Abstract
The aim of the present study was to investigate the effect of experimental and chronic neck–shoulder pain on the magnitude of cycle-to-cycle variability of task timing, kinematics and muscle activation during repetitive arm movement performed for 3 or 5 min. In an experimental part, acute muscle pain was induced in healthy subjects by intramuscular injection of hypertonic saline in trapezius (n = 10) and infraspinatus (n = 10) muscles. In a clinical part, workers with (n = 12) and without (n = 6) chronic neck–shoulder pain were compared. Cycle-to-cycle standard deviations of task duration, arm and trunk movement in 3D and surface electromyographic (EMG) root mean square activity were computed to assess the degree of variability. The variability in task timing increased in presence of both experimental and chronic pain (P < 0.05) compared with non-painful conditions. Experimental pain increased the variability of the starting position of the arm (P < 0.05), the arm range of motion (P < 0.01), the arm and trunk movement area (P < 0.01) and the acceleration of the arm (P < 0.01). In the chronic pain condition, the variability of arm and trunk acceleration (P < 0.01) and EMG activity (P < 0.05) was decreased compared with healthy controls. These results indicate that pain alters the magnitude of motor variability, and that the transition from acute to chronic pain is accompanied by changes in motor patterns. Experimental pain likely resulted in a quest for a motor solution reducing nociceptive influx, while chronic pain was characterised by a diminished motor flexibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Discomfort and pain in the neck–shoulder region is particularly prevalent among workers engaged in repetitive work where a short work cycle is repeated over and over again for long periods of time (Bernard 1997; Punnett and Wegman 2004). Muscle pain influences motor control strategies via central mechanisms (Le Pera et al. 2001; Thunberg et al. 2002), and the effects of muscle pain on motor patterns have been widely studied both during isometric and dynamic contractions (Arendt-Nielsen et al. 1996; Birch et al. 2000). During dynamic contractions, neck–shoulder pain status, i.e., acute, sub-chronic or chronic, has been shown to be associated with different motor adaptation mechanisms that can explain the transitions of pain status (Madeleine et al. 2003b). Acute experimental pain induced by hypertonic saline injection was found in another part of the same study to induce a slower working rhythm, a decreased electromyographic (EMG) activity of the painful muscle and an increased amplitude of arm movements (Madeleine et al. 1999). Sub-chronic pain (pain developed after 6 months of work) resulted in lower force level, higher EMG activity, decreased arm movement amplitude and, increased trunk movement amplitude (Madeleine et al. 2003a), while chronic pain led to a slower working rhythm, an increased background EMG activity of the trapezius muscle and an increased amplitude of arm movements (Madeleine et al. 1999).
In studies of motor adaptation to pain, one aspect of motor control has often been neglected: motor variability. The size and structure or components of motor variability in a standardised task reveal attributes of the motor control strategies, e.g., the extent that degrees of freedom are used (Latash et al. 2002; Sosnoff et al. 2006). Temporal and spatial variation in postures and muscle activity has been suggested to be a decisive determinant of risk for the development of work-related musculoskeletal disorders (Mathiassen 2006), and it has been hypothesised that individuals who perform a particular work task in a more stereotyped fashion than others, and thus do not fully benefit from the redundancy of the motor system, are more at risk (Mathiassen et al. 2003). This theory may be particularly relevant to neck–shoulder disorders since the shoulder region comprises a very complex construct of muscles and thus a large potential for achieving the same external result through different synergies. The ability and readiness to take on different muscle synergies in the neck–shoulder region has been demonstrated using biofeedback techniques (Palmerud et al. 1995), and also as a response to neck–shoulder pain (Madeleine et al. 1999). Recent studies have suggested that even a reorganisation of EMG activity among subdivisions of the trapezius muscle occurs in the presence of acute experimental pain, and that this may well explain the spreading of pain observed in clinical conditions (Falla et al. 2006; Madeleine et al. 2006).
Acute experimental muscle pain affects feed-forward control of muscles in a complex way beyond a general inhibition (Ervilha et al. 2004, 2005; Hodges et al. 2003). At chronic pain stages, motor variability is changed too as compared to a pain-free state, but both increases and decreases in motor variability have been reported (Lamoth et al. 2006). Individuals with a larger motor variability show a higher probability of returning to normal postural strategies after experimental pain than subjects with less flexibility (Moseley and Hodges 2006). Thus, recent studies have demonstrated that motor variability can be modulated by muscle pain and that the size of the motor variability may have important clinical implications.
We hypothesised that the magnitude of cycle-to-cycle motor variability in a stereotyped repetitive task decreases during the course of pain transitions; from an increased degree of variability during acute experimental pain to a decrease in chronic pain conditions. For this purpose, secondary analysis was performed on data set collected for a previous study (Madeleine et al. 1999).
Subjects and methods
Subjects
In total, 38 volunteers took part in this study which is composed of two parts. The first, experimental part involved 20 healthy participants with no pain or history of injuries in the neck–shoulder region [mean age 26.1 years (SD 2.6), weight 76 kg (SD 6.6), height 181.6 cm (SD 10.3)]. In the second, clinical part, 12 butchers suffering from chronic neck–shoulder pain participated [mean age 48.6 years (SD 7.2), weight 78.9 kg (SD 12.3), height 173.9 cm (SD 8.3)] together with 6 controls [mean age 43.8 years (SD 6.7), weight 83.0 kg (SD 8.5), height 181.2 cm (SD 7.5)]. The local Ethical Committee approved the studies (VN 94/184 and 95/3352), and informed consent was obtained from all participants. The studies were conducted in accordance with the Declaration of Helsinki.
Protocol
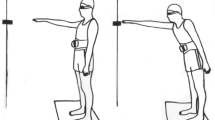
Motor recordings were performed during a standardised motor task in a laboratory setting. The subjects were instructed that they were to perform a task representing a common work task in the meat industry (Fig. 1). The task was time-paced using a tone generator triggered every second, and it consisted of five events (Madeleine et al. 1999): (1) pressing a sensor with the left hand; (2) micro-pause; (3) applying a 30 N force in the diagonal direction with the right hand; (4) applying a 30 N force in parallel to the frontal plane with the right hand; force feedback was given to the experimenter via a multimeter (Fluke 37, Tilburg, The Netherlands) and fed back verbally to the subject; and (5) pressing a sensor with the left hand. The task was thus standardised with respect to the movement amplitude and the load level. The subjects practiced until they were able to perform the task correctly and with ease. Following this practice period, the subjects performed the experimental task according to the following protocol, during which motor patterns were recorded (see “Motor recordings and analyses”):
Example of a task cycle for subject #10 in the experimental part; pain free state. From the top, task events [event (1) dashed line, events (3)–(4) solid line and event (5) dashed-dotted line) with their onset and offset (T on and T off); arm and trunk movement (°) in the flexion–extension (Flex–Ext), abduction–adduction (Abd–Add) and rotation (Rot) directions, corrected for offset in initial position; rectified electromyographic activity (mV) from the right trapezius, infraspinatus, deltoideus anterior and deltoideus medius muscles with the onsets and offsets used to calculate root mean square values (RMSnon-active and RMSactive)
Experimental part
First, the subjects performed the task for 3 min in a pain-free state. After 5 min rest, experimental muscle pain was induced by means of intramuscular injection of 0.75 ml of sterile hypertonic saline (6%) injected over 15 s. A 27Gx1-1/2″ cannula was inserted into the muscle belly (1.5–2 cm) of the right trapezius muscle (2 cm laterally from the mid-distance between cervical vertebra C7 and acromion) or of the right infraspinatus muscle (two finger breadths below the medial portion of spine of scapula). Immediately after the bolus injection, the subject started performing the task and continued for 5 min. The mean pain intensities for the right trapezius and infraspinatus muscles measured across these 5 min were 4.9 (SD 2.0) and 4.4 (SD 1.0), respectively, on a 0–10 point visual analogue scale (Madeleine et al. 1998). Data from the two injection sites were pooled to show the overall effect of neck–shoulder pain.
Clinical part
Both groups performed the task for three times 3 min with 5 min pause in between. The mean pain intensity referred to the right trapezius was measured during the motor task on a 0–10 point visual analogue scale. The butchers with chronic neck–shoulder pain and the healthy controls reported intensities of 4.8 (SD 1.8) and 0 (SD 0), respectively (Madeleine et al. 1998).
Motor recordings and analyses
Task timings from the left hand were sampled at 500 Hz by means of force sensing resistor® device (Toptronic, Echternach, Luxembourg). The durations of the task events (1) through (5) [except event (2)] as well as the total duration were computed for each cycle. T on and T off of each event were obtained using a level detection technique. Strain gauges mounted on a knife were used to measure the applied cutting force during events (3) and (4) (sampling frequency 500 Hz). The degree of variability of the task events and the whole cycle was measured by computing the standard deviation of their durations.
The movements of the upper right arm and trunk were recorded using a 3D motion analysis system at a sampling rate of 30 Hz (McReflex, Qualysis A/S, Partille, Sweden). Three cameras were used and acted as infrared illuminator and detector of passive reflective markers. Two complexes of three markers were attached on the back and around the upper right arm. Each marker was tracked in 3D by direct linear transformation. Reference recordings with the subjects in an upright anatomical position in the laboratory coordinate system were performed prior to work simulation recordings. After the tracking procedure, the markers’ 3D coordinates were low-pass filtered using a Butterworth filter (order 4, cut-off frequency 3 Hz). Relative motion between the right arm and the trunk were computed and expressed for the upper arm as anatomical flexion–extension, abduction–adduction, and inward–outward rotation, and for the trunk as flexion–extension, right–left lateral flexion, and rotation (Madeleine et al. 1999). For each cycle, four kinematic parameters were determined in 3D for both the right arm and the trunk: (1) the starting position (°), (2) the integrated acceleration through the cycle (° s−2), as obtained by double differentiation, (3) the range of motion (°, ROM) and, (4) the total area under the movement curve versus time (°s). The cycle-to-cycle standard deviations of these four parameters were used to express aspects of kinematic variability. Thus, the degree of variability was assessed at a whole-cycle level, but not with respect to sub-events within the cycle (Chau et al. 2005).
Surface EMG recordings were collected from four muscle of the right shoulder girdle. Bipolar EMG surface electrodes (Medicotest® N-10–E, Ølstykke, Denmark) were aligned (inter-electrode distance 2 cm) on abraded ethanol-cleaned skin along the direction of the muscle fibres. The four electrode centres were placed, (1) halfway between the lateral 1/3 of clavicula and the insertion of deltoideus (deltoideus anterior), (2) halfway between acromion and the insertion of deltoideus medius (deltoideus medius), (3) infraspinous fossa, two finger breadths below medial portion of the spine of scapula (infraspinatus) and, (4) 2 cm laterally from the mid-point between cervical vertebra C7 and acromion (trapezius). EMG signals were pre-amplified 100 times and amplified 2,000 times in total, band-pass filtered 10–400 Hz, and sampled at 1,000 Hz. Surface EMG activity was monitored by measuring the root mean square (RMS) values. Background RMS EMG normalised values RMSnon-active (%) were computed for each muscle during a non-active part of the task cycle (300 ms epoch) and normalised to a reference value measured with the right arm resting on the workbench. RMS normalised values RMSactive (%) were also computed for each muscle and for each cycle during active phases [events (3) and (4)] and were normalised to the reference force in the same arm position. The RMSactive/RMSnon-active absolute ratio (RMSratio) was also computed for each cycle. The standard deviations of RMSnon-active, RMSactive and RMSratio were then computed as measures of the amplitude of cycle-to-cycle variability in surface EMG activation during the task.
Statistics
Mann–Whitney test (test of the two groups) and two-way analyses of variance (ANOVA) [factors: (1) time event or muscle or direction of movement, (2) pain status] with Student–Newman–Keuls (SNK) method for multiple comparisons were applied. P < 0.05 was considered significant.
Results
Variability magnitude during acute experimental pain
The standard deviation of task event durations increased during experimental pain from 0.08 s (SE 0.006) to 0.09 s (SE 0.007) (F 3,19 = 90.9, P < 0.001, η2 = 0.97). There was a significant interaction between pain status and time event (F 3,57 = 10, P < 0.001, η2 = 0.19); the standard deviation of event (3) was greater during experimental pain than before pain [0.16 s (SE 0.01) vs. 0.14 s (SE 0.01), respectively, P < 0.001, SNK].
Table 1 presents the results of the two-way ANOVA of the standard deviations of arm and trunk starting position, range of motion, total area and, movement acceleration. For the arm starting position, range of motion, total area (Fig. 2) and, acceleration (Fig. 3), values were greater during experimental pain than before pain [6.4° (SE 1.0) vs. 3.0° (SE 0.2), 6.4° (SE 0.7) vs. 3.1° (SE 0.2), 14.4°s (SE 1.6) vs. 6.2°s (SEM 0.4), and 5.6°s−2 (0.5) vs. 3.9°s−2 (SE 0.3), respectively, P < 0.05]. For the arm, there was a significant interaction between pain status and direction of movement, the standard deviation of the starting position was greater during experimental pain compared with before pain for the flexion and rotation directions (P < 0.05, SNK).
Variability magnitude (SD) of the total movement area (°s) in the flexion–extension, abduction–adduction and rotation directions before and during acute experimental neck–shoulder pain (N = 20). Each filled diamond represents the movement variability amplitude of a single subject, while each filled square illustrates the group mean. * Indicates a significant difference between pain states (P < 0.001, SNK)
Variability magnitude (SD) of the movement acceleration (°s−2) in the flexion–extension, abduction–adduction and rotation directions before and during acute experimental neck–shoulder pain (N = 20). Each filled diamond represents the movement variability amplitude of a single subject, while each filled square illustrates the group mean
For the trunk, the standard deviation of the total area of movement was greater during experimental pain compared with before pain [7.9° (SE 1.0) vs. 4.6° (SE 0.4), respectively, P < 0.05]. There was significant interaction between pain status and direction of movement, the standard deviation of the total area of movement was greater during experimental pain compared with before pain for the flexion direction (Fig. 2, P < 0.001, SNK).
Table 2 presents the results of the two-way ANOVA for RMSnon-active, RMSactive, and RMSratio standard deviations. There was significant interaction between pain status and muscle; the standard deviation of the RMSnon-active was smaller during experimental pain compared with before pain for the deltoideus anterior (Fig. 4, P < 0.001, SNK).
Variability magnitude (SD) of the normalised surface electromyographic (EMG) root mean square (RMS) values (%) during the non-active part of the task before and during acute experimental neck–shoulder pain (upper panel, N = 20), as well as for patients with chronic neck–shoulder pain (N = 12) and controls (N = 6) in the clinical part of the study (lower panel). * Indicates a significant difference between conditions or groups (P < 0.05, SNK)
Variability magnitude among workers with chronic neck–shoulder pain
The standard deviation of the task cycle duration was greater among patients with chronic neck–shoulder pain than among healthy controls [0.32 s (SE 0.02) vs. 0.26 s (SE 0.04), respectively, P = 0.042].
The standard deviation of the arm acceleration was smaller for the patients than for the controls [4.2°s−2 (SE 0.2) vs. 6.2°s−2 (SEM 0.4), respectively, P < 0.001]. There was significant interaction between pain status and direction of movement, the standard deviation of acceleration was smaller for the patients than for the controls for the flexion and rotation directions (Fig. 5, P < 0.05, SNK).
Variability magnitude (SD) of the movement acceleration (°s−2) in the flexion–extension, abduction–adduction and rotation directions for patients with chronic neck–shoulder pain (N = 12) and controls (N = 6). Each filled diamond represents the movement variability amplitude of a single subject (three recordings for each subject), while each filled square illustrates the group mean. * Indicates a significant difference between groups (P < 0.05, SNK)
The standard deviation of the trunk acceleration was smaller for the patients than for the controls [2.5°s−2 (SE 0.1) vs. 4.1°s−2 (SEM 0.3), respectively, P < 0.001]. As for the arm, there was significant interaction between pain status and direction of movement, the standard deviation of acceleration was smaller for the patients than for the controls for the flexion, abduction and rotation directions (Fig. 5, P < 0.05, SNK).
The standard deviation of the RMSnon-active level was smaller for the patients than for the controls [14.8% (SE 0.9) and 19.3% (SE 1.4), respectively, P < 0.01, Table 2]. There was significant interaction between pain status and muscle, the standard deviation of the RMSnon-active was smaller for the patients than for the controls for the infraspinatus muscle (Fig. 4, P < 0.001, SNK). The standard deviation of the RMSratio was smaller for the patients than for the controls [1.01 (SE 0.07) and 1.25 (SE 0.09), respectively, P < 0.05].
Discussion
This study investigated the magnitude of cycle-to-cycle variability in task timing, kinematics and muscle activation during repetitive standardised arm movement performed with or without pain in the neck–shoulder region. Acute experimental pain led to an increase in the variability amplitude of the task timing and of several arm and trunk movement parameters. In contrast, the cycle-to-cycle variability amplitude of arm and trunk acceleration, baseline EMG activity and the ratio between EMG during active and non-active task periods were smaller among patients with chronic neck–shoulder pain compared with healthy controls, while the task cycle duration variability was increased.
Methodological considerations
The motor task performed by the subjects was not a carbon copy of a real occupational task, but it simulated generic attributes of tasks found in the meat industry such as short-cycle repetitive movements at a rather constant load. The issue of whether the observed changes in motor patterns during the experimental task would also occur in real meat-cutting is therefore of concern.
The size of the control group in the clinical study was small, but we believe that these controls were representative of healthy butchers. The fact that we found significant differences in the magnitude of motor variability between patients and controls even in this limited material suggests a quite coherent change of motor strategy in response to chronic pain. While the chronic pain and control groups differed slightly in age and work experience, both of which are known to influence motor strategies (Enoka et al. 2003; Madeleine et al. 2003a; Sosnoff and Newell 2006), we judge these differences to be too small to fully account for the motor difference between the groups. In the experimental study, subjects acted as their own controls, and we suggest the response to experimental pain to represent a general change in motor strategy.
We assessed the size of motor variability by the most common measure of statistical dispersion, i.e., standard deviation. Standard deviation or variance of kinematic or kinetic summary measures have been widely used for assessing motor variability due to their simplicity (Chau et al. 2005; Christ et al. 1999; Mathiassen et al. 2003; Moseley and Hodges 2006). However, more advanced measures of the structure of motor variability and its possible change are required for analyzing motor control strategies in depth (Chau et al. 2005; Sosnoff et al. 2006). These methods include, e.g., uncontrolled manifold analyses, approximate or sample entropy, bootstrap prediction bands of register curves, and drift-diffusion techniques (Frank et al. 2006; Latash et al. 2002; Sosnoff et al. 2006; Sosnoff and Newell 2006; van Mourik et al. 2006). The primary purpose of the present study was to present descriptive data to guide further research on pain-related changes in motor variability, and thus we refrained from using these methods at this stage.
Effects of neck–shoulder pain on the size of motor variability
Chronic and acute experimental neck–shoulder pain has been shown to decrease the rhythm of the present experimental task, which suggests a protective response (Madeleine et al. 1999, 2003b). In the present study, increases in the variability amplitude of the durations of task events and the entire cycle were found in presence of acute experimental and chronic neck–shoulder pain, respectively. The latter result, which contradicts our hypothesis of decreased variability in chronic pain, is likely to be explained by the concomitant decrease in rhythm observed in both acute and chronic pain conditions. Unilateral experimental pain in the neck–shoulder region caused bilateral changes in the realisation of the motor task since the durations of both left and right hand movements were affected. This is in line with previous results indicating that supraspinal and/or spinal mechanisms may mediate a spread of motor effects to the contralateral pain-free body side through, e.g., reflex mediated pathways (Madeleine et al. 1999; Thunberg et al. 2002).
The starting position and movement amplitude of the arm and trunk have been found to increase during acute experimental pain, suggesting a positive feedback loop that could explain the transition to a chronic stage (Madeleine et al. 1999). For the arm, chronic neck–shoulder pain has lead to increased movement amplitude, while a decrease has been found among workers with sub-chronic pain (Madeleine et al. 1999, 2003b). For the trunk, the opposite pattern was observed. According to the present results, the magnitude of arm and trunk motor variability increased in the experimental pain condition while a decrease was found with chronic pain. A decreased arm and trunk movement variability amplitude is in line with recent results obtained at an early sub-chronic stage (Madeleine et al. 2007). Thus, our results support that regional motor behaviour differs with pain at different stages and/or different origins.
Muscle pain has also been shown to influence muscle synergies in the neck–shoulder region in previous analyses of the present and other materials (Falla et al. 2006; Madeleine et al. 1999). Experimental neck–shoulder pain appears to cause a decreased level of activity during both active and non-active parts of the task, which can be interpreted as a protective adaptation (Lund et al. 1991; Madeleine et al. 1999). At sub-chronic or chronic neck–shoulder pain stages, the level of EMG activity has, however, been reported to be higher than if pain is not present (Madeleine et al. 1999, 2003b; Veiersted et al. 1990). In the present study, the variability amplitudes of the non-active EMG level and the EMG ratio were smaller in chronic pain than without pain, which is in line with results from sub-chronic pain (Madeleine et al. 2007). Thus, the pain adaptation model (Lund et al. 1991) is more consistent with our results during experimental pain, while theories predicting muscular hyperactivity (Johansson and Sojka 1991; Schmidt et al. 1981; Travell et al. 1942) are better supported at chronic pain stages. The present effects of neck–shoulder pain on the degree of EMG variability indicate a dynamic reorganisation of muscle activity under painful conditions, and that new modulated synergies develop as pain changes from acute to chronic stages.
Motor variability amplitude and the risk of developing musculoskeletal disorders
In the present study, the magnitude of motor variability increased during acute experimental pain. This is consistent with the notion that the pain presented thoroughly new conditions for performing the task. In this case, motor patterns are adjusted by the central nervous system on the basis of integration and adaptation of sensory information so as to improve performance with respect to the goals set by the task. This is obtained by engaging and testing motor programs involving new muscle synergies (Kargo and Nitz 2003). During the acute experimental pain period, the central nervous system is presumably still in an experimenting state, and has not yet settled on a tuned strategy taking into account the influx of nociceptive input. Motor skill learning has been suggested to follow “the principle of abundance” in which redundant degrees of freedom are not decreased to a minimum but used by the central nervous system to ensure flexible and stable motor performance (Latash and Anson 2006). Thus, while motor variability would be particularly high in the learning phase, some will remain even in a fully known context. Some of this variability can be seen as “bad” in the sense that it affected performance to the worse (Latash and Anson 2006). On the other hand, it may also contain “good” components in the sense that it facilitates the return to a normal motor strategy after experimental pain, as suggested in a clinical study of postural strategies in the back (Moseley and Hodges 2006).
At a chronic pain stage, the magnitude of variability decreased compared with healthy controls. Whether this is a long-term adaptation to the pain state per se, or a sign that workers with less variable motor patterns are more at risk for developing pain than those with a larger variability cannot be inferred from cross-sectional study like this one. However, the latter “prognostic” hypothesis is supported by the finding that experienced, pain-free butchers show a larger motor variability than inexperienced controls (Madeleine et al. 2007). It is also supported by a few studies suggesting positive effects of a larger motor variability on the development of fatigue (Farina et al. 2006; Madeleine and Farina 2007; van Dieen et al. 1993) and neck–shoulder disorders (Kilbom and Persson 1987).
Conclusion
The amplitude of motor variability during a standardised repetitive motor task increased during acute experimental pain compared with before pain while it decreased among patients with chronic neck–shoulder pain compared with healthy controls. The present results support that variability is an important element in motor control and provide a possible general transition path from acute towards chronic pain stages: acute pain would lead the central nervous system to search for the least painful biomechanical solution, while at chronic stage the chosen solutions would be characterised by a reduced flexibility of the motor system.
References
Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P (1996) The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain 64:231–240
Bernard BP (1997) Musculoskeletal disorders and worplace factors: a critical review of epidemiologic evidence for work-related musculoskeletal disorders of the neck, upper extremity and low back. US Department of Health and Human Services, Public Health Service, Center for Disease Control and Prevention, National Institute for Occupational Safety and Health
Birch L, Christensen H, Arendt-Nielsen L, Graven-Nielsen T, Sogaard K (2000) The influence of experimental muscle pain on motor unit activity during low-level contraction. Eur J Appl Physiol 83:200–206
Chau T, Young S, Redekop S (2005) Managing variability in the summary and comparison of gait data. J Neuroeng Rehabil 29:2–22
Christ M, Seyffart K, Wehling M (1999) Attenuation of heart-rate variability in postmenopausal women on progestin-containing hormone replacement therapy. Lancet 353:1939–1940
Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL (2003) Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13:1–12
Ervilha UF, Arendt-Nielsen L, Duarte M, Graven-Nielsen T (2004) The effect of muscle pain on elbow flexion and coactivation tasks. Exp Brain Res 156:174–182
Ervilha UF, Farina D, Arendt-Nielsen L, Graven-Nielsen T (2005) Experimental muscle pain changes motor control strategies in dynamic contractions. Exp Brain Res 164:215–224
Falla D, Graven-Nielsen T, Farina D (2006) Experimental muscle pain results in the reorganization of coordination among trapezius muscle subdivisions during repetitive shoulder flexion. Exp Brain Res. doi:10.1007/s00221-006-0746-6
Farina D, Leclerc F, Arendt-Nielsen L, Butteli O, Madeleine P (2006) The change in the spatial distribution of upper trapezius muscle activity is correlated to endurance time in static contraction. J Electromyogr Kinesiol. doi:10.1016/j.jelekin.2006.08.005
Frank TD, Friedrich R, Beek PJ (2006) Stochastic order parameter equation of isometric force production revealed by drift-diffusion estimates. Phys Rev. e 74.051905
Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC (2003) Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res 151:262–271
Johansson H, Sojka P (1991) Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses 35:196–203
Kargo WJ, Nitz DA (2003) Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J Neurosci 23:11255–11269
Kilbom A, Persson J (1987) Work technique and its consequences for musculoskeletal disorders. Ergonomics 30:273–279
Lamoth CJC, Meijer OG, Daffertshofer A, Wuisman PIJM, Beek PJ (2006) Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. Eur Spine J 15:23–40
Latash ML, Anson JG (2006) Synergies in health and disease: Relations to adaptive changes in motor coordination. Phys Ther 86:1151–1160
Latash ML, Scholz JP, Schoner G (2002) Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 30:26–31
Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L (2001) Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol 112:1633–1641
Lund JP, Donga R, Widmer CG, Stohler CS (1991) The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol 69:683–694
Madeleine P, Farina D (2007) Time to task failure in shoulder elevation is associated to increase in amplitude and to spatial heterogeneity of upper trapezius mechanomyographic signals. Eur J Appl Physiol. doi:10.1007/s00421-007-0589-2
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L (1998) Sensory manifestations in experimental and work-related chronic neck–shoulder pain. Eur J Pain 2:251–260
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L (1999) Shoulder muscle co-ordination during chronic and acute experimental neck–shoulder pain. An occupational study. Eur J Appl Physiol 79:127–140
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L (2003a) Standardized low-load repetitive work: evidence of different motor control strategies between experienced workers and a reference group. Appl Ergon 34:533–542
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L (2003b) The effects of neck–shoulder pain development on sensory–motor interaction among female workers in poultry and fish industries. A prospective study. Int Arch Occup Environ Health 76(1):39–49
Madeleine P, Leclerc F, Arendt-Nielsen L, Ravier P, Farina D (2006) Experimental muscle pain changes the spatial distribution of upper trapezius muscle activity during sustained contraction. Clin Neurophysiol 117:2436–2445
Madeleine P, Voigt M, Mathiassen SE (2007) Cycle-to-cycle variability in biomechanical exposure among butchers performing a standardized cutting task (submitted)
Mathiassen SE (2006) Diversity and variation in biomechanical exposure: what is it, and why would we like to know? Appl Ergon 37:419–427
Mathiassen SE, Moller T, Forsman M (2003) Variability in mechanical exposure within and between individuals performing a highly constrained industrial work task. Ergonomics 46:800–824
Moseley GL, Hodges PW (2006) Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci 120:474–476
Palmerud G, Kadefors R, Sporrong H, Järvholm U, Herberts P, Högfors C, Peterson B (1995) Voluntary redistribution of muscle activity in human shoulder muscle. Ergonomics 38:806–815
Punnett L, Wegman DH (2004) Work-related musculoskeletal disorders: the epidemiologic evidence and the debate. J Electromyogr Kinesiol 14:13–23
Schmidt RF, Kniffki K-D, Schomburg ED (1981) Der Einfluss kleinkalibriger Muskelafferenzen auf den Muskeltonus. In: Bauer H, Koella WP, Struppler H (eds) Therapie der Spastic. Verlag for angewandte Wissenschaft, München, pp 71–86
Sosnoff JJ, Newell KM (2006) Are age related increases in force variability due to decrements in strength? Exp Brain Res 174:86–94
Sosnoff JJ, Valantine AD, Newell KM (2006) Independence between the amount and structure of variability at low force levels. Neurosci Lett 392:165–169
Thunberg J, Ljubisavljevic M, Djupsjobacka M, Johansson H (2002) Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp Brain Res 142:319–326
Travell JG, Rinzler S, Herman M (1942) Pain and disability of the shoulder and arm. JAMA 120:417–422
van Dieën JH, Vrielink HHEO, Housheer AF, Lotters FBJ, Toussaint HM (1993) Trunk extensor endurance and its relationship to electromyogram parameters. Eur J Appl Physiol 66:388–396
van Mourik AM, Daffertshofer A, Beek PJ (2006) Deterministic and stochastic features of rhythmic human movement. Biol Cybern 94:233–244
Veiersted KB, Westgaard RH, Andersen P (1990) Pattern of muscle activity during stereotyped work and its relation to muscle pain. Int Arch Occup Environ Health 62:31–41
Acknowledgments
The authors are grateful to Birthe Lundager (Social Medicine Unit, Aalborg Hospital) and Michael Voigt [Centre for Sensory-Motor Interaction (SMI), Aalborg University] for the help during data acquisition. This work was financially supported by Arbejdsmiljøforskningsfond, Sygekassernes Helsefond, Norma og Frode S. Jacobsens Fond, and the Danish National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madeleine, P., Mathiassen, S.E. & Arendt-Nielsen, L. Changes in the degree of motor variability associated with experimental and chronic neck–shoulder pain during a standardised repetitive arm movement. Exp Brain Res 185, 689–698 (2008). https://doi.org/10.1007/s00221-007-1199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1199-2