Abstract
The purpose of this investigation was to examine the relationship between strength and the magnitude and time sequential structure of force variability. Young and old adults produced isometric force via index finger abduction to a visually presented target corresponding to a constant force level of 5 or 25% maximal voluntary contraction (MVC). Cluster analysis was used to divide subjects into groups based on age and strength. The variability of older adults was greater and showed more time dependent structure than their younger counterparts. The force output of weaker subjects was also more variable and had a stronger sequential structure. Indeed, when MVC was controlled for there was no significant age effect on force variability. The relationship between strength and variability remained significant, however, when chronological age was controlled for. The findings revealed that the established age-related changes in force variability are more fundamentally due to the association between strength and force variability and provide a further challenge to using chronological age as a marker of the biological aging process in studies of motor control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well documented that as individuals age there is a pronounced decrease in muscular strength (Doherty 2003; Hughes et al. 2001). Additionally, older adults exhibit greater levels of variability that has more time and frequency dependent structure (Galganski et al. 1993; Vaillancourt and Newell 2003). This age difference in force variability has been found to be greatest at low force levels in tasks involving the digits of the hand (Christou and Tracy 2005; Enoka et al. 2003). The interaction between force and age-related variability has led to a considerable amount of work examining the possible contributions of various neurophysiological mechanisms (Erim et al. 1999; Laidlaw et al. 2000). It has been proposed that age differences in force variability are due to several mechanisms, some of which remain to be elucidated (Enoka et al. 2003).
Although there is an ongoing debate as to the most appropriate function that captures the force-force variability relation, it is agreed that force variability scales to force magnitude, with greatest relative variability [coefficient of variation (CV)] occurring at low force levels (Christou et al. 2002; Moritz et al. 2005; Slifkin and Newell 1999, 2000). Consequently, to insure that subjects with different degrees of strength such as young and old adults are producing force at the same relative value, researchers utilize force targets based on percentages of maximal voluntary contraction (MVC). Otherwise, the use of absolute force targets will result in weaker subjects (that tend to be older adults) producing force at greater relative force levels and consequently artificially increasing the differences in the absolute level of force on which variability is assessed. Additionally, modeling has suggested that the use of relative force targets provides a more parsimonious account of the force variability magnitude relationship (Christou et al. 2002).
Unfortunately the use of relative force targets creates its own potential methodological confound. Since older adults are weaker, force produced at low % MVC targets corresponds to lower absolute levels of force output. The production of low magnitude force is inherently more variable regardless of age (Christou et al. 2002; Slifkin and Newell 1999, 2000; Taylor et al. 2003). It has been proposed that relative force variability is greatest at low forces because there are very few motor units contributing to the total force and as a result fluctuations in their firing rate or other properties [i.e., motor unit synchrony (Yao et al. 2000)] have proportionally a greater effect on the force output (Fuglevand et al. 1993; Moritz et al. 2005; Taylor et al. 2003). Recently, Hamilton et al. (2005) have shown that weaker muscles are more variable and proposed that this is due to smaller number of motor units being active and higher firing rate.
The hypothesis that there is a strength and force variability relationship is based on several empirical findings. First, in reports that find minimal age differences in force control between young and old adults there are typically no age differences in strength (Christou and Carlton 2001; Shinohara et al. 2005; Sosnoff and Newell 2006). Second, strength and activity training that led to increases in strength has been found to decrease age differences in force variability (Keen et al. 1994; Kornatz et al. 2005; Yan 1999). Third, the fitness level (a proxy index of strength) of older subjects is often relied upon to explain discrepancies in age effects between studies (Enoka et al. 2003; Tracy and Enoka 2000). Lastly, since chronological age is a poor marker of biological aging (Austad 2001; Busse 1969; Wohlwill 1970) it is logical to assume that age in and of itself, is not driving changes in the control of force output, but rather there is some other age-associated factor driving the performance decrement.
Consequently, the purpose of the current experiment is to examine the relationship between muscle strength and force variability as a function of chronological age in older adults. The hypothesis investigated was that weaker subjects regardless of age will be more variable and have more time dependent structure in their force output. In order to test this hypothesis secondary analysis was performed on a data set collected for an investigation examining if age differences in force variability are related to age-deficits in visual motor processing speed (Sosnoff and Newell 2006). The original analysis revealed that although older adults’ force production was more variable, it was not related to decreases in visual motor processing speed. It was also observed that the older adults were not only more variable but also weaker. This observation served as a primary motivation in the current investigation.
Methods
Participants
A total of 48 participants (24 males and 24 females) ranging in age from 20–29 years (n=15, mean=24.9 years, SD=3.8 years, 9 males and 6 females) and 60–79 years (n=33, mean=70.9 years, SD=5.6 years, 15 males and 18 females) took part in the study. To be included in the study, participants met the following selection criteria: (1) lived independently;(2) were free of any neuromuscular disorders, severe arthritis, cognitive impairments, diabetes; (3) had normal or corrected to normal vision; (4) were able to locomote without the use of an assistive device; (5) right hand dominant and (6) reported no falls in the last year. The subjects signed informed consent forms that had been approved by the University Institutional Review Board.
Apparatus
Isometric force recording
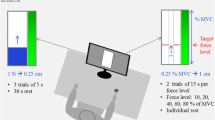
Participants were seated in a chair facing a 17-in. video monitor (CTX International, California), with their dominant hand placed in a prone position in an apparatus designed to isolate abduction force of the index finger. Orthogonal to the index finger and 36 cm from the participant’s midline was an Eltran EL-500 load cell (diameter 1.27 cm). The load cell measured compressive force produced by the index finger. The lateral side of the distal portion of the index finger was in constant contact with the load cell producing compressive force.
Voltage changes from the load cell were amplified by a Coulbourn (V72-25) resistive bridge strain amplifier with an excitation voltage of 10 V and an amplifier gain of 100. The analog force signal was sampled at 140 Hz by a 16-bit analog to digital converter. The smallest increment of change in force that could be detected was 0.0016 N. Prior to being saved on the hard drive of the computer the force signal was passed through an 8th pole Butterworth lowpass filter (858L8B-1, Frequency Devices, inc.) with a cutoff frequency of 25.6 Hz.
At each sampling interval the force produced was presented on the monitor. The monitor had a viewing area of 1,200 horizontal pixels and 1,000 vertical pixels. The monitor was approximately 50 cm from the volunteer’s eyes and 100 cm from the ground. The display to control gain was set at 50 pixels/N. On the monitor, subjects viewed a red target line, which spanned the width of the monitor and a series of illuminated yellow pixels both on a black background. The red target line served as the force target and the yellow pixels depicted the force trajectory produced by the subject.
Procedures
Estimation of maximal voluntary contraction
The participant’s maximal voluntary contraction (MVC) strength was determined at the start of the experimental session. The participant was instructed to produce the maximal amount of isometric force possible by abducting their index finger. The participant produced maximal force while pressing against the load cell. The force applied to the load cell was displayed on the monitor to provide visual feedback. Three 6 s maximal contractions were recorded with 30 s rest between each contraction. The participant’s MVC was determined to be the highest force produced over the three trials.
Experimental design and instructions
The participant adjusted their force output to match a red target line displayed on the monitor and viewed online feedback of their performance in the form of a series of yellow dots which corresponded to the force trajectory that moved left to right across the screen with time. The target line corresponded in independent conditions to a constant force level of 5 and 25% of the participant’s MVC.
The experiment (Sosnoff and Newell 2006) from which the data were extracted consisted of two blocks of 27 trials. In the original investigation the amount of visual information in the form of visual feedback rate was manipulated over nine levels, for the purpose of this report only the condition in which visual feedback was provided at 25 Hz will be examined. Three 25 s trials were performed at each unique force condition. Half of the subjects performed the lower force target block first, followed by the higher force target block. In order to minimize fatigue, participants were given a 30 s rest between trials and 2 min rest between condition blocks.
Data analysis
The initial 4 s and final second of force data from each trial were removed prior to analysis to avoid the initial force stabilization and/or premature cessation of force production. All data processing was performed using software written in Matlab v7 (The Mathworks, Inc.).
Magnitude and structure of force variability
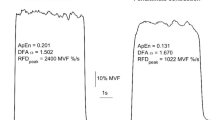
The magnitude of force variability was indexed with the within trial coefficient of variation (CV=SD/mean). The time domain structure of force output was assessed with approximate entropy (ApEn) (Pincus 1991). ApEn yields a single value that quantifies the regularity (complexity) of a time series. A very regular signal with relatively greater time dependent structure such as an ideal sinewave would have an ApEn value approaching zero while a random time series (white noise) would have a value close to 2. Increases in ApEn have been interpreted as an increase in the signal’s time domain complexity (Pincus 1991). The appendix in Slifkin and Newell (1999) provides a brief explanation of how ApEn is calculated.
Statistical analysis
In order to examine the relationship between strength, age and force variability several analyses were performed. First, the traditional approach of dividing the 48 Ss into groups based on chronological age (20–29 year olds, 60–79 year olds) and then examining differences in the magnitude and structure of force variability with two-way ANOVAs (age X force level) was conducted. Second, the subjects were divided into groups (strong and weak) based on their MVC and then the two-way ANOVAs (strength X force level) were run. Third, since MVC is known to co-vary with age, ANCOVAs (analysis of covariance) were performed, with MVC or chronological age entered as a covariate into the appropriate two-way ANOVAs. Additionally, partial eta squared (η2) was calculated to provide an estimate of the amount of variance accounted for by each factor in the ANOVAs. All statistics were evaluated as significant when there was less than a 5% chance of making a Type I error (P<0.05). Statistical analyses were completed using SPSSv12 statistical package (SPSS, Inc.). Data reported are mean and standard deviations unless otherwise noted.
Results
Two step cluster analysis was used to divide subjects into age and strength groups. As expected subjects were divided into a young age (n=15, mean=24.9 years, SD=3.8 years, 9 males and 6 females years old) and old age group (n=33, mean=70.87 years, SD=5.61 years, 15 males and 18 females). Cluster analysis on MVCs revealed that the subject pool was equally divided into 2 groups based on strength (strong and weak). The strong group was composed of 24 Ss (10 young and 14 old) and had a mean MVC of 24.36 N (SD 3.46 N), while the weak group was composed of 24 Ss (5 young and 19 old) and had a mean MVC of 14.71 N (SD 3.00 N). These age and strength groupings were then used to examine the relationship between strength and age on the magnitude and structure of force variability.
Aging and the magnitude of force variability
Figure 1a shows the influence of age and force level on force CV. A main effect for force level [F(1,46)=10.52; P<0.05; η2=0.19] and age [F(1,46)=6.17; P<0.05; η2=0.12 ] was found. The 5% force level had a higher CV than the 25% level [0.06 (SD 0.04) vs. .04 (SD 0.02), respectively]. The young age group (0.04 (SD 0.02)) had less relative variability than the old age group [0.06 (SD 0.04)].
Figure 1b depicts the influence of strength and force level on CV. The two-way ANOVA revealed main effects for strength [F(1,46)=10.97; P<0.05, η2=0.19] and force level [F(1,46)=13.75; P<0.05, η2=0.23] as well as an interaction between strength and force level [F(1,46)=4.95; P<0.05, η2=0.10]. The strong group (0.04 (SD 0.02)) was found to have smaller CV than the weak group (0.07 (SD 0.05)). Also the 5% force level had greater relative variability [0.07 (SD 0.05) vs. 0.04 (SD 0.03)]. The two-way interaction between strength group and force level was due to the lack of an effect for strength group at the 25% force level.
An examination of Fig. 1a, b reveals that there is a similarity between the effect of age and strength on the magnitude of force variability. It is possible that the well known decrease in maximal strength with advanced age explains this similarity. In order to examine if there is a significant relation between MVC and chronological age in the current subject pool a correlational analysis was conducted. This analysis revealed a significant though moderate relationship [r(48)=−0.34; P<0.05], with strength decreasing with age.
Individual subject’s CVs were plotted as a function of both chronological age and MVC to further examine if there was a covariation between strength and age. Figure 3a, b shows that chronological age does not have a strong relationship with force variability. It should be noted that a lack of middle age subjects possibly influenced this relationship. On the other hand, Fig. 2c, d illustrates that strength is negatively related to the magnitude of variability, irrespective of age.
a, b Coefficient of variation as a function of chronological age in the 5% MVC and 25% MVC condition. Each data point represents an individual subject’s performance. c, d Coefficient of variation as a function of MVC in the 5% MVC and 25% MVC conditions. Each data point represents an individual subject’s CV
To further examine if the effect of age was due to the covariation with strength, a two-way ANCOVA with age group and force level as main factors with MVC as a covariate was performed. Figure 3a shows the effect of force level and age group on CV when MVC is controlled for. Statistical analysis revealed a main effect for force level [F(1,46)=19.71; P<0.05, η2=0.31], with the lower force level having greater CV [0.06 (SD 0.05) vs. 0.04 (SD 0.03), respectively]. Importantly, there was no significant effect for age group (P>0.05).
To examine if chronological age influences the effect of strength on CV, a two-way ANCOVA with strength and force level as main factors and chronological age as the covariate was performed. Figure 3b shows that even when chronological age is controlled for, the effect of strength [F(1,45)=7.2; P<0.05, η2=0.14] on CV still remains. The strong group (0.04 (SD 0.02)) was found to have less variability than the weak group (0.07 (SD 0.05). Additionally, a two-way interaction between strength group and force level was observed [F(1,45)=4.69; P<0.05, η2=0.09]. The interaction between strength and force level was a result of the lack of effect for force level in the strong group, while the weak group had greater CV in the 5% (0.09) than the 25% force level (0.04).
Aging and the structure of force variability
Figure 4a shows the effect of age and force level on ApEn. A main effect for force level [F(1,46)=34.58; P<0.05, η2=0.43] was found and there was also a significant trend for age [F(1,46)=4.17; P<0.05, η2=0.08]. The lower force level was found to have greater ApEn [0.46 (SD 0.14) vs. 0.34 (SD 0.13), respectively]. The young age group (0.44 (SD 0.145)) had greater ApEn than the old age group (0.36 (SD 0.13)). Figure 4b shows the effect of strength and force level on ApEn. It is clear that the lower force level has greater ApEn [0.46 (SD 0.14) vs. 0.34 (SD 0.13), respectively] [F(1,46)=47.95; P<0.05, η2=0.51]. The weak group (0.35 (SD 0.13)) had lower ApEn than the strong group (0.42 (SD 0.13)) [F(1,46)=4.19; P<0.05, η2=0.08].
To examine if the covariation between strength and age influenced the relation between age and ApEn, individual subject’s ApEn values were plotted as a function of both chronological age and MVC. Figure 5a, b shows that chronological age does not have a strong relationship with force irregularity. In contrast, Fig. 5c, d illustrates that strength is positively related to ApEn, irrespective of age.
To investigate if the relationship between age and strength influenced their effects on the structure of force variability, 2 two-way ANCOVAs with main factors of either age group and force level or strength group and force level combined with either MVC or chronological age as the covariate were performed. Figure 6a depicts the influence of age and force level when MVC is controlled for. Statistical analysis revealed no effect for age (P=0.17, η2=0.04). There was a trend for a two-way interaction between force and age, but a traditional level of significance was not reached (P=0.11, η2=0.06). This trend appeared to be due to the young age group (0.38) having greater ApEn than the old group (0.30) at the higher force level.
Figure 6b shows the influence of strength on ApEn when chronological age is controlled for. There was a trend for two-way interactions between strength group and force level (P=0.07, η2=0.07). The strength group by force level interaction was a result of the strong group having greater ApEn than the weak group only at the lower force level (0.49 vs. 0.40, respectively).
Discussion
This study examined if the reported deficits in force control with advanced age (Galganski et al. 1993; Vaillancourt and Newell 2003) are confounded with age-associated muscle weakness. The findings clearly showed that the force output of the older age group was more variable and had a stronger time dependent sequential structure (Galganski et al. 1993; Vaillancourt and Newell 2003). However, when the magnitude and structure of force variability was examined as a function of MVC, the weaker group had a higher level of variability that also had greater time sequential structure. To further address this issue, ANCOVAs on force variability with MVC and chronological age as covariates were performed. There was no effect of age on force variability and its structure when MVC was controlled for. However, strength still mediated force variability when chronological age was controlled for. Collectively, these findings support the notion that observations of age-related changes in the magnitude and structure of force variability are more fundamentally documenting weakness related variability.
Cross-sectional (Shock and Norris 1970) and longitudinal (Kallman et al. 1990) data are not consistent in regard to the extent of strength loss with aging, but it is well established that there is a significant decrease in strength with advanced age (Doherty 2003; Hughes et al. 2001). It is currently maintained this loss is strength with advanced age is the result of the interplay of multiple factors including decreases in muscle mass (i.e., sarcopenia) (Doherty 2003), number of functioning motor units (Doherty and Brown 1993), increases in connective tissue within the muscle (Arking 1998) and other metabolic and cellular changes. Additionally, the loss of strength with aging is dependent on multiple factors including the physical fitness level of the individual (Barry and Carson 2004) the muscle group under investigation (Bosco and Komi 1980; Petrofsky et al. 1975), and the type of contraction (Vandervoort et al. 1990).
Although absolute force targets are more functionally relevant since activities of daily living are not typically scaled to ability, investigations of the aging process on the control of force production have dealt with the age-related loss of strength by utilizing force targets as a percentage of the individual subject’s MVC. This approach is based on the finding that force variability scales with force magnitude (Christou et al. 2002; Slifkin and Newell 1999, 2000). If relative force targets were not used it would not be clear if differences in variability between age groups were due to subjects producing force at varying relative levels of difficulty or rather some other difference. The use of % MVC targets has revealed that older subjects are more variable and have greater time sequential structure in their force output, especially at low force levels (Galganski et al. 1993; Vaillancourt and Newell 2003). However, the use of relative force targets is to some degree biased against weak subjects because they are required to produce force at the lower levels of force production that are close to the limits of their system output. Additionally, low forces are also relatively the most variable portion of the force-variability function (Slifkin and Newell 1999, 2000). The result and outcome of this practice is the possible artificial increase in the observed force variability at low force levels of subjects with low muscle strength (e.g., older adults).
Given that there are distinct contributions of neuromuscular mechanisms at various portions of the force production curve (Fuglevand et al. 1993; Milner-Brown et al. 1973), the age-force level variability interaction has lead to the proposition that various neurophysiological mechanisms are responsible for age-related force output changes. However, currently there are several discrepancies in the literature involving aging and the neurophysiological control of force production. For instance, Laidlaw et al. (2000) proposed that age related differences in motor unit firing rate variability drive the increase in variability of older adults. This position has recently received support from the work of Moritz et al. (2005) who demonstrated that motor unit firing rate variability is a major determinant of force fluctuations at low force levels. Additionally, Tracy et al. (2005) have shown that motor unit firing rate variability is only a determinant of force variability in older adults.
However, there have also been studies that have documented increased force variability in older adults and not found motor unit firing rate differences (Erim et al. 1999; Vaillancourt et al. 2003). For instance, Vaillancourt et al. (2003) demonstrated that rhythmical oscillations in intramuscular EMG contribute to force control deficits in older adults, but did not find any relation between force variability and firing rate variability. Importantly, Vaillancourt et al. (2003) looked at a small sample of motor units and not the entire motor unit pool. These discrepancies have led to the hypothesis that multiple neuromuscular mechanisms contribute to age associated differences in force control (Enoka et al. 2003; Taylor et al. 2003). However, the findings of the current study suggest that there is not an age related deficit in force control but rather a muscle weakness related deficit. The central hypothesis of this investigation is consistent with recent findings which have shown that weaker muscles are more variable at a given force (Hamilton et al. 2005). As such it is possible that differences in neuromuscular contributions to force control between age groups could be a result of examining subject populations with varying levels of strength.
The weakness related variability highlighted in the current investigation is in line with documentations of older adults improvements in force control with increases in strength via training (Keen et al. 1994; Tracy et al. 2004; Yan 1999). Kornatz et al. (2005) have shown that training decreases motor unit firing rate variability and increases hand function in older adults. It has also been shown that chronic activity can influence the amount of motor unit synchronization (Semmler and Nordstrom 1998). Both of these processes have been proposed to contribute to increased force variability (Laidlaw et al. 2000; Yao et al. 2000). Yet, it is not clear if the relationship between muscle weakness and variability is due to differences in neuromuscular processes between strong and weak subjects or rather that the production of low force magnitudes is inherently more difficult. Fluctuations in force at low levels are believed to be due to several factors including the recruitment of additional motor units, the unfused firing of newly recruited motor units, firing rate variability and motor unit synchrony (Fuglevand et al. 1993; Milner-Brown et al. 1973; Mortiz et al. 2005; Yao et al. 2000). Jones, Hamilton and Wolpert (2002) have provided evidence that there is a small constant contribution of the mechanical components of force generation to force variability. However, since this study only examined force output from 20–80% MVC, it is possible that at low levels of force production there is a relatively large impact of the mechanics of muscular contraction.
It is not clear, based in part on the behavioral nature of the current investigation, if the strength-variability relationship is due to either a shared underlying cause or methodological concerns. For instance, it is possible that increases in contractile tissue in the muscle of older adults results in their weakness (Arking 1998) as well as increased force variability via an enhanced influence of muscle mechanics (Jones et al. 2002). It is also possible as stated previously that the use relative force targets biases weaker subjects to produce force at inherently more difficult absolute force targets. As such further experimentation is needed to determine between these two possible mechanisms.
Additionally, it is logical to suggest that subjects who had lower MVCs were less motivated to perform in the experimental protocol and that the increased variability was not due to physiological deficits. However, several experimental procedures were followed to insure that subjects remain motivated throughout the experimental session. First, subjects were given a performance score at the end of each trial. Second they were given adequate rest between trials to minimize mental fatigue. Lastly, the subjects were all volunteers and were interested in the research. Additionally, the removal of the 4 most variable older subjects who also have fairly low MVCs (see Fig. 2c, d) does not substantially change the overall findings - that force variability is mediated by muscle strength. The results of this investigation highlight the importance of attaining a “true” MVC when testing subjects of varying strengths.
The lack of evidence for an age-related deficit in force control in the current study is congruent with the established finding that chronological age is a poor index of the biological aging process (Austad 2001; Busse 1969; Wohlwill 1970). In contrast, decreases in strength have been found to be a predictive marker of frailty, morbidity and mortality (Fried et al. 2004). As such the proposed concept of weakness related variability is congruent with the use of muscular strength as a valid biomarker of the aging process (Arking 1998). The association between muscle weakness and force variability also offers indirect evidence for the theoretical link between increased variability and risk for mortality (Rowe and Kahn 1987; Lipsitz 2002).
Finally, an increasing common practice in neuropsychology of aging is to examine the neural correlates of behavior in ability matched young and old subjects (Reuter-Lorenz 2002). This practice allows researchers to discuss the impact of aging on neural activation unencumbered by behavioral differences. This behavior matching is in line with the utilization of control groups composed of age matched subjects when studying the effects of a particular disease which predominately inflict older adults (i.e., Parkinson’s Disease). We propose that it would be useful to extend this behavior matching paradigm to examinations of the aging process on motor control in general and force control specifically in order to gain a clear picture of what specific mechanisms are impacted by the aging process.
References
Arking R (1998) Biology of aging. Sinauer Associates, Inc., Sunderland
Austad SN (2001) Concepts and theories of aging. In: EJ Masoro, Austad SN (eds) Handbook of the biology of aging. Academic, Amsterdam, pp 3–18
Barry BK, Carson RG (2004) The consequence of resistance training for movement control in older adults. J Gerontol A Biol Sci 59:M730–M745
Bosco C, Komi PV (1980) Influence of the aging on the mechanical behavior of leg extensor muscles. E J Appl Physiol 45:209–219
Busse EW (1969) Theories of aging. In: EW Busse, E Pfeiffer (eds) Behavior and adaptation in late life. Little, Brown, Boston, pp 11–31
Christou EA, Carlton LG (2001) Old adults exhibit greater motor output variability than young adults only during rapid discrete isometric contractions. J Gerontol A Bio Sci Med Sci 56:B524–B532
Christou EA, Tracy BL (2005) Aging and variability in motor output. In: Davids K, Bennet S, Newell KM (eds) Movement system variability. Human Kinetics, Champaign, pp 199–218
Christou EA, Grossman M, Carlton LG (2002) Modeling variability of force during isometric contractions of the quadriceps femoris. J Mot Behav 34:67–81
De Luca CJ, LeFever RS, McCue MP, Xenakis AP (1982) Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329:129–142
Doherty TJ (2003) Invited review: aging and sarcopenia. J Appl Physiol 95:1717–1727
Doherty TJ, Brown WF (1993) The estimated numbers and relative sizes of thenar muscle motor units as selected by multiple point stimulation in young and older adults. Muscle Nerve 16:355–366
Enoka RM, Christou EA, Hunter SK et al (2003) Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kines 13:1–12
Erim Z, Beg MF, Burke DT, De Luca CJ (1999) Effects of aging on motor-unit control properties. J Neurophysiol 82:2081–2019
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G (2004) Untangling the concepts of disability, frailty and comorbidity: Implications for improved targeting and care. J Gerontol Med Sci 59:255–263
Fuglevand AJ, Winter D, Patla AE (1993) Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70:2470–2488
Galganski ME, Fuglevand AJ, Enoka RM (1993) Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol 69:2108–2115
Hamliton AFdC, Jones KE, Wolpert DM (2005) The scaling of motor noise with muscle strength and motor unit number. Exp Brain Res 157:417–430
Hughes VA, Frontera WR, Wood M et al (2001) Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J Gerontol Bio Sci Med Sci 56:B209–B217
Jones KE, Hamilton AFdC, Wolpert DM (2002) Sources of signal-dependent noise during isometric force production. J Neurophysiol 88:533–544
Kallman DA, Plato CC, Tobin JD (1990) The role of muscle loss in the age-related decline in grip strength: Cross-sectional and longitudinal perspectives. J Gerontol Med Sci 45:M82–M88
Keen DA, Yue GH, Enoka RM (1994) Training-related enhancement in the control of motor output in elderly humans. J App Physiol 77:2648–2658
Kornatz KW, Christou EA, Enoka RM (2005) Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol 98:2072–2080
Laidlaw DH, Bilodeau M, Enoka RM (2000) Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23:600–612
Lipsitz LA (2002) Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Bio Sci Med Sci 57:B115–B125
Milner-Brown HS, Stein RB, Yemm R (1973) Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol (Lond) 230:371–390
Moritz CT, Barry BK, Proscoe MA, Enoka RM (2005) Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93:2449–2459
Petrofsky JS, Burse RL, Lind AR (1975) Comparison of women and men to isometric exercise. J Appl Physiol 38:863–868
Pincus SM (1991) Approximate entropy as a measure of system complexity. Proc Nat Acad Sci 88:2297–2301
Reuter-Lorenz PA (2002) New visions of the aging mind and brain. Tr Cog Sci 6:394–400
Rowe JW, Kahn RL (1987) Human aging: usual and successful. Science 237:143–149
Semmler JG, Nordstrom MA (1998) Motor unit discharge and force tremor in skill-and strength-trained individuals. Exp Brain Res 119:27–38
Shinohara M, Keenen KG, Enoka RM (2005) Fluctuations in motor output during steady contractions are weakly related across contraction types and between hands. Muscle Nerve 31:741–750
Shock NW, Norris AH (1970) Neuromuscular coordination as a factor in age changes in muscular exercise. Med Sport 4:92–99
Slifkin AB, Newell KM (1999) Noise, information transmission and force variability. J Exp Psychol Hum Percep Perf 25:837–851
Slifkin AB, Newell KM (2000) Variability and noise in continuous force production. J Mot Behav 32:141–150
Sosnoff JJ and Newell KM (2006) Aging, visual intermittency and variability in isometric force output. J Gerontol Psychol Sci 61:P117–P124
Taylor AM, Christou EA, Enoka RM (2003) Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol 90:1350–1361
Tracy BL, Enoka RM (2000) Older adults are less steady during submaximal isometric contractions with the knee extensors muscles. J Appl Physiol 92:1004–2000
Tracy BL, Byrnes WC, Enoka RM (2004) Strength training reduces force fluctuations during anisometric contractions of the quadriceps femoris muscles in old adults. J Appl Physiol 96:1530–1540
Tracy BL, Maluf KS, Stephenson JL, Hunter SK, Enoka RM (2005) Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 32:533–540
Vaillancourt DE, Newell KM (2003) Aging and the time and frequency structure of force output variability. J Appl Physiol 94:903–912
Vaillancourt DE, Larsson L, Newell KM (2003) Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and Hz EMG activity. Neurobiol Aging 24:25–35
Vandervoort AA, Kramer JF, Wharram ER (1990) Eccentric knee strength of elderly females. J Gerontol Biol Sci 45:B125–B128
Wohlwill JF (1970) The age variable in psychological research. Psych Rev 77:49–64
Yan JH (1999) Tai Chi practice reduces movement force variability for seniors. J Gerontol Med Sci 54:M629–M634
Yao W, Fuglevand RJ, Enoka RM (2000) Motor-unit synchronization increases EMG amplitude and decreases force steadiness of stimulated contractions. J Neurophysiol 83:441–452
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sosnoff, J.J., Newell, K.M. Are age-related increases in force variability due to decrements in strength?. Exp Brain Res 174, 86–94 (2006). https://doi.org/10.1007/s00221-006-0422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0422-x