagittal plane perturbation but also a rotational perturbation consistent with, but less forceful than, that elicited during reciprocal arm movements. We found the anticipatory muscle activity during this task differed from the pattern that was used during reciprocal arm movements. In our study, the children simultaneously activated the right and left erector spinae muscles and showed anticipatory activity of the BF muscle on the ipsilateral side and the RF on the contralateral side. With regard to the muscles serving the ankle joint, we noted the EMG activity of the soleus on the ipsilateral side was suppressed while the soleus on the contralateral side showed anticipatory EMG activity.
For the children in this study, the selected pattern of muscle activation may have acted to minimize rotation in the transverse plane. Using the trunk muscles symmetrically, to stabilize the body, while activating reciprocal leg and ankle muscle groups, dorsal on the ipsilateral and ventral on the contralateral side of the body, may be a plausible strategy to counteract rotational forces. This strategy, similar to the one used during reciprocal arm movements, may add further support to the hypothesis that the CNS, in children who are typically developing, is able to select directionally specific solutions to maintain balance in anticipation of the effect of perturbing tasks.
In adults, the existence of such a counter-rotation strategy is supported by accelerometer and EMG data (Bouisset and Zattara 1981; Bouisset et al. 2000) and motion capture and EMG data (Hodges et al. 2000). The outcome of these studies involving healthy adults verifies the existence of the anticipatory counter-rotation between ipsilateral and contralateral body segments (shoulder, trunk, hips, thigh and shank) during unilateral shoulder flexion. These studies demonstrate that the body segments on the ipsilateral side were moving in the direction of the rotation while the body segments on the contralateral side were moving in opposite direction. It is postulated that reciprocal muscle patterns may be used to simplify the control process during asymmetrical movements and to counteract the effect of a rotational perturbation on balance (Bouisset et al. 2000).
Onset of EMG activity
Muscle sequencing was task specific for all experimental conditions. The children in this study sequenced muscle activity similar to that described in studies with healthy adults (Friedli et al. 1984; Zattara and Bouisset 1988) with the BF muscles activating just prior or together with ES muscles. In the shoulder extension task, the ventral muscles were activated in anticipation of the arm movement as described by Friedli et al. (1984) with the RA preceding the activity of the RF.
With respect to the unilateral shoulder flexion tasks, the children also performed in a manner similar to that of healthy adults (Belen’kii et al. 1967; Bouisset and Zattara 1981; Zattara and Bouisset 1988; Gantchev and Dimitrova 1996) with ipsilateral BF activity preceding activity in the bilateral ES muscles, while the contralateral BF was activated just prior to the initiation of the arm.
Finally, for the reciprocal arm movement tasks, which incorporate simultaneous shoulder flexion and extension, the anticipatory muscle activity demonstrated by the children was a combination of what was observed during both BLSF and BLSE. Similar results describing how muscles were activated, were previously described in adults (Aruin et al. 2001a; Shiratori and Aruin 2004). However, to the best of our knowledge, there are not studies in adults or children, which describe sequencing of anticipatory muscle activity in similar tasks.
Directional specificity of anticipatory COP displacement
In this study, the subjects demonstrated anticipatory posterior displacements of COP during bilateral shoulder flexion and anticipatory anterior COP displacements during bilateral shoulder extension. These same directional displacements have been documented in adults (Aruin and Latash 1995a, b; Latash et al. 1995), in adults and children with typical motor development (Riach and Hayes 1987, 1990; Hay and Redon 2001), and in children with cerebral palsy (Liu et al. 2007).
Limitations
While our hypotheses were supported, this study has certain limitations. First, the wide range in the age of the subjects (see Table 1) should be taken into consideration when reading this study. Although the younger children showed similar patterns of anticipatory muscle activity, muscle sequencing, and COP displacements as those seen in the older children in this study and in healthy adults in other studies (Bouisset and Zattara 1981; Aruin and Latash 1995a, b), age may have contributed to the variability of their EMG activity (number of EMG bursts before, during and after T0) and variability of the onset of their APAs. We recommend this variability in the younger children be kept in mind when considering the conclusions of this study.
Second, the sample size in this study was relatively small (n = 10), which did not allow us to describe the longitudinal development of APAs by grouping the subjects’ data by age. As such, we see the need for a follow-up study with a larger sample of subjects grouped into age intervals of 2–3 years. This study design would have the potential to make an important contribution to the developmental literature by documenting the longitudinal acquisition of APAs in children who are typically developing and by pinpointing the earliest age at which mature APAs are present during self-induced perturbations in standing.
Conclusion
The results of the study indicate that by at least age seven, children who are typically developing are able to generate task-dependent anticipatory postural adjustments, to sequence muscle activity with respect to the task and to differentiate between sagittal plane and transverse plane perturbations. In addition, the muscle activation and suppression patterns were similar to those reported for healthy adults.
References
Adkin AL, Frank JS et al (2002) Fear of falling modifies anticipatory postural control. Exp Brain Res 143(2):160–170
Alexandrov AV, Frolov AA et al (2005) Feedback equilibrium control during human standing. Biol Cybern 93(5):309–322
Angulo-Barroso RM, Wu J et al (2008) Long-term effect of different treadmill interventions on gait development in new walkers with Down syndrome. Gait Posture 27(2):231–238
Aruin AS, Latash ML (1995a) Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res 103(2):323–332
Aruin AS, Latash ML (1995b) The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res 106(2):291–300
Aruin AS, Nicholas JJ et al (1997) Anticipatory postural adjustments during standing in below-the-knee amputees. Clin Biomech (Bristol, Avon) 12(1):52–59
Aruin AS, Forrest WR et al (1998) Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol 109(4):350–359
Aruin AS, Ota T et al (2001a) Anticipatory postural adjustments associated with lateral and rotational perturbations during standing. J Electromyogr Kinesiol 11(1):39–51
Aruin AS, Shiratori T et al (2001b) The role of action in postural preparation for loading and unloading in standing subjects. Exp Brain Res 138(4):458–466
Assaiante C, Woollacott M et al (2000) Development of postural adjustment during gait initiation: kinematic and EMG analysis. J Mot Behav 32(3):211–226
Barela JA, Jeka JJ et al (1999) The use of somatosensory information during the acquisition of independent upright stance. Infant Motor Behavior 22(1):87–102
Basmajian JV (1980) Electromyography–dynamic gross anatomy: a review. Am J Anat 159(3):245–260
Belen’kii VE, Gurfinkel VS et al (1967) Control elements of voluntary movements. Biofizika 12(1):135–141
Bleuse S, Cassim F et al (2005) Vertical torque allows recording of anticipatory postural adjustments associated with slow, arm-raising movements. Clin Biomech (Bristol, Avon) 20(7):693–699
Bouisset M, Zattara M (1981) A sequence of postural reactions precedes voluntary movements. Neurosci Lett 22:263–270
Bouisset S, Zattara M (1987) Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech 20(8):735–742
Bouisset S, Richardson J et al (2000) Do anticipatory postural adjustments occurring in different segments of the postural chain follow the same organisational rule for different task movement velocities, independently of the inertial load value? Exp Brain Res 132(1):79–86
Burtner PA, Woollacott MH et al (1999) Stance balance control with orthoses in a group of children with spastic cerebral palsy. Dev Med Child Neurol 41(11):748–757
Dickstein R, Shefi S et al (2004) Anticipatory postural adjustment in selected trunk muscles in post stroke hemiparetic patients. Arch Phys Med Rehabil 85(2):261–267
Dufosse M, Hugon M et al (1985) Postural forearm changes induced by predictable in time or voluntary triggered unloading in man. Exp Brain Res 60(2):330–334
Friedli WG, Hallett M et al (1984) Postural adjustments associated with rapid voluntary arm movements 1. Electromyographic data. J Neurol Neurosurg Psychiatry 47(6):611–622
Gantchev GN, Dimitrova DM (1996) Anticipatory postural adjustments associated with arm movements during balancing on unstable support surface. Int J Psychophysiol 22(1–2):117–122
Grasso R, Assaiante C et al (1998) Development of anticipatory orienting strategies during locomotor tasks in children. Neurosci Biobehav Rev 22(4):533–539
Haas G, Diener HC et al (1989) Development of feedback and feedforward control of upright stance. Dev Med Child Neurol 31(4):481–488
Hadders-Algra M, Brogren E et al (1998) Development of postural control–differences between ventral and dorsal muscles? Neurosci Biobehav Rev 22(4):501–506
Hahn ME, Farley AM et al (2005) Neural network estimation of balance control during locomotion. J Biomech 38(4):717–724
Hay L, Redon C (1999) Feedforward versus feedback control in children and adults subjected to a postural disturbance. Exp Brain Res 125(2):153–162
Hay L, Redon C (2001) Development of postural adaptation to arm raising. Exp Brain Res 139(2):224–232
Hedberg A, Carlberg EB et al (2005) Development of postural adjustments in sitting position during the first half year of life. Dev Med Child Neurol 47(5):312–320
Hedberg A, Schmitz C et al (2007) Early development of postural adjustments in standing with and without support. Exp Brain Res 178(4):439–449
Henry SM, Fung J et al (1998) EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol 80(4):1939–1950
Hodges PW, Cresswell AG et al (2000) Three dimensional preparatory trunk motion precedes asymmetrical upper limb movement. Gait Posture 11(2):92–101
Horak FB, Dimitrova D et al (2005) Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol 193(2):504–521
Jover M, Schmitz C et al (2006) Anticipatory postural adjustments in a bimanual load-lifting task in children with Duchenne muscular dystrophy. Neurosci Lett 403(3):271–275
Jucaite A, Fernell E et al (2003) Deficient coordination of associated postural adjustments during a lifting task in children with neurodevelopmental disorders. Dev Med Child Neurol 45(11):731–742
Latash ML, Aruin AS et al (1995) Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry 58(3):326–334
Ledebt A, Bril B et al (1998) The build-up of anticipatory behaviour. An analysis of the development of gait initiation in children. Exp Brain Res 120(1):9–17
Lee LJ, Coppieters MW et al (2009) Anticipatory postural adjustments to arm movement reveal complex control of paraspinal muscles in the thorax. J Electromyogr Kinesiol 19(1):46–54
Li X, Aruin AS (2008) Anticipatory postural adjustments in conditions of simulated reduced gravity. Gait Posture 28(4):538–544
Liu W, Zaino CA et al (2007) Anticipatory postural adjustments in children with cerebral palsy and children with typical development. Pediatr Phys Ther 19:188–195
Macpherson JM, Horak FB et al (1989) Stance dependence of automatic postural adjustments in humans. Exp Brain Res 78(3):557–566
Maki BE, McIlroy WE (1996) Postural control in the older adult. Clin Geriatr Med 12(4):635–658
Malouin F, Richards CL (2000) Preparatory adjustments during gait initiation in 4–6-year-old children. Gait Posture 11(3):239–253
Martineau J, Schmitz C et al (2004) Impairment of a cortical event-related desynchronisation during a bimanual load-lifting task in children with autistic disorder. Neurosci Lett 367(3):298–303
Massion J (1992) Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 38(1):35–56
McFadyen BJ, Malouin F et al (2001) Anticipatory locomotor control for obstacle avoidance in mid-childhood aged children. Gait Posture 13(1):7–16
Park S, Horak FB et al (2004) Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res 154(4):417–427
Riach CL, Hayes KC (1987) Maturation of postural sway in young children. Dev Med Child Neurol 29(5):650–658
Riach CL, Hayes KC (1990) Anticipatory postural control in children. J Mot Behav 22(2):250–266
Riach C, Lucy SD (1987) Adjustments to posture prior to arm movement. In: Jonsson B et al (eds) International series on biomechanics, biomechanic X-A. Human Kinetics, Champaign, pp 459–463
Roncesvalles MN, Woollacott MH et al (2001) Development of lower extremity kinetics for balance control in infants and young children. J Mot Behav 33(2):180–192
Santos MJ, Aruin AS (2008) Effects of lateral perturbations and changing stance conditions on anticipatory postural adjustment. J Electromyogr Kinesiol
Santos MJ, Kanekar N et al (2010) The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol 20(3):388–397
Schmitz C, Martin N et al (1999) Development of anticipatory postural adjustments in a bimanual load-lifting task in children. Exp Brain Res 126(2):200–204
Schmitz C, Martin N et al (2002) Building anticipatory postural adjustment during childhood: a kinematic and electromyographic analysis of unloading in children from 4 to 8 years of age. Exp Brain Res 142(3):354–364
Schmitz C, Martineau J et al (2003) Motor control and children with autism: deficit of anticipatory function? Neurosci Lett 348(1):17–20
Shiratori T, Aruin AS (2004) Anticipatory postural adjustments associated with rotational perturbations while standing on fixed and free-rotating supports. Clin Neurophysiol 115(4):797–806
Shiratori T, Latash M (2000) The roles of proximal and distal muscles in anticipatory postural adjustments under asymmetrical perturbations and during standing on rollerskates. Clin Neurophysiol 111(4):613–623
Shiratori T, Latash ML (2001) Anticipatory postural adjustments during load catching by standing subjects. Clin Neurophysiol 112(7):1250–1265
Shumway-Cook A, Woollacott MH (1985) The growth of stability: postural control from a development perspective. J Mot Behav 17(2):131–147
Shumway-Cook A, Hutchinson S et al (2003) Effect of balance training on recovery of stability in children with cerebral palsy. Dev Med Child Neurol 45(9):591–602
Slijper H, Latash M (2000) The effects of instability and additional hand support on anticipatory postural adjustments in leg, trunk, and arm muscles during standing. Exp Brain Res 135(1):81–93
Sveistrup H, Woollacott MH (1996) Longitudinal development of the automatic postural response in infants. J Mot Behav 28(1):58–70
Sveistrup H, Woollacott MH (1997) Practice modifies the developing automatic postural response. Exp Brain Res 114(1):33–43
Ulrich DA, Lloyd MC et al (2008) Effects of intensity of treadmill training on developmental outcomes and stepping in infants with Down syndrome: a randomized trial. Phys Ther 88(1):114–122
Vallis LA, McFadyen BJ (2005) Children use different anticipatory control strategies than adults to circumvent an obstacle in the travel path. Exp Brain Res 167(1):119–127
van der Fits IB, Klip AW et al (1999a) Postural adjustments during spontaneous and goal-directed arm movements in the first half year of life. Behav Brain Res 106(1–2):75–90
van der Fits IB, Otten E et al (1999b) The development of postural adjustments during reaching in 6- to 18-month-old infants. Evidence for two transitions. Exp Brain Res 126(4):517–528
Westcott SL, Burtner PA (2004) Postural control in children: implications for pediatric practice. Phys Occup Ther Pediatr 24(1–2):5–55
Winter DA, Prince F et al (1996) Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol 75(6):2334–2343
Witherington D, von Hofsten C, Rosander K, Robinette A, Woollacott MW, Bertenthal BI (2002) The developmental of anticipatory postural adjustments in infancy. Infancy 3(4):495–517
Woollacott M, Shumway-Cook A et al (2005) Effect of balance training on muscle activity used in recovery of stability in children with cerebral palsy: a pilot study. Dev Med Child Neurol 47(7):455–461
Wu J, Looper J et al (2007) Exploring effects of different treadmill interventions on walking onset and gait patterns in infants with Down syndrome. Dev Med Child Neurol 49(11):839–845
Wu J, Ulrich DA et al (2008) Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with Down syndrome after different treadmill interventions. Exp Brain Res 186(2):261–272
Zattara M, Bouisset S (1988) Posturo-kinetic organisation during the early phase of voluntary upper limb movement. 1. Normal subjects. J Neurol Neurosurg Psychiatry 51(7):956–965
Acknowledgments
We would like to thank the children and families who participated in this study and also Shruti Joshi, PT, MS, Xioyan Li, PhD, Marcio Santos, PT, PhD and Veena Iyengar, PT, MS for assistance with data collection. This study was supported in part by a grant from the Neuro-Developmental Treatment Association, Laguna Beach, CA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girolami, G.L., Shiratori, T. & Aruin, A.S. Anticipatory postural adjustments in children with typical motor development. Exp Brain Res 205, 153–165 (2010). https://doi.org/10.1007/s00221-010-2347-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2347-7