Abstract
The aims of this study were to (1) characterize anticipatory and reactive postural strategies in typically developing (TD) children and adolescents; (2) determine if TD youth shift from reactive to anticipatory mechanisms based on knowledge of platform movement; and (3) determine whether TD youth further modify postural strategies when additional information about the perturbation is provided. Sixteen typically developing youth aged 7–17 years stood with eyes open on a movable platform that progressively translated anteroposteriorly (20 cm peak-to-peak) through four speeds (0.1, 0.25, 0.5, and 0.61 Hz). Participants performed two trials each of experimenter-triggered and self-triggered perturbations. Postural muscle activity (1000 Hz) of the tibialis anterior, gastrocnemius, quadriceps and hamstrings and 3D whole body kinematics (100 Hz) were recorded. The Anchoring Index and marker-pair trajectory cross-correlations were calculated as indications of body stabilization. The number of steps taken to regain balance/avoid falling were counted. Transition states and steady states were analyzed separately. Generally, the higher frequencies resulted in more steps being taken, lower correlations coupled with greater temporal lags between marker trajectories, and postural muscle activity similar to older adults. The provision of self-triggered perturbations allowed participants to make the appropriate changes to their balance by use of anticipatory postural control mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balance strategies used by children and adolescents involve two functional principles (Assaiante et al. 2005). First, there is identification of a frame of reference and, for static balance this can be organized from the support surface in an ascending fashion or from the head to the feet in a descending fashion. Second, children and adults need to simultaneously control large numbers of degrees of freedom of the various body joints. In static or perturbed balance, the task permits the control of superimposed modules such as the head–trunk unit which can be controlled more or less independently from other segment pairs. Stabilization of the head can thus occur in two ways: (a) it can be stabilized on the trunk referred to as the head stabilization on trunk strategy (HSTS) (Assaiante et al. 2005) or (b) it can be stabilized in space, referred to as the head stabilized in space strategy (HSSS) (Assaiante et al. 2005; Assaiante and Amblard 1995). The strategy used depends, in part, on the dynamic constraints determining task difficulty and the developmental characteristics of the person (Assaiante et al. 2005). For example, in scenarios with more destabilizing effects, HSTS might be selected as a preferred method of stabilization to provide a stable reference frame, while scenarios in which the person is comfortable with their ability to deal with a perturbation results in a preference for HSSS. Thus, the Anchoring Index (AI) can be used to provide an indication of which method of stabilization (HSTS or HSSS) is being employed, as it compares the stabilization of a segment with respect to both external space and its inferior segment (Mesure et al. 1999; Amblard et al. 1997).
Studies examining the development of postural control suggest specific observable stages of control (cf Fujiwara et al. 2011; Assaiante et al. 2005). By about 7 years of age, children should begin to exhibit adult-like performance in terms of maintaining balance and posture (Woollacott and Shumway-Cook 1990) with the frame of reference organized in a descending fashion (Assaiante 1998). If the postural disturbance is large enough, it is likely that a step must be taken to avoid falling (Burtner et al. 2007). An inadequate feet-in-place response can often be recovered within one effectively placed step (Roncesvalles et al. 2000). Studies have identified thresholds for step initiation induced by support surface translations with predictions that stepping would occur if the state space threshold is breached (Pai and Patton 1997; Pai et al. 1998, 2003). If the postural disturbance is small, however, balance can be maintained through modulation of joint torque by activating the muscles of the lower leg (e.g., gastrocnemii and tibialis anterior) appropriately, termed ankle strategy. If the postural disturbance is somewhere in between, the result is likely to be controlled about the hip, termed hip strategy, with proximal to distal activation of postural muscles. It is entirely likely that the response used lies somewhere in between the ankle and hip strategies and depends on multiple factors including the required force or torque to maintain stability, the support configuration, body morphology and initial position, and muscular strength (McCollum and Leen 1989).
Prior knowledge or experience of a disturbance has been shown to result in habituation whereby preparation occurs in an attempt to counteract the upcoming perturbation, resulting in postural muscle activations in advance of or coincident with the perturbation (Kennedy et al. 2013; Schmid et al. 2011; Bugnariu and Sveistrup 2006; Pavol and Pai 2002). Motor adaptations to balance-challenging perturbations occur with the repetition of successive, separate perturbation trials (Buchanan and Horak 1999; Hansen et al. 1988; Perrin et al. 1998; Kennedy et al. 2013). The oscillating platform paradigm provides an experimental approach to perturb the support surface at different frequencies and amplitudes where the initial perturbation elicits a reactive response mechanism and as the platform continues to oscillate, the participant can switch to an anticipatory mechanism. Adaptations to the increasingly predictable perturbations can occur within just a few cycles of sinusoidal platform translations (Schmid et al. 2011; Laessoe and Voigt 2008; Bugnariu and Sveistrup 2006). Changes in the frequency of platform oscillation results in a sudden perturbation and the participant must use a reactive mechanism to respond to this change before switching again to the anticipatory mechanism once they are stabilized.
In the present study, we asked (1) whether typically developing children and adolescents would shift from a head stabilized on trunk strategy to a head stabilized in space strategy when the perturbation characteristics became known; and (2) whether children and adolescents would predict and shift to an earlier use of head stabilized in space postural strategy if they were able to control the perturbation onset.
Materials and methods
The study was approved by the University of Ottawa Health Sciences and Science Research Ethics Board, conforming to the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2), and fully informed consent was obtained from parents and adolescents. Assent was obtained from the younger children.
Participants
Sixteen typically developing (TD) children and adolescents aged 7–17 years (7 boys, 9 girls) participated in this study. Mean age, height, and weight (± SD) were 12.56 years (± 3.16), 160.47 cm (± 19.59), and 53.94 kg (± 16.82).
Dynamic balance protocol
Participants stood with their eyes open and bare feet shoulder-width apart on a hydraulic movable platform (CAREN platform, Motek Medical, B.V., Amsterdam). They were instructed to maintain their balance while avoiding taking steps unless absolutely necessary. When a step was taken, participants were told to regain their balance and reposition their feet to the initial position that was marked on the platform with stickers.
The platform translated 20 cm peak-to-peak in the anterior/posterior direction. The sinusoidal oscillations commenced at a frequency of 0.1 Hz. At intervals of 80–100 s, the frequency was increased successively to 0.25, 0.5, and 0.61 Hz. Trials were approximately 342 s long and consisted of at least 10 cycles at 0.1 Hz, 20 cycles at 0.25 Hz, 40 cycles at 0.5 Hz, and 50 cycles at 0.61 Hz. Participants performed 2 trials where the change in frequency of the platform oscillation was initiated by the experimenter (externally triggered perturbation: ETP). Participants then performed two trials where the increase in oscillation frequency was self-cued (self-triggered perturbation: STP). Regular rests were provided.
Data acquisition
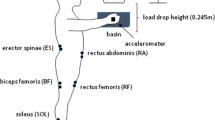
Participants were instrumented with retroreflective markers (14 mm) to obtain full body kinematics (modified Plug-in Gait model). Motion analysis software recorded body movements at 100 Hz using 7 Vicon T13 cameras (Vicon, Oxford, UK). Surface electromyography (sEMG) data were collected at 1000 Hz using Delsys Bagnoli EMG (Delsys Inc., Natick, USA) systems. Postural muscle activity was recorded by attaching surface electrodes to the tibialis anterior (TA), gastrocnemius (G), quadriceps (Q), and hamstrings (H) muscles of the left side of the body. A ground electrode was placed on the left iliac crest.
Data were processed offline. Trials were individually reconstructed, digitally labeled, and filtered for noise reduction in Vicon Nexus 1.8.5, then exported for data analysis. Exported data files were processed for each dependent variable using MATLAB version R2015a (Mathworks Inc., USA).
Data reduction and analysis
In each trial, the first three to five consecutive cycles without stepping at each frequency were considered ‘transition state periods’ and were analyzed for reactive postural responses. In the last half of the trial at each frequency, a series of 3–5 consecutive cycles without stepping at 0.1 Hz and a series of 8–10 consecutive cycles without stepping at the remaining frequencies was considered the ‘steady-state period’ when anticipatory postural responses would be expected (Fig. 1).
Perturbation protocol depicting platform oscillation and corresponding EMG signals a from tibialis anterior (TA), gastrocnemius (GAS), quadriceps (Q), and hamstring (H) muscles during the transition- and steady-state periods at 0.5 Hz. b Depicts a participant’s posture during backward platform displacement at 0.5 Hz in transition (left) and steady states (right). Expanded head–neck stick figure shows a shift to head stabilization in space strategy. c A participant with markers and EMG electrodes
Stepping responses
The number of steps taken by each subject at every frequency was documented.
Anchoring Index
The absolute angles (with respect to the external axis) around the transverse axis were computed for the head and trunk. These values were used to compute the AI for the head and to determine the stabilization of the head with respect to both external space and the trunk (Amblard et al. 1997, 2001; Mesure et al. 1999). The AI was calculated for transition- and steady-state periods in the pitch plane as follows:
where σa is the standard deviation of the absolute angular distribution of the head relative to vertical, and σr is the standard deviation of the angular distribution of the head relative to the trunk. A negative AI indicates a preference for stabilization of a segment on its inferior segment (e.g., head stabilization on trunk strategy/HSTS), whereas a positive AI indicates a preferred stabilization with respect to the external space (e.g., head stabilization in space strategy/HSSS).
Cross-correlation functions
The cross-correlation coefficients of the anterior–posterior linear displacements of the ankle and head markers, hip and head markers, and ankle and hip markers were computed for each trial. To identify the maximum cross-correlation (CCmax), and the temporal relationship (lag/lead), each signal correlation was calculated for up to ± 50% time shift of one cycle by shifting one signal temporally one data point at a time. The CCmax was recorded as well as the normalized time lag at which it occurred (CClag/lead). Cycles during which the participants took steps were excluded from the CC analysis.
Postural muscle burst and tonic activity
Postural muscle activity was identified as the first burst of activity associated with a perturbation that lasted more than 50 ms and that was greater than two standard deviations above the baseline. To be included in the calculations of group muscle activity, responses had to be present in at least 30% of the directionally specific perturbations at each frequency (i.e., anterior muscles for backward perturbations, posterior muscles for forward perturbations) for transition state periods, and 50% for steady-state periods. For the 0.1 Hz frequency, this recruitment threshold was reduced to 20% of perturbations.
Tonic postural muscle activity was expressed as a percentage of the baseline tonic activity level in ETP SS 0.1 Hz. This was determined during quiet stance at a point mid-cycle in the lowest frequency where no burst activity was present.
Statistical analysis
Participant demographics and stepping data were summarized using descriptive analyses. Statistical analyses were performed using SPSS v 23.0.0.2 (IBM Corp.). The data were determined to be non-normal through inspection of skewness and kurtosis, histograms, and Shapiro–Wilk tests of normality. Non-parametric inferential testing using the Wilcoxon signed rank test for within group differences identified significant differences between transition- and steady-state periods at each frequency in both ETP and STP conditions (separately). The dependent variables tested were: CCmax and CClag/lead of marker trajectories (ankle–head, ankle–hip, hip–head), the AI, and postural muscle activity (tonic and bursting activity). Comparisons were also made between condition (ETP and STP) for all variables in each of transition and steady states. Results were considered significant at p < 0.0125 (Bonferroni adjusted for multiple comparisons).
Results
Stepping responses
The total number of steps, the number of participants who stepped, and the range of steps taken immediately following each change of frequency are reported in Table 1. In ETP, no participants stepped during the first two frequencies. Four participants took a total of 26 steps at 0.5 Hz and five participants recorded 11 steps at the highest frequency. The number of steps taken by any individual participant was less at 0.61 Hz than at 0.5 Hz.
Fewer steps were recorded in the STP condition. A single participant took two steps in the transition to 0.25 Hz. There were no steps recorded at 0.5 Hz and a single participant took two steps at 0.61 Hz.
Children shift postural responses with experience in a task: transition state vs steady state
Anchoring Index
There were no significant differences in the AI between the transition- and steady-state periods at any frequency in either ETP or STP condition (Fig. 2). However, for both conditions, there was a trend towards greater head stabilization in space, as the platform oscillation frequency increased. This was noted in both transition and steady states except for a marked decrease in the AI during the steady-state period at 0.61 Hz in STP.
Anchoring Index (AI) values of transition and steady states (solid and broken lines, respectively) across the four platform oscillation frequencies (mean ± SE). The externally triggered condition is presented in a, and the self-triggered condition is presented in b. A positive AI value indicates a head stabilization in space strategy (HSSS), while a negative AI value indicates a head strapped to trunk strategy (HSTS)
Inter-joint cross-correlation coefficients
Externally triggered perturbation
All CCmax remained above 0.8 between transition and steady states at the lower frequencies (Fig. 3), which, in combination with the short time lag of less than 4% of the cycle, suggests keeping the body relatively rigid. At 0.5 and 0.61 Hz, the ankle–head CCmax was significantly lower in the steady state compared to transition state (0.5 Hz: z = 3.206, p = 0.001; 0.61 Hz: z = 2.585, p = 0.010). Temporally, the head segment led the ankle at 0.1 Hz, suggesting a slight lean forward in transition state (z = 3.002, p = 0.003). Although the head lagged the ankle segment at both higher frequencies, the difference between the transition- and steady-state periods was significant only at 0.5 Hz (0.5 Hz: z = 2.844, p = 0.004; 0.61 Hz: z = 1.655, p = 0.098).
Mean (± SE) cross-correlation function peak values (CCmax—a, c, e, left) and time lags (CClag/lead—b, d, f, right) for the hip–head, ankle–hip, and ankle–head marker pairs trajectories in transition (solid lines, filled bars) and steady (dashed lines, open bars) states in the externally triggered condition. Asterisks denote significant differences
Although there was a significant difference in CCmax for the ankle–hip pair in ETP at 0.1 Hz (z = 3.413, p = 0.001), the difference was small (transition state: M = 0.98, SD = 0.02; steady state: M = 0.99, SD = 0.01). This finding was likely due to the extremely tight coupling between joints with limited variability between individuals and periods. Though not significant, the time lag, however, changed from little hip lag in transition state (M = − 0.038, SD = 1.09%cycle) to the hip lagging the ankle more in steady state (M = − 0.84, SD = 1.13%cycle) at 0.25 Hz (z = 2.017, p = 0.044). This effect was reversed at 0.5 Hz (z = 2.482, p = 0.013), where the hip lagged the ankle less in steady state (M = − 1.42, SD = 2.45%cycle) than it did in transition state (M = − 2.79, SD = 1.29%cycle), while no significant difference was observed at 0.61 Hz.
The CCmax and temporal relationships for the hip–head pair did not differ significantly from transition- to steady-state periods at any frequency in the ETP condition. There was, however, a tendency for the CCmax to decrease in both transition- and steady-state periods with the higher frequencies. While not significant, there was also a shift with the head lagging the hip less in steady state (M = − 4.55, SD = 3.56%cycle) than in transition state (M = − 6.32, SD = 4.47%cycle) at 0.5 Hz (z = 2.217, p = 0.030).
Self-triggered perturbation
In the STP condition, the ankle–head CCmax differed significantly between transition state (M = 0.83, SD = 0.14) and steady state (M = 0.89, SD = 0.09) at 0.1 Hz (z = 3.181, p = 0.001), indicating the two trajectories became more correlated with a shift to the steady-state period (Fig. 4). With an increase in frequency to 0.61 Hz, the marker traces were less correlated in the steady-state period (M = 0.39, SD = 0.3) than they were in the transition-state period (M = 0.56, SD = 0.19), although this was not found to be significant (z = 2.329, p = 0.020). No significant differences were found for the ankle–head cross-correlation temporally, however, transition state (M = − 5.82, SD = 3.19% cycle) and steady state (M = − 3.08, SD = 3.67% cycle) at the 0.5 Hz frequency approached significance (z = 2.329, p = 0.020), indicating the head was lagging the ankle less in steady state.
Mean (± SE) cross-correlation function peak values (CCmax—a, c, e, left) and time lags (CClag/lead—b, d, f, right) for the hip–head, ankle–hip, and ankle–head marker pairs trajectories in transition (solid lines, filled bars) and steady (dashed lines, open bars) states in the self-triggered condition. Asterisks denote significant differences
In STP, the CCmax and CClag/lead analysis revealed significant differences for the ankle–hip marker pairs across two of the four frequencies. The two marker traces were more correlated in steady state (M = 0.97, SD = 0.03) than they were in transition state (M = 0.96, SD = 0.03) at 0.1 Hz (z = 2.556, p = 0.011). The timing was also affected at 0.1 Hz (z = 2.551, p = 0.011), with the hip lagging the ankle less in steady state (M = − 0.2, SD = 0.57% cycle) than in transition state (M = − 0.97, SD = 1.06% cycle). The same effect was observed at 0.25 Hz with the correlation between the two marker traces (z = 2.726, p = 0.006) increasing from transition state (M = 0.94, SD = 0.05) to steady state (M = 0.96, SD = 0.03), and a timing shift (z = 2.755, p = 0.006) from slight hip lead in transition state (M = 0.35, SD = 1.6% cycle) to slight hip lag in steady state (M = − 0.38, SD = 1.1% cycle). No significant differences were found for amplitude or timing at 0.5 and 0.61 Hz.
No significant differences were found for the hip–head cross-correlations (CCmax or CClag/lead) between transition- and steady-state periods at any frequency in the STP condition.
EMG
Onset latencies
In the ETP condition, the onset latencies did not differ significantly between transition and steady states for any muscle. In the STP condition, the difference between transition and steady states in the G approached significance at 0.25 Hz (z = 2.354, p = 0.019), with the muscle onset occurring earlier in steady state (M = − 0.56, SD = 0.26% half cycle) than in transition state (M = − 0.18, SD = 0.38% half cycle). Figure 5 illustrates the differences in onset latencies for the muscles (directionally specific) at the four frequencies for both ETP and STP conditions, as well as transition and steady states. Though not significant, there is subtle shift to earlier activations in all muscles but the hamstrings in the steady-state periods in ETP and STP conditions.
Postural muscles onset latencies (mean ± SE) during forward (a) and backward (b) perturbations at the four frequencies of platform oscillation. Onset latencies are expressed as a percentage of half cycle time for muscles normally associated with forward (TA and Q in a) or backward (G and H in b) perturbations. Results from transition and steady states are represented by open and filled icons, respectively, while ETP and STP are represented by diamonds and circles, respectively. Zero (0) represents the time at which the platform changed direction and the platform begins to slow down at the − 50% half cycle mark. Transition and steady state icons are offset for clarity purposes. Q and H onset latencies not presented for 0.1 Hz as these muscles did not meet minimum activation requirements
Tonic activity
EMG tonic activity was calculated as a percentage of the baseline value in steady state at 0.1 Hz. Generally, the tonic activity in all muscles increased as a function of frequency in both ETP and STP conditions, but tended to decrease from transition to steady state (Fig. 6). In ETP, tonic activity was greater in the TA at 0.5 Hz (z = 2.543, p = 0.011) in transition state (M = 146.35% baseline, SD = 84.66) than in steady state (M = 110.18% baseline, SD = 43.81). It was greater in the G at 0.5 Hz (z = 2.543, p = 0.011) in transition state (M = 130.66% baseline, SD = 79.61) than in steady state (M = 97.53% baseline, SD = 36.20). In the STP condition, tonic activity was greater in transition state for TA at 0.61 Hz (z = 2.613, p = 0.009), Q at 0.61 Hz (z = 3.010, p = 0.003), and H at 0.5 Hz (z = 3.408, p = 0.001) and 0.61 Hz (z = 2.691, p = 0.007).
EMG tonic activity for (top to bottom panels) tibialis anterior (TA), gastrocnemius (G), quadriceps (Q), and hamstring (H) muscles in ETP (left side) and STP (right side) conditions. Comparisons were made to baseline tonic activity of each muscle in the steady-state period at 0.1 Hz in ETP. Asterisks denote significant differences between transition and steady states
In summary, in both ETP and STP conditions, there was a trend towards HSSS as the oscillation frequency increased, with the exception of 0.61 Hz in steady state. This was accompanied by the head lagging the ankle and hip less during SS at the higher frequencies. No significant differences were observed between transition and steady states in postural muscle onset latencies; however, there was a tendency to shift towards earlier activations in steady state. Tonic activity increased with oscillation frequency, and tended to decrease from transition to steady state.
Children shift postural responses if they have knowledge about perturbation timing: externally vs self-triggered perturbation comparisons
Anchoring Index
No significant differences between ETP and STP conditions were observed for the AI at any frequency in either transition- or steady-state period.
Ankle–head trajectory cross-correlation
The ankle–head trajectories (CCmax) at 0.1 Hz were found to be more correlated (z = 3.408, p = 0.001) in transition state of the ETP condition (M = 0.91, SD = 0.07) compared to the STP condition (M = 0.83, SD = 0.14), as well at 0.25 Hz (z = 3.010, p = 0.003) (ETP: M = 0.88, SD = 0.07; STP: M = 0.79, SD = 0.13). While no significant differences were found at the lower frequencies, at 0.5 Hz the head lagged the ankle more (z = 3.067, p = 0.002) in the ETP condition (M = − 9.11, SD = 3.86% cycle) compared to the STP condition (M = − 5.82, SD = 3.19% cycle). Though not significant, similar results were found at 0.61 Hz (z = 2.272, p = 0.023) where the ETP condition yielded more head lag in the ETP condition (M = − 11.05, SD = 7.00% cycle) than the STP condition (M = − 7.65, SD = 3.52% cycle). Meanwhile, at 0.25 Hz in steady state, the ankle and head marker traces were significantly more correlated (z = 3.237, p = 0.001) in the ETP condition (M = 0.86, SD = 0.23) than in the STP condition (M = 0.7, SD = 0.34). Moreover, there was a preference for less head lag in STP (M = − 3.08, SD = 3.67) than in ETP (M = − 6.61, SD = 4.34) at 0.5 Hz (z = 2.840, p = 0.005).
Ankle–hip trajectory cross-correlation
The difference found for the CCmax between ETP (M = 0.98, SD = 0.02) and STP (M = 0.97, SD = 0.03) conditions in transition state approached significance at the 0.1 Hz frequency (z = 2.385, p = 0.017). Differences were revealed in CClag/lead at 0.5 Hz (z = 3.352, p = 0.001), indicating a shift from hip lag in the ETP condition (M = − 2.79, SD = 1.13% cycle) to slightly less hip lag in the STP condition (M = − 1.28, SD = 1.41% cycle).
In steady state, only the 0.1 Hz frequency saw a significant difference (z = 3.067, p = 0.002) in CCmax, indicating slightly more correlated trajectories in the ETP condition (M = 0.99, SD = 0.01) than in STP (M = 0.97, SD = 0.03). Furthermore, while not significant, the hip was found to lag the ankle less in STP (M = − 1.65, SD = 2.6% cycle) than it did in ETP (M = − 3.00, SD = 3.13% cycle), but only at 0.61 Hz (z = 2.442, p = 0.015).
Hip–head trajectory cross-correlation
In transition state, the hip was found to have greater correlation at 0.1 Hz (z = 3.124, p = 0.002) in ETP (M = 0.94, SD = 0.07) than in STP (M = 0.78, SD = 0.42). At 0.25 Hz, the correlation in ETP (M = 0.92, SD = 0.06) was greater than that of STP (M = 0.75, SD = 0.45) (z = 2.897, p = 0.004). In steady state, this correlation was greater (z = 2.897, p = 004) in ETP (M = 0.91, SD = 0.22) than in STP (M = 0.69, SD = 0.59).
Cross-correlation analysis also revealed significant differences in the CClag/lead for the hip–head marker traces at the higher frequencies. At 0.5 Hz, the head lagged the hip significantly more (z = 2.528, p = 0.011) in the ETP condition (M = − 6.32, SD = 4.47% cycle) than in the STP condition (M = − 3.71, SD = 4.21% cycle) and again at 0.61 Hz [(z = 3.17, p = 0.002); M = − 10.03, SD = 6.70% cycle in ETP versus M = − 5.92, SD = 3.20% cycle in STP]. In steady state, however, CC analysis only revealed significant difference for timing between ETP (M = − 4.554, SD = 3.56) and STP (M = − 2.62, SD = 3.18% cycle) conditions at 0.5 Hz (z = 2.67, p = 0.008), indicating less head lag in the STP condition.
EMG
Onset latencies
In transition state, only the quadriceps were activated earlier in STP (M = − 0.11, SD = 0.08% half cycle) than in ETP (M = − 0.06, SD = 0.09% half cycle) at 0.61 Hz; however, this was not significant (z = 2.201, p = 0.028). Conversely, in steady state, the gastrocnemius at 0.25 Hz (z = 2.701, p = 0.007) were activated earlier in ETP (M = − 0.60, SD = 0.11% half cycle) than in STP (M = − 0.41, SD = 0.11% half cycle).
Tonic activity
Though both transition and steady states generally saw an increase in tonic activity with an increase in oscillation frequency, the tonic activity observed in ETP was greater than in STP only during transition state. At 0.1 Hz, the tonic activity in the G was greater (z = 2.731, p = 0.006) in ETP (M = 101.87, SD = 25.01% baseline) than in STP (M = 88.19, SD = 21.45% baseline), as was the H (ETP M = 108.97, SD = 15.41% baseline; STP: M = 90.21, SD = 15.76% baseline; z = 2.528, p = 0.011). The TA and Q exhibited greater tonic activity at 0.5 Hz (z = 2.668, p = 0.008; z = 2.856, p = 0.004, respectively) in ETP (M = 146.36, SD = 84.66% baseline; M = 161.24, SD = 76.40% baseline, respectively) than STP (M = 108.65, SD = 43.06% baseline; M = 136.10, SD = 72.26% baseline, respectively). No tonic activity differences were observed between ETP and STP in steady state.
In summary, few differences were observed between ETP and STP conditions. The AI was similar between conditions. The head lagged the ankle and hip more in ETP during transition state at the high frequencies, while the hip lagged the ankle less in STP at 0.5 Hz in ETP and STP. As oscillation frequency increased, tonic activity tended to increase in ETP during transition state only. Generally, no differences were found in onset latencies between conditions.
Discussion
We characterized the displacement of and relationships between the head, ankle and hip, the anchoring of the head on the trunk, and postural muscle activity to explain how children and adolescents respond to and anticipate a continuous perturbation. We initially hypothesized that there would be an effect of period type on these characteristics. The data suggest potential biomechanical constraints and reduced abilities to take advantage of platform movement at the higher frequencies.
The ability to use a step as a compensatory response for balance emerges in young children, as they gain walking experience usually between 18 and 24 months (Roncesvalles et al. 2000). In conditions with discrete perturbations, a young adult stepping response may consist of a single step in the axis of the perturbation to regain control. Older adults, however, will use multiple steps to regain balance. Moreover, the older adult will often direct their steps laterally to regain stability (Mcllroy and Maki 1993, 1996). The need for stepping and number of steps required to regain stability are influenced by the perturbation amplitude as well as the ability to regulate mediolateral stability. As expected, higher frequencies experienced in the oscillating paradigm elicited the most stepping responses in both ETP and STP conditions with most steps recorded following the changes to 0.5–0.61 Hz in the ETP condition. The transition period from 0.25 to 0.5 Hz was the largest increase in perturbation, as oscillation frequency doubled at this point. The subsequent shift to 0.61 Hz appeared to be less destabilizing.
Low frequency perturbations are compensated through stiffness
The increase in difficulty of perturbation due to increase in oscillation frequency was also reflected in the amount of correlation between marker trajectory pairs decreasing. At the lower frequencies, the high CCmax values and low CClag/lead suggest the participants’ abilities to maintain balance were not sufficiently challenged, and thus they were able to stand erect (‘riding’ the platform—cf. De Nunzio and Schieppati (2007)), modulating their balance control through the use of the ankle strategy. This changed, however, with the increase in oscillation frequency. At 0.5 and 0.61 Hz, where the greatest threats to postural stability were evident, the ankle and head marker traces become less coupled and more temporally displaced, while the ankle–hip pair remains more correlated, suggesting the use of a hip strategy (or at least a dissociation somewhere in the upper half of the body). Previous research suggests that allowing the upper body to follow the platform translation at higher frequencies would be counterproductive, as the required muscle activity to counteract body inertia at the extremes of the platform translation would produce its effects too late, resulting in loss of balance as the platform changes direction on its return path (Corna et al. 1999). Furthermore, in transition state, the high CCmax at relatively low temporal lag values reflect a tight coupling of the marker pairs, suggestive of the participants’ unsuccessful attempts to maintain a rigid body while on the platform indicated with increased stepping responses. However, as the participants became more comfortable with the frequency oscillations (i.e., shift to a steady-state period), the lower body segments follow the platform movement, while a head in space strategy is maintained. This is reflected in the lower CCmax values in the steady-state periods (compared to the transition state periods) at 0.5 and 0.61 Hz, as well as the increase in temporal lag of the hip and head.
Children use different response strategies under anticipatory situations
Providing the participants with the ability to control/determine when the platform changes frequency (i.e., the STP condition) appears to have allowed them to better stabilize their bodies for the upcoming change in frequency. The overall number of steps taken—and the total number of participants who stepped—in the STP condition decreased compared to ETP. This suggests that (1) the perturbations following change in frequency did not pose as much a threat to the participants’ balance as they did in the ETP condition and/or (2) the participants were better able to prepare for the upcoming change in frequency by taking advantage of knowing when the change would occur. The latter is supported by the increased muscle onset latencies, which provides an indication that postural muscles are activated slightly more in advance in the STP condition.
There is some consistency in terms of postural muscle activity with previous reports in the literature. For example, there was some evidence of trends of adaptation between the transition and steady states (Fig. 6) which could be due to transfer of prior experience (Kennedy et al. 2013; Schmid et al. 2011; Van Ooteghem et al. 2008; Dietz et al. 1993). However, children appear to behave more like older adults in terms of postural muscle activity in this situation, since the timing of the activations occurred generally around the − 25% half cycle mark [compared to the ~ − 50% half cycle mark observed in young adults by Bugnariu and Sveistrup (2006)]. This would suggest that the participants were able to shift to anticipatory mechanisms in steady state, but were not able to take full advantage of the platform slowing down to prepare for the upcoming change in direction. While the orders of activation were generally consistent with an ankle strategy (i.e., distal to proximal organization) (Horak and Nashner 1986), the kinematic data are suggestive of a hip strategy. Therefore, we postulate the resulting strategy must be a combination of the two. There is also the possibility that compensation for the perturbation is made through knee flexion (Santos et al. 2010), though joint angles at the knee level were not investigated in this study.
Influence of attentional demands and fear on response strategies
Studies have shown that there are significant attentional requirements for postural control (Woollacott and Shumway-Cook 2002) and that in multitask conditions, the inability to allocate sufficient attention to the maintenance of balance is a contributing factor to falls, especially in the elderly, however, not all cognitive tasks affect postural control equally. Shumway-Cook and Woollacott (2000) demonstrated that there may be a hierarchy of attentional demands with respect to postural control. Simple additional tasks tend to be associated with feet-in-place strategies (i.e., ankle or hip strategy), whereas more complex problems elicit more drastic measures like the stepping strategy. An increase in attentional demands results in decreased postural muscle activity during balance recovery, in this case from platform perturbations, which can prompt the use of an alternate response strategy, such as stepping (Rankin et al. 2000). It could be that an increase in cognitive demand associated with the higher frequencies is responsible for the children attempting to revert to a ‘ride’ solution as a head in space strategy may have otherwise required the allocation of cognitive demands. This could also explain the increased tonic postural muscle activity in that the final cycles of the steady state would most likely include some sort of preparation for the upcoming change in frequency. This preparation would then be considered a cognitive loading, leading to postural muscle activity closer to (instead of well in advance of) the perturbation onset, as well as the stiffening observed in the kinematics.
Increasing the level of postural threat can also play a large role in the selection of a strategy to maintain (or regain) balance. Adkin et al. (2000) and Carpenter et al. (2004) have found that by placing subjects on high platforms, thereby inducing an element of fear of falling, the CNS adopts tighter control over postural stability. This control is scaled to the level of threat, as well as the order in which the threat to posture is experienced by the subject, suggesting both physiological and psychological factors influence postural control. This may explain the observed changes (large decrease in the number of steps taken by participants, kinematic strategy chosen, earlier postural muscle onsets) when given control over the change in frequency.
In summary, children and adolescents were subjected to oscillatory antero-posterior postural perturbations at various frequencies. Generally, the higher frequencies resulted in more steps being taken, lower correlations coupled with greater temporal lags between marker trajectories, and postural muscle activity similar to older adults. The provision of self-triggered perturbations allowed participants to make the appropriate changes to their balance by use of anticipatory postural control mechanisms.
References
Adkin AL, Frank JS, Carpenter MG, Peysar GW (2000) Postural control is scaled to level of postural threat. Gait Posture 12(2):87–93. https://doi.org/10.1016/S0966-6362(00)00057-6
Amblard B, Assaiante C, Fabre J-C et al (1997) Voluntary head stabilization in space during trunk movements in weightlessness. Exp Brain Res 114:214–225
Amblard B, Assaiante C, Vaugoyeau M, Baroni G, Ferrigno G, Pedotti A (2001) Voluntary head stabilisation in space during oscillatory trunk movements in the frontal plane performed before, during and after a prolonged period of weightlessness. Exp Brain Res 137(2):170–179. https://doi.org/10.1007/s002210000621
Assaiante C (1998) Development of locomotor balance control in healthy children. Neurosci Biobehav Rev 22(4):527–532
Assaiante C, Amblard B (1995) An ontogenetic model for the sensorimotor organization of balance control in humans. Hum Mov Sci 14(1):13–43. https://doi.org/10.1016/0167-9457(94)00048-J
Assaiante C, Mallau S, Viel S, Jover M, Schmitz C (2005) Development of postural control in healthy children: a functional approach. Neural Plast 12(2–3):109–118. https://doi.org/10.1155/NP.2005.109
Buchanan JJ, Horak FB (1999) Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol 81:2325–2339
Bugnariu N, Sveistrup H (2006) Age-related changes in postural responses to externally- and self-triggered continuous perturbations. Arch Gerontol Geriatr 42(1):73–89. https://doi.org/10.1016/j.archger.2005.05.003
Burtner PA, Woollacott MH, Craft GL, Roncesvalles MN (2007) The capacity to adapt to changing balance threats: a comparison of children with cerebral palsy and typically developing children. Dev Neurorehabil 10(3):249–260. https://doi.org/10.1080/17518420701303066
Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JHJ (2004) Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 92(6):3255–3265. https://doi.org/10.1152/jn.01139.2003
Corna S, Tarantola J, Nardone A, Giordano A, Schieppati M (1999) Standing on a continuously moving platform: is body inertia counteracted or exploited? Exp Brain Res 124(3):331–341. https://doi.org/10.1007/s002210050630
De Nunzio AM, Schieppati M (2007) Time to reconfigure balancing behaviour in man: changing visual condition while riding a continuously moving platform. Exp Brain Res 178(1):18–36. https://doi.org/10.1007/s00221-006-0708-z
Dietz V, Trippel M, Ibrahim IK, Berger W (1993) Human stance on a sinusoidally translating platform: balance control by feedforward and feedback mechanisms. Exp Brain Res 93(2):352–362. https://doi.org/10.1007/BF00228405
Fujiwara K, Kiyota T, Mammadova A, Yaguchi C (2011) Age-related changes and sex differences in postural control adaptability in children during periodic floor oscillation with eyes closed. J Physiol Anthropol 30(5):187–194. https://doi.org/10.2114/jpa2.30.187
Hansen PD, Wollacott M, Debu B (1988) Postural responses to changing task conditions. Exp Brain Res 73:627–636
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55(6):1369–1381. https://doi.org/10.1152/jn.1986.55.6.1369
Kennedy A, Bugnariu N, Guevel A, Sveistrup H (2013) Adaptation of the feedforward postural response to repeated continuous postural perturbations. Neurosci Med 4(1):45–49. https://doi.org/10.4236/nm.2013.41007
Laessoe U, Voigt M (2008) Anticipatory postural control strategies related to predictive perturbations. Gait Posture 28(1):62–68. https://doi.org/10.1016/j.gaitpost.2007.10.001
McCollum G, Leen TK (1989) Form and exploration of mechanical stability limits in erect stance. J Mot Behav 21(3):225–244
Mcllroy WE, Maki BE (1993) Task constraints on foot movement and the incidence of compensatory stepping following perturbation of upright stance. Brain Res 616:30–38
Mcllroy WE, Maki BE (1996) Age-related changes in compensatory stepping in response to unpredictable perturbations. J Gerontol 51(6):M289–M296
Mesure S, Azulay J, Pouget J, Amblard B (1999) Strategies of segmental stabilization during gait in Parkinson’s disease. Exp Brain Res 129:573–581
Pai Y, Patton J (1997) Center of mass velocity-position predictions for balance control. J Niomech 30(4):347–354
Pai Y, Rogers MW, Patton J, Cain TD, Hanke TA (1998) Static versus dynamic predictions of protective stepping following waist—pull perturbations in young and older adults. J Biomech 31:1111–1118
Pai Y, Wening JD, Runtz EF, Iqbal K, Pavol MJ (2003) Role of feedforward control of movement stability in reducing slip-related balance loss and falls among older adults. J Neurophysiol 90:755–762
Pavol MJ, Pai YC (2002) Feedforward adaptations are used to compensate for a potential loss of balance. Exp Brain Res 145(4):528–538. https://doi.org/10.1007/s00221-002-1143-4
Perrin P, Schneider D, Deviterne D, Perrot C (1998) Training improves the adaptation to changing visual conditions in maintaining human posture control in a test of sinusoidal oscillation of the support. Neurosci Lett 245:155–158
Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA (2000) Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A Biol Sci Med Sci 55(3):M112–M119. https://doi.org/10.1093/gerona/55.3.M112
Roncesvalles MNC, Woollacott MH, Jensen JL (2000) The development of compensatory stepping skills in children. J Mot Behav 32(1):100–111
Santos MJ, Kanekar N, Aruin AS (2010) The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol 20(3):398–405. https://doi.org/10.1016/j.jelekin.2010.01.002
Schmid M, Bottaro A, Sozzi S, Schieppati M (2011) Adaptation to continuous perturbation of balance: progressive reduction of postural muscle activity with invariant or increasing oscillations of the center of mass depending on perturbation frequency and vision conditions. Hum Mov Sci 30(2):262–278. https://doi.org/10.1016/j.humov.2011.02.002
Shumway-Cook A, Woollacott M (2000) Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci 55(1):M10–M16. https://doi.org/10.1093/gerona/55.1.M10
Van Ooteghem K, Frank JS, Allard F, Buchanan JJ, Oates AR, Horak FB (2008) Compensatory postural adaptations during continuous, variable amplitude perturbations reveal generalized rather than sequence-specific learning. Exp Brain Res 187(4):603–611. https://doi.org/10.1007/s00221-008-1329-5
Woollacott M, Shumway-Cook A (1990) Changes in posture control across the life span—a systems approach. Phys Ther 70(12):799–807
Woollacott M, Shumway-Cook A (2002) Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16(1):1–14. https://doi.org/10.1016/S0966-6362(01)00156-4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mills, R.S., Sveistrup, H. Kinematics and postural muscular activity during continuous oscillating platform movement in children and adolescents. Exp Brain Res 236, 1479–1490 (2018). https://doi.org/10.1007/s00221-018-5228-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5228-0