Abstract
We examined the effects of aging on the predictive control of grip force during object manipulation under various external force fields. Participants rhythmically moved a hand-held object (m = 0.4 kg) in the horizontal plane under three experimental conditions: (1) with an elastic cord attached to the upper arm (ARM), (2) with the elastic cord attached to the object (OBJECT), and (3) without any elastic cord (NO ELAST). Performance was evaluated in terms of both metric and spectral characteristics of the grip force (GF) profile, in relation to the movement-induced variations in load at the object-finger interface (LFO). The performance of a group of 12 older adults (mean age = 66.3 years) was compared to the performance of a group of 12 young adults (mean age = 25.0 years), whose metric characteristics were reported earlier (Exp. Brain Res. 172:331, 2006). Although elderly participants exerted a larger mean GF, a tight linear coupling between GF and LFO was found for both groups in OBJECT. In ARM and NO ELAST, coefficients of cross-correlations were markedly lower, the more so for the elderly participants. Adjustments in GF occurred slightly in advance of variations in LFO in young adults (+7 ms) and somewhat delayed in the elderly (−26 ms). Spectral analyses revealed that in OBJECT, LFO and GF varied primarily at the frequency of movement. In ARM and NO ELAST, where LFO varied at twice this frequency, GF modulations contained a substantial frequency component at the frequency of movement, with this effect being more pronounced for the elderly participants. We conclude that both young and older adults demonstrate a predictive control of GF, capable of separating external force fields acting on the arm or on object–finger interface. However, in the presence of variations in LFO occurring at twice the frequency of movement, the spectral profile of GF exhibits a non-functional component of variation at the frequency of movement. Aging amplifies this latter effect, thereby affecting the efficiency of the predictive control of grip force.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to grasp and manipulate an object maybe the most important function of the hand, and any deterioration in this ability can severely impair a person’s independence in activities of daily living (Kinoshita and Francis 1996; Ranganathan et al. 2001). Frequently, a decline in manual dexterity is reported in older adults in their 60s and 70s as compared to young adults. This view is supported by self reports in elderly (Falconer et al. 1991), as well as experimental investigations during force-tracking tasks (Ranganathan et al. 2001; Shinoara et al. 2004; Barry et al. 2005; Voeckler-Rehage and Alberts 2005), pinching tasks (Ranganathan et al. 2001), lifting tasks (Cole 1991), and peg-board tasks (Ranganathan et al. 2001). Among the possible factors responsible, this decline in manual dexterity could result from changes in mechanical properties of the skin (Cole 1991; Kinoshita and Francis 1996), loss in muscle strength (Kallman et al. 1990; Bemben et al. 1996; Ranganathan et al. 2001), deterioration in tactile sensitivity (Kenshalo 1986; Cole 1991; Cole and Rotella 2001; Ranganathan et al. 2001), as well as changes within the central nervous system (Kinoshita and Francis 1996; Mattay et al. 2002; Ward and Frackowiak 2003). The goal of the present study is to further explore this last possibility by investigating grip force modulations during object manipulation tasks.
When holding an object with a precision grip, a minimal grip force (normal to the contact surfaces) must be applied to prevent the object from slipping under the influence of external forces. According to Newton’s second law, accelerating an object creates an inertial force. How does the central nervous system (CNS) deal with such mechanical constraints? Earlier studies have shown that, for self-produced hand-and-arm movements, grip force adjustments occur simultaneously with (or slightly ahead of) movement-induced fluctuations in object load. To account for these observations, it has been suggested that the CNS uses the motor command of the arm in conjunction with an internal (forward) model incorporating both the arm and the object to anticipate the resulting load force and thereby adjust grip force appropriately (Johansson and Cole 1992; Flanagan and Wing 1995, 1997; Blakemore et al. 1998; Flanagan et al. 2003). The main objective of the current study is to investigate whether the predictive control of grip force is affected (or not) in older adults.
Many reports can be found on the control of grip force during manipulative tasks in elderly (Cole 1991; Kinoshita and Francis 1996; Cole et al. 1998, 1999; Cole and Rotella 2001, 2002; Lowe 2001; Gilles and Wing 2003). A large subset of these studies focused on the control of the grip force used to hold an object stable following a lifting task (Cole 1991; Kinoshita and Francis 1996; Cole et al. 1998, 1999). A consistent finding across experiments was that elderly participants used excessive grip force as compared to young adults (Cole 1991; Kinoshita and Francis 1996; Cole et al. 1998, 1999). Although relevant, such analyses provide little information about the ability of older participants to predict the fluctuations in object load resulting from movement. To our knowledge, only three studies have specifically addressed this question. In the first, using a lift-and-hold task, Gilles and Wing (2003) found that elevated grip force in elderly was not accompanied by a fundamental change in the synchronization of grip force modulation with load force fluctuations. In the second, using a grasp-and-lift task, Cole and Rotella (2002) reported that old age impairs the ability to use visual cues for predictive control, but, as suggested by their Fig. 1, without compromising the coordination between grip force and load force. In the third study, using a slightly different task (a pulling force tracking task), Lowe (2001) also found no statistically significant age effects in the way grip force was modulated with respect to load force. Overall, at this stage, there is little evidence that aging would impair the internal (forward) model underlying the control of grip force.
Despite this body of evidence in favour of the maintenance of a predictive mechanism in the elderly, a number of questions remain. In particular, it remains unclear why the coupling between grip and load forces has been found to vary—to a considerable extent—as a function of the environment in which movement is produced. Indeed, when the strength of this coupling is assessed through linear regression (a standard method in this field), considerable differences can be found depending on the external force field present (Flanagan and Wing 1997; Gilles and Wing, 2003; Descoins et al. 2006). For instance, when young adults horizontally oscillated an object attached to the wall with an elastic cord, Descoins et al. (2006) found coefficients of cross-correlation between 0.85 and 0.90, indicating a tight linear coupling between grip and load forces. However, in conditions in which the variations in the load on the object were exclusively determined by the movement-induced inertial force (elastic cord attached to the arm and without elastic cord), the coefficients of cross-correlation were only on the order of 0.5. In a task requiring rhythmical movement of an object in the vertical plane (with load therefore varying as a function of combined inertial and gravitational forces), Gilles and Wing (2003) also reported rather low coefficients of correlation between maximum grip and load forces (on the order of 0.4) for both younger and older adults. Unfortunately, in this latter study no other types of load were tested for comparison purposes. Similarly, during discrete horizontal movements performed by young adults, Flanagan and Wing (1997) found stronger correlations under a viscous load than under an inertial load (0.90 versus 0.76).
To date, the considerable variations in the correlation between grip and load forces remain unexplained. On the basis of the time-varying signals of both these forces, available under each of the three afore-mentioned conditions for both younger and older adults, we therefore studied this coupling in more detail, complementing the standard regression analysis with spectral analyses of the constituent signals. Indeed, while an inertial load is arguably the most common motion-dependent load experienced in everyday life, the above-mentioned studies suggest that the modulation of grip force does not co-vary linearly with such an inertial load force. Yet, when other loads supersede the inertial load, relatively high linear correlations are to be observed, at least for young adults (Flanagan and Wing 1997; Descoins et al. 2006).
We hypothesized that these effects could be due to frequency at which different loads vary when an object is moved. In the case of discrete movement (Flanagan and Wing 1997), the object is first accelerated (leading to a peak in inertial load) and then decelerated (leading to another peak in inertial load). When such a discrete movement is performed in a viscous environment, the velocity-dependent viscous load leads to a single peak, occurring at the moment of peak velocity (see Fig. 2 in Flanagan and Wing 1997). Thus, the presence of the viscous environment influenced the frequency content of the load signal, leading it to vary once per movement rather than twice, as in the inertial condition. For these discrete movements, coefficients of correlation between grip and load forces were higher in the viscous condition than in the inertial condition. In the case of smooth rhythmical movements, accelerations/decelerations peaks occur at the movement extremes (Guiard 1997; Mottet and Bootsma 1999). As a result, inertial load demonstrates a peak at each extreme, and thereby varies twice during a movement cycle. In contrast, with an elastic cord of sufficient stiffness between the hand-held object and the wall, as in the study of Descoins et al. (2006), the total load on the object is characterized by a single peak during a movement cycle. For these rhythmic movements, coefficients of cross-correlation between grip and load forces were higher in the elastic condition than in the inertial condition.
Together, these observations suggest that grip force does not co-vary linearly with load force when variations in load occur at frequencies higher than the frequency of movement (such as in a pure inertial environment). In the present contribution we therefore addressed the following questions: (1) What are the characteristics of the grip force signal when the total load at the object–finger interface varies at once (predominantly elastic origin) or twice (predominantly inertial origin) the movement frequency, and (2) Are the spectral characteristics of the grip force signal modified by aging in either one of these situations? Our working hypothesis, taken from the work on coordination dynamics, was that participants may have a tendency to vary grip force at the frequency of arm movement, due the intrinsic stability of such a 1:1 frequency ratio (De Guzman and Kelso 1991).
A subsidiary goal of this study was to investigate the neural processes involved in the anticipation of the resulting load force in elderly participants. Anticipation of the resulting load force at the object–finger interface requires (at least) two successive neural processes: (1) to predict the kinematic characteristics of the unfolding arm trajectory, and (2) to predict the load force at the object–finger interface associated with this arm trajectory. The former operation requires knowledge about the force field in which the arm is moved, whereas the latter requires knowledge about the force field in which the object is moved. Are these two operations based on distinct representations of the external forces? One way to address this issue is to explore whether humans can still anticipate adequately the resulting load force when the arm is submitted to a load that does not contribute to the object. Two recent reports demonstrate that adequate grip force control is preserved in young adults: (1) when the arm (not the object) was constrained by an elastic cord (Descoins et al. 2006), or (2) when the inertia of the arm (not the object) was increased by wearing a brace (White et al. 2005). However, it is presently unclear whether this conclusion can be extended to older adults.
To summarize, the goal of the present contribution was to investigate the effects of aging on the predictive control of grip force during object manipulation. A first (primary) issue was to investigate the possible mechanisms leading to the alteration of the grip/load force coupling under inertial load. A second (subsidiary) issue was to investigate how external forces acting on the arm and/or the object are taken into account in the process of anticipating the resulting load force. To address these issues we have tested a group of elderly participants following the same experimental procedure as in our recent study (Descoins et al. 2006). Briefly, this group of elderly participants was asked to rhythmically move an object with (or without) an elastic cord attached to the arm or to the object. Data analysis focused on the comparison between the performance (in terms of metric and spectral characteristics of the grip force profile) of this elderly group and the performance of our earlier set of younger adults.
Materials and methods
Participants
Two groups of unpaid participants took part in the experiment. The first group (YG) consisted of 12 young healthy adults aged 22–32 years (6 men and 6 women, 25.0 ± 2.4 years of age). A previous report has been published on the performance of this group (Descoins et al. 2006). This means that some of the results (the metric characteristics) exposed in the present report are redundant with our previous study. Note, however, that new data processing, such as spectral frequency analysis, was specifically performed for the goal of the present study. The second group (EG) comprised 12 older adults aged 61–74 (5 men and 7 women, 66 ± 3.6 years of age). The mean body height and mass of the participants were 1.73 ± 0.08 m and 65.8 ± 9.2 kg in YG and 1.65 ± 0.09 m and 70.4 ± 9.9 kg in EG, respectively. The group of older adults was tested approximately 6 months after the group of young adults. All young and older adults were right-handed according to their preferential use of the right hand during writing and eating.
The young participants were students or junior staff members at the University of the Mediterranean. The elderly participants were recruited from the general community nearby the laboratory; they travelled independently to and from the laboratory for the test session. All participants were asked to fill out a general information questionnaire. All of them claimed to perform activities of daily living with no apparent difficulty and to be healthy. None of the participants reported any musculoskeletal hand or neurological problem that might have affected manual dexterity. Participants described themselves as having normal visual acuity (uncorrected or corrected with lenses) and gave informed consent according to the procedures approved by the University of the Mediterranean.
Apparatus
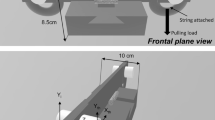
The grip device that the participants manipulated has been described in more detail previously (Descoins et al. 2006). Five unidirectional sensors (ELPM-T1M-25N, Entran) were used to measure the forces exerted by each of the fingers. With participants grasping the object between the thumb and the four remaining fingers, each sensor measured the normal force component (i.e. the force perpendicular to the sensor’s surface). The configuration was comfortable for all participants. The surface of each transducer was covered with medium grain sandpaper. To measure the horizontal acceleration induced by the oscillatory movement, the object was equipped with an accelerometer (EGAS-FS-5, Entran, 5 g range). An infrared camera (C2399, Hamamatsu) tracking an infrared LED mounted on the upper part of the object served to measure the horizontal (X) and vertical (Y) displacement of the object in the sagittal plane. The total mass of the grip device was 0.4 kg (or 3.92 N). However, within the task space, the weight of the device was neutralized: the object was suspended from the ceiling by a 2-m long compliant elastic cord (stiffness = 3.8 N/m). Depending on the experimental conditions another set of elastic cords, much stiffer (stiffness = 37.2 N/m), could be attached to the object or to the participant’s upper arm (see Fig. 1). In the latter condition, a rigid plastic cuff, attached to the dorsal side of the participant’s upper arm, allowed the elastic cords to be connected very close to the axis of rotation of the elbow. A force sensor (ELPM-T1M-125N, Entran) was placed between the wall and the elastic cords to determine the traction force exerted by the participant. A LabView (National Instrument, Austin, TX) program was used to collect the signals from each sensor at a sampling frequency of 1,000 Hz.
Procedure
The procedure is illustrated in Fig. 1. During testing, the participant was seated on a chair facing the apparatus. At the beginning of each trial, the arm posture was the following: the shoulder was at about 50° of flexion and the elbow at 40° of flexion (0° corresponding to full extension). Using the right hand, the participants grasped the instrumented object between the thumb and the four remaining fingers. The task was to move the object at a prescribed frequency of 1.2 Hz over an amplitude of 20 cm, indicated by two targets. The movement was performed along a horizontal axis that was parallel to the sagittal plane. Participants were instructed to synchronise movement reversals in the vicinity of the targets with the beeps of the metronome. The duration of a trial was 20 s.
Schematic drawing of a participant holding the grasping device, suspended from the ceiling by a compliant elastic cord. During OBJECT, a stiffer set of elastic cords was placed between the grasping device and the wall facing the participant. During ARM, the set of elastic cord was attached to the back to the participant’s upper arm. During NO ELAST, the set of elastic cord was removed. During OBJECT and ARM, a unidirectional force sensor measured the elastic load. See “Materials and methods” for further details
Depending on the experimental conditions two experimental factors were manipulated. The first factor (TYPE) corresponded to the type of external load applied. In the first condition, the elastic cord was attached to the upper arm (ARM), meaning that the arm was submitted to the elastic load but not the object. In contrast in the second condition, the elastic cord was attached to the object (OBJECT), so that both the arm and the object were submitted to the elastic load. The length of the cords was adjusted so as to equalize the magnitude of the elastic load in ARM and OBJECT. In the third condition, the elastic cord was removed (NO ELAST), so that no elastic load was applied on the object or on the arm. The second experimental factor (ZONE) was determined by the location in space where the movement was performed. Two sets of targets were used (see Fig. 1). When the participant performed the task between the first set of targets (zone Z1), the average elastic load was about 9 N (range 5–13 N). When the task was performed between the second set of targets (zone Z2), the average elastic load was about 13 N (range 9–17 N). The overlap between Z1 and Z2 was 10 cm. When the participant switched from one zone to the other, the chair was displaced by 10 cm so as to preserve a constant posture of the arm.
In order to estimate the effects of our experimental manipulations on the net muscle torque required at the shoulder level, a two-link inverse dynamic model has been developed (for more details see Appendix in Descoins et al. 2006). This model demonstrated that, because the movement was performed with the hand and arm in front of the longitudinal plane through the shoulder, the effect of the gravitational forces acting on the two arm segments was to pull the arm in. In contrast, the force created by the elastic cord (when being attached to the arm or to the object) was oriented in the opposite direction (pulling the arm outward). Overall, the presence of the elastic force field reduced the magnitude of the required net muscle torque at the shoulder.
Each participant performed a block of five trials in each of the six (3 TYPES × 2 ZONES) experimental conditions. The order of the blocks was pseudo-randomized and counterbalanced across participants. Prior to the experiment, each participant performed two practice trials (NO ELAST × Z1) to become familiarized with the task. Participants were neither suggested useful strategies nor were they given instructions regarding suitable normal forces (Ohki et al. 2002). Whenever necessary, resting periods were introduced to prevent possible effects of muscular fatigue. Overall the duration of the experiment was about one hour per participant.
Once the main experimental conditions described above had been completed, participants performed several post-experimental trials in order to evaluate the minimal grip force needed to hold the object. During these trials, the elastic cord was connected to the object and the elastic load was close to 6 N. Participants were asked to gradually release their grasp until the object slipped.
Data analysis
Several MATLAB routines were developed to analyze the data. The kinematic and kinetic signals were first filtered using a second-order dual-pass Butterworth digital filter with a low-pass cut-off frequency of 10 Hz. For the present purposes, grip force was defined as the sum of all finger forces. The total load force at the object–finger interface (LFO) was computed as the absolute value of the algebraic sum of the elastic (EL) and inertial loads (IL), that is LFO = ABS (EL + IL). Figure 2 provides examples for each experimental condition. During ARM and NO ELAST trials, EL was always equal to zero, but IL could be either positive or negative depending on the phase of movement (see Fig. 2a). Because LFO is actually the absolute value of IL, LFO varies twice per cycle of movement in ARM and NO ELAST. During OBJECT trials, EL was always positive (pulling the object away from the participant), and much larger than IL (see Fig. 2b). When the object was close to the participant, EL was small and IL was positive. In contrast, when the object was far from the participant, EL was large but IL was negative. Overall, during OBJECT, LFO varies only once per cycle of movement (with an amplitude that is reduced as compared to EL).
Schematic drawing showing how total load force (LFO) relates to movement kinematics in each experimental condition: a without any elastic cord (NO ELAST) or with the elastic cord attached to the object (OBJECT); b with the elastic cord attached to the upper arm (ARM). EL elastic load (position dependent load), IL inertial load (acceleration dependent load)
For analysis purposes, each trial was segmented into cycles on the basis of the horizontal position signal. By definition, a cycle corresponded to a movement of the object performed toward the participant, followed by a movement away from the participant. Only the first 20 cycles of each trial were retained for further analysis. Thus, with 5 trials per condition, for each participant a total of 100 cycles was analysed for each experimental condition. The following dependent variables were extracted within each cycle. The amplitude of the horizontal and vertical displacement and the duration of each cycle served to evaluate whether participants abided by the task instructions. At the kinetic level, we computed the mean of LFO and grip force (GF) signals. To evaluate the strength of the coupling between grip and total load force, cross-correlations were computed between GF and LFO. When the cross-correlation was significant, the lag was kept for further analysis; a positive lag indicated that GF preceded LFO. The percentage of cycles that satisfied our criteria (i.e. a significant r value) was taken as an additional index of the coupling between GF and LFO. Before statistical analysis, each dependent variable was averaged over 100 values (100 cycles per condition and per participant).
The Fast Fourier Transform (FFT) method was applied to LFO and GF normalized signals (each cycle being normalized to 1,000 points). The rational was to compare the frequency contents of LFO signal to those of the GF signal. For each signal, we extracted the component at the frequency of movement (F1 corresponding to 1.2 Hz), and the component at twice the frequency of the movement (F2 corresponding to 2.4 Hz). The amplitude F1 and F2 for LFO and GF signals was the average of five values (one for each of the five trials in each experimental condition). In the NO ELAST and ARM conditions, given that fluctuations in load originated from fluctuations in inertial force, we expected a much larger component of LFO at F2 as compared to F1 (see Fig. 3b, c). In contrast, in the OBJECT condition, given the way fluctuations in inertial load are combined with fluctuations in elastic load (see earlier), a much larger component of LFO at F1 than at F2 was expected (see Fig. 3a). If predictive control is accurate in all experimental conditions we expect that GF and LFO spectral patterns demonstrate similar main frequency components. In other words, GF should vary predominantly at F1 in OBJECT, whereas in ARM and NO ELAST, GF should vary predominantly at F2. In case LFO spectral pattern includes both F1 and F2 as main frequency components, strong correlations between LFO and GF signals can only occur if the ratio between GF/LFO components at F1 remains close to the ratio at F2.
Mean power spectra for the LFO and GF signals in each experimental condition: a with the elastic cord attached to the object (OBJECT); b without the elastic cord (NO ELAST); c with the elastic cord attached to the upper arm (ARM). Data are pooled across zones. Data relative to the young and older participants are presented respectively by the left and right columns. Note that with no elastic cord present (NO ELAST and ARM), the total load applied at the object–finger interface (LFO) is characterized by a main frequency component at F2 (2.4 Hz). In the OBJECT condition, the main component of LFO is found at F1 (1.2 Hz)
In the post-experimental control trials, we sought to identify the minimal grip force (GFmin) necessary to prevent the object from slipping between the fingers. Initiation of slipping was determined with respect to the first time derivative of the accelerometer signal. We used a critical value of −1 g/s to determine whether the object was slipping or not (Danion 2004). The grip force at that specific value was taken as an estimate of GFmin. For each GFmin value, an individual estimate of the friction coefficient (μ) was obtained by dividing the load on the object by GFmin. For the YG, we found μ = 0.90 ± 0.10, a value comparable to those reported elsewhere for sandpaper (Burstedt et al. 1999; Gilles and Wing 2003; Danion 2004). For the EG, we found a statistically significant lower (P < 0.01) coefficient of friction (μ = 0.75 ± 0.14). Slightly higher values have been reported elsewhere for elderly participants (μ = 0.86 for sandpaper in Gilles and Wing 2003).
Statistical analysis
Three-way repeated measures analyses of variance (ANOVAs) were used as the main tool for statistical analysis of the data. Factors included AGE (young versus elderly), TYPE (ARM versus OBJECT versus NO ELAST conditions) and ZONE (Z1 versus Z2). Because coefficients of (cross) correlation do not follow a normal distribution, we used a logarithmic transformation (z-score) before conducting statistical procedures on this variable. Post hoc tests (Newman–Keuls) were used whenever a main effect of TYPE (or an interaction) was found. All tests were performed with P < 0.05 as significance criterion.
Results
Mean values (and inter-individual standard deviations) of the variables discussed in the subsequent sections are presented in Table 1 for all the experimental conditions. Results are presented in two sections. The first one deals with parameters (movement kinematics, LFO) that follow from the task requirements (frequency/amplitude of movement). The second section deals with the characteristics of grip force whose modulations were not prescribed by the task.
Task performance
Amplitude of movement
Elderly participants produced slightly larger amplitudes of movement than younger participants (22.3 ± 1.7 cm versus 21.1 ± 0.9 cm, respectively). This observation was corroborated by a main effect of the factor AGE (F (1,22) = 8.5; P < 0.01). The ANOVA also revealed a main effect of TYPE (F (2,44) = 11.7; P < 0.001) and an interaction between AGE and TYPE (F (2,44) = 5.9; P < 0.01). Post hoc analysis revealed similar amplitudes of movement under all experimental conditions for the YG. Compared to YG, EG realized a slightly larger amplitude in NO ELAST and ARM, increasing in the OBJECT condition (23.3 ± 1.6 cm). For the vertical movement component, amplitude was rather small as compared to horizontal component (1.6 cm versus 21.7 cm). However, as for horizontal amplitude, an ANOVA revealed a main effect of AGE (F (1,22) = 24.2; P < 0.001) such that EG performed larger vertical movements as compared to YG (2.2 ± 0.9 cm versus 1.0 ± 0.4 cm).
Cycle duration
The duration of a movement cycle did not vary over groups and experimental conditions. The ANOVA revealed no significant main effects of the factors AGE (F (1,22) = 0.5; ns), TYPE (F (2,44) = 0.70; ns) and ZONE (F (1,22) = 1.4; ns), nor any significant interactions. The average duration of a cycle was 829 ± 13 ms, close to the 833 ms cycle duration of the metronome.
Mean load force
Despite slight changes in the movement kinematics (amplitude), EG and YG experienced similar loads at the object site (see Fig. 4a): the ANOVA did not reveal a main effect of the factor AGE (F (1,22) = 0.9; ns). However, load varied over condition as revealed by a main effect of TYPE (F (2,44) = 30,699; P < 0.001). Mean LFO had similar values in NO ELAST (1.5 ± 0.1 N) and ARM (1.5 ± 0.1 N). Of course with the elastic cord attached to the object, mean LFO increased, reaching 11.2 ± 1.8 N. Similarly as expected, the factor ZONE affected LFO in the OBJECT condition only, reaching 9.5 ± 0.4 and 13.0 ± 0.4 N in Z1 and Z2, respectively. These latter observations were validated by a significant main effect of the factor ZONE (F (1,22) = 801; P < 0.001) and a significant TYPE × ZONE interaction (F (2,44) = 788; P < 0.001).
Means of the kinetic variables under the NO ELAST, OBJECT and ARM conditions for the YG (open symbols) and for EG (filled symbols), when executing the rhythmical task in zones Z1 (discontinuous line) and Z2 (continuous line): a mean load force at the object–finger interface (LFO); b mean grip force (GF). Error bars indicate inter-individual standard deviations
Spectral analysis of load force
As anticipated, spectral analysis of load force supported the view that LFO varied essentially at the movement frequency (F1) in OBJECT, whereas it varied primarily at twice the movement frequency (F2) in ARM and NO ELAST. Let us start with the amplitude of the F1 component in the LFO signal. Figure 5a presents this variable for the two groups in all the experimental conditions. The ANOVA revealed no statistical effects of AGE (main effect F (1,22) = 1.2; ns, no significant interactions). However, the amplitude of F1-LFO varied significantly across experimental conditions, as revealed by a significant main effect of the factor TYPE (F (2,44) = 51.2; P < 0.001). As was to be expected due to the presence of the elastic cord in the OBJECT condition (with elastic force varying at the frequency of movement), F1-LFO was larger in OBJECT (3.2 ± 1.2 N) as compared to NO ELAST (0.3 ± 0.2 N) and ARM (0.2 ± 0.1 N). Due to the presence of the elastic cord, a main effect of ZONE (F (1,22) = 389; P < 0.001) together with an interaction between ZONE and TYPE (F (2,44) = 185; P < 0.001) also appeared. Indeed, in the OBJECT condition, the amplitude of F1-LFO differed significantly across zones (2.7 ± 2.0 N in Z1 and 3.7 ± 2.0 N in Z2), whereas this was not the case in NO ELAST and ARM.
The pattern of results for the amplitude of the double frequency (F2) component of LFO (presented in Fig. 5b) was the inverse of that observed for the F1-LFO. Again, the ANOVA did not reveal an effect of AGE (main effect F (1,22) = 0.5; ns). Due to the interaction between inertial and elastic loads (see “Apparatus” in “Materials and methods”), values of F2-LFO were markedly reduced in OBJECT (0.6 ± 0.3 N) as compared to NO ELAST (1.8 ± 0.2 N) and ARM (1.8 ± 0.2 N). This observation was confirmed by a main effect of the factor TYPE (F (2,44) = 616; P < 0.001) and post hoc analyses.
Means of the variables extracted from the spectral analysis of the LFO signals under the NO ELAST, OBJECT and the ARM experimental conditions for the YG (open symbols) and for EG (filled symbols), when executing the rhythmical task in zones Z1 (discontinuous line) and Z2 (continuous line): a mean amplitude of the F1 component; b mean amplitude of the F2 component. Error bars indicate inter-individual standard deviations
Grip force
Mean grip force
As can be seen from Fig. 4b, participants from the group of elderly adults exerted a larger mean GF than participants from the group of younger adults. In addition to a main effect of AGE (F (1,22) = 9.1; P < 0.01), the ANOVA revealed significant main effects of the factors TYPE (F (2,44) = 211; P < 0.001) and ZONE (F (1,22) = 32.1; P < 0.001), as well as an interaction TYPE × ZONE (F (2,44) = 48.8; P < 0.001) and an interaction AGE × TYPE × ZONE (F (2,44) = 3.8; P < 0.05). Post hoc analysis of the effects of the factor TYPE indicated no significant differences between the conditions NO ELAST and ARM (9.8 ± 4.4 and 10.3 ± 4.3 N for YG; 18.3 ± 10.0 and 18.0 ± 7.1 N for EG, respectively). Mean GF was higher under the condition OBJECT, where it also varied as a function of ZONE. This influence of the factor ZONE was somewhat more pronounced for the YG than for the EG. For the YG, mean GF in the OBJECT condition reached 28.6 ± 5.7 N in Z1 and 40.0 ± 7.7 N in Z2, compared to 40.0 ± 13.3 N in Z1 and 46.1 ± 10.3 in Z2 for the EG.
GF–LFO coupling: percentage of cycle retained
Figure 6 presents the three indices that were used to characterize the relationship between GF and LFO. As shown in Fig. 6a, the percentage of cycles in which a significant GF–LFO cross-correlation appeared differed considerably across experimental conditions. The ANOVA revealed significant main effects of AGE (F (1,22) = 8.4; P < 0.01) and TYPE (F (2,44) = 34.3; P < 0.001) and a significant interaction between the two (F (2,44) = 8.1; P < 0.001). Post hoc analysis demonstrated that for both groups in OBJECT almost all cycles could be retained, with percentages reaching 98% and above. In contrast, under ARM and NO ELAST, the percentage of cycles with a significant LFO–GF cross-correlation was significantly lower in both groups. This decrease was more pronounced for the group of elderly participants (61 and 66%, for ARM and NO ELAST, respectively) as compared to the group of younger participants (respectively, 85 and 88%). There was no main effect of ZONE (F (1,22) = 0.7; ns), nor any interaction with this factor.
GF–LFO coupling: coefficients of correlation
Although calculated using only the cycles demonstrating a significant cross-correlation, the mean coefficients of cross-correlation (r) between GF and LFO were found to differ considerably across experimental conditions (see Fig. 6b). The ANOVA revealed significant main effects of TYPE (F (2,44) = 351; P < 0.001) and ZONE (F (1,22) = 57; P < 0.001) and a significant interaction between them (F (2,44) = 18.4; P < 0.001). Post hoc analysis demonstrated similar results in the NO ELAST (r = 0.48 ± 0.1) and ARM (r = 0.45 ± 0.09) conditions, with the magnitude of the coefficient not varying over zones. In the OBJECT condition, the coefficient of cross-correlation between GF and LFO increased, reaching 0.85 ± 0.06 in Z1 and 0.90 ± 0.05 in Z2. Although there was no main effect of AGE (F (1,22) = 1.1; ns), there was a significant AGE × TYPE interaction (F (2,44) = 5.2; P < 0.01). Post hoc analysis demonstrated several marginally significant differences. Elderly participants tended to have lower r-values as compared to young adults in NO ELAST (EG = 0.44, YG = 0.53, P = 0.09) and ARM (EG = 0.40, YG = 0.50; P = 0.08), whereas the inverse held in OBJECT (EG = 0.90, YG = 0.88; P = 0.09).
Mean indices characterizing the relationship between GF and LFO under NO ELAST, OBJECT and ARM experimental conditions for the YG (open symbols) and for EG (filled symbols): a mean percentage of cycles retained (i.e. cycles in which a significant cross-correlation was found between GF and LFO); b mean coefficient of correlation in the retained cycles; c mean lag in the retained cycles. A positive value indicates that GF precedes LFO. Error bars indicate standard error of the mean
GF–LFO coupling: lag
As can be seen in Fig. 6c, for all the experimental conditions the lag between GF and LFO was close to zero. However, it nevertheless varied over experimental conditions. Indeed, the ANOVA revealed a main effect of TYPE (F (2,44) = 23.9; P < 0.001). Post hoc analysis demonstrated that the GF–LFO lag was larger in OBJECT (45.7 ± 40.1 ms) than in either ARM (−12.9 ± 50.3 ms) or NO ELAST (−6.5 ± 42.8 ms). There was no main effect of AGE (F (1,22) = 1.7; ns), but there was a significant AGE × TYPE interaction (F (2,44) = 3.4; P < 0.05). Post hoc analysis demonstrated that while both groups demonstrated a comparable lag in OBJECT (YG = 35.8 ms, EG = 55.6 ms), this was not the case in ARM and NO ELAST (P < 0.05). In these latter two experimental conditions GF adjustments in the group of older participants were delayed by about 30 ms relative to the group of young adults (see Fig. 6c). There was no main effect of ZONE (F (1,22) = 1.4; ns), nor any interaction with this factor.
Spectral analyses of GF
In this section we explore the frequency content of the GF (and LFO) signals, in order to understand the reasons underlying the appearance of factually low correlation coefficients under the NO ELAST and ARM conditions, as compared to the OBJECT condition. With frequency normalized across participants so as to have 1 correspond to the frequency of movement (F1), the modulations in load and grip forces as evoked by the different experimental conditions can be analysed in more depth.
A condition sine qua non for a high correlation between two signals is that their spectral pattern must carry the same fundamental frequencies, and in similar proportions (see “Materials and methods”). As can be seen in Fig. 3a, in the OBJECT condition load varied primarily at the frequency of movement for both groups. Grip force also demonstrated a larger contribution of the F1 component (4.4 ± 1.9 N for YG and 8.9 ± 3.6 N for EG) than of the F2 component (1.0 ± 0.6 N for YG and 0.7 ± 0.4 N for EG), thus fulfilling the necessary (but not sufficient) condition mentioned. As clearly shown in Fig. 6b, the OBJECT condition gave rise to a high correlation between LFO and GF for both groups (0.88 for YG and 0.90 for EG). In contrast under the NO ELAST and ARM conditions, this correlation fell to 0.5 for YG and 0.4 for EG (see Fig. 6b). Inspection of Fig. 3b revealed that in NO ELAST LFO varied predominantly at F2. Yet, GF varied more at F1 than at F2, thereby not fulfilling the necessary condition. Note that this dominating contribution of the F1 component was actually more pronounced in older participants (F1 = 4.0 ± 1.1 N and F2 = 1.5 ± 0.9 N) than in younger participants (F1 = 1.4 ± 0.9 N and F2 = 1.1 ± 0.6 N). Inspection of the ARM condition (Fig. 3c) reveals the same pattern of results.
At a more general level, a main effect of AGE was observed at F1 (F (1,22) = 84; P < 0.001) and F2 frequency components (F (1,22) = 8.5; P < 0.01). In both cases, GF modulations were larger in older participants (F1 = 5.9 ± 3.1 N and F2 = 1.7 ± 1.1 N) as compared to younger ones (F1 = 2.4 ± 1.9 and F2 = 0.9 ± 0.5 N). Expressed as a percentage, the overall increase in GF modulations ranged from 88% at F1 to 146% at F2.
Mean ratio of the amplitude of GF over LFO under NO ELAST, OBJECT and ARM experimental at movement frequency F1 (filled symbols) and F2 (open symbols). Panels a and b refer, respectively, to YG and EG. Data are pooled across zones. Error bars indicate standard error of the mean. Note that all participants exhibited a different ratio at F1 and F2, except under OBJECT
In order to test the similarity (or dissimilarity) between GF and LFO spectral patterns, we computed the ratio between GF and LFO components at each frequency. Our rational was that if participants exhibit a similar ratio at F1 and F2, this means that the corresponding GF and LFO spectral patterns differ only by a scaling factor, thereby preserving the possibility to reach a high correlation between GF and LFO. Figure 7 presents the average ratio in each experimental condition for the young (panel a) and elderly group (panel b). A three-way ANOVA (AGE × TYPE × FREQ) revealed a main effect of TYPE (F (2,44) = 11.1; P < 0.001), FREQ (F (1,22) = 47.5; P < 0.001), as well as an interaction between them (F (2,44) = 36.8; P < 0.001). Post hoc analysis demonstrated that, in OBJECT, the ratio of GF over LFO was not significantly different at F1 and F2 (2.6 versus 3.6; ns), whereas in both ARM and NO ELAST, this ratio was found to be much larger at F1 compared to F2 (respectively 13.6 and 0.6; P < 0.001). Finally, we observed a marginal interaction between AGE, TYPE, and FREQ (F (2,44) = 2.9; P = 0.06), consistent with the view that in ARM and NO ELAST the discrepancy between GF/LFO ratios at F1 and F2 was greater in older participants (F1 = 17.7, F2 = 0.8) than in younger ones (F1 = 9.5, F2 = 0.5).
Discussion
The goal of this study was to examine the possible effects of aging on the predictive control of grip force during object manipulation under various external force fields. In the general framework of the present study, we can distinguish three types of results. First, the results that replicate earlier observations obtained with elderly participants, such as the larger grip force employed by elderly as compared to young adults. Second, the results that extend the conclusions formulated in our previous study (Descoins et al. 2006) to the elderly population. Two results fall into this category: (1) grip force modulations in elderly were not affected by the presence of the elastic cord attached to the arm (ARM versus NO ELAST), (2) the grip to load force coupling, as quantified by correlation coefficients, was weaker under ARM and NO ELAST compared to OBJECT. Finally, three results should be considered as novel findings: (1) grip force modulations were larger in elderly adults as compared to young adults; (2) under ARM and NO ELAST the lag between the grip and load force signal was different in young and elderly adults; (3) when the load varied at twice the frequency of movement (NO ELAST and ARM), the grip force profile demonstrated the persistence of a movement-frequency component. Let us now discuss these results in more detail and consider their implications with respect to the questions formulated in the “Introduction”.
Excessive grip force in elderly
A clear finding in this study was that elderly participants deployed a considerably larger grip force when moving the object than young participants. Expressed as a percentage, the average supplement of grip force across all conditions corresponded to 63%, reaching 80% in the ARM and NO ELAST conditions. Even though our experimental paradigm was rather different from those used in earlier studies, these percentages remain within the range of values reported in the literature (+143% in Cole 1991; +86% in Kinoshita and Francis 1996; +35–73% in Cole et al. 1999; +14% in Lowe 2001; +84% in Cole and Rotella 2001; +20% in Gilles and Wing 2003), thereby suggesting that excessive grip force in elderly is a robust phenomena. Skin slipperiness was found to increase with age, as in most of the earlier studies (except in Cole and Rotella 2001), but this contribution cannot, by itself, account for the entire change in grip force. For instance, in the present experiment the change in skin slipperiness approximated 20%, whereas the overall change in grip force was more than three times larger. Rather similar conclusions can be drawn from the reports of Cole (1991, 2001), Cole and al. (1999), and Kinoshita and Francis (1996). Overall, the origin of excessive grip force is still under debate. When the tactile sensitivity is reduced (Cole et al. 1998), the capability to detect a slipping of the object is also altered (Cole and Rotella 2001), and maintaining a larger safety margin could be considered as a wise strategy (Kinoshita and Francis 1996). Alternatively, excessive grip force could help compensating for the reduced sensitivity of the finger (Cole 1991) by providing a stronger afferent signal (via stronger compression of the finger).
Global effects of aging on the predictive control of grip force
The analysis of the lag between GF and LFO revealed that grip force adjustments occur simultaneously with (or in the vicinity of) movement-induced fluctuations in object load. This observation is consistent with many earlier reports (Johansson and Cole 1992; Flanagan and Wing 1995; Blakemore et al. 1998; Flanagan et al. 2003) and confirms the existence of a predictive mechanism; note that even when GF lags behind LFO by about 30 ms, a reactive mode of control cannot be suspected because sensory feedback loops take at least 60 ms (Cole and Abbs 1988). Overall, the value of this lag was identical for the two groups under OBJECT condition, but not under the ARM and NO ELAST conditions. In these last conditions, GF preceded LFO by 7 ms in young adults, whereas GF lagged behind LFO by 25 ms in elderly. This observation contrasts with the report of Gilles and Wing (2003) who found no consistent changes in the temporal coupling of GF and LFO under an inertial load. However, their evaluation of the lag focused on specific events in the signals, such as the times of GF and LFO onsets and maxima, whereas in the present study the cross-correlation technique included the full GF and LFO signals. From our point of view, there is no obvious reason to believe that the predictive control of grip force exists only at LFO onsets or maxima, thereby making unclear why lag evaluation should be performed on such a small fraction of the data. Overall, this comparison across studies suggests that our method is more powerful in discriminating older participants from younger ones. Interestingly, using a method very similar to ours, Blank et al. (2001) observed a similar trend between children (3- to 6-year) and young adults that were asked to shake objects at intermediate frequencies (0.6–1 Hz). The delay between GF and LFO was virtually zero in children, whereas GF preceded LFO by 16 ms in adults. Development thus appears to influence the timing on GF modulation. Finally, coming back to the elderly participants, it is worth noting that anticipatory postural adjustments associated with fast voluntary arm movements are delayed in elderly by about 60 ms (Bleuse et al. 2006). All together, this second comparison suggests that the effect of aging could lead to similar deteriorations in the predictive control of posture and grip force.
Although both groups synchronized their grip force modulations with the load force fluctuations, elderly participants exhibited significantly larger grip force modulations as compared to young participants. As can be seen in Table 1, this was true for each experimental condition, and for each frequency component. Averaged across all conditions grip force modulations were about twice as large in elderly participants as compared to young adults (+146% at F1, and +88% at F2). Overall, this means that, even if elderly participants use an excessively high safety margin, they still modulate their grip force with respect to load force fluctuations, thereby suggesting that their control of grip force remains effective. This view is coherent with the study of Lowe (2001), who compared the grip force profiles of young and older adults during a rhythmical pulling task. Indeed, when the amplitude of grip force modulation was normalized to the maximum grip force (i.e. GF/GFmax), no significant differences were found between the two groups. One possibility of accounting for these observations would be that grip force modulations are scaled with respect to background grip force. However, experimental data collected by Flanagan and Wing (1995) suggest that this scheme is incorrect. Indeed, when participants are asked to increase their baseline grip force, the counter part is that grip force modulations decrease in terms of amplitude. An alternative option would be to consider that older participants use larger grip force modulations so as to compensate for their reduced tactile sensitivity. However, experimental data collected by Nowak and Hermsdorfer (2003) suggest that this scheme is also incorrect. Indeed, following cooling of the fingertips, participants increased their baseline grip force, but this was not accompanied by larger grip force modulations (see the example provided in their Fig. 2). At this stage it remains unclear why our elderly participants exhibit larger grip force modulations.
Functional separation of external forces
A subsidiary goal of the present study was to determine whether elderly participants could effectively separate the external forces acting at the object site and those acting on the moving arm only. To address this issue we proposed to investigate grip force modulations when external forces acting on the arm and the object are experimentally dissociated. In both the NO ELAST and ARM conditions, the fluctuations in the total load at the object–finger interface resulted from the variations in the acceleration of the object. However, an important difference between these conditions was that the arm movement was further constrained by an elastic cord in the ARM condition. Despite this salient difference, results showed that behaviour was very similar under both conditions. First, the pattern movement was hardly affected by the elastic constraint on the arm, since we found no differences in terms of the resulting LFO (average values and frequency contents). Second, even when confronted with different magnitudes of the elastic force (Z1 versus Z2), average GF as well as its frequency content remained comparable in ARM and NO ELAST. Third, no significant changes were observed when we analyzed the phase lag, and the correlation between GF and LFO. Overall, these results demonstrate that all participants continued to adequately anticipate the load force at the object–finger interface under the force dissociation protocol. With respect to the population of young adults, these results are consistent with the study of White et al. (2005), and demonstrate that more rigorous analyses such as spectral analyses of the GF and LFO signals (not reported previously) do not alter our earlier observations (Descoins et al. 2006). All together, we conclude that the neural processes involved in the prediction of the arm trajectory and those involved in the prediction of the object load can take into account different external force fields, and that this is the case for the group of young adults as well as the group of older adults.
Differential accommodation of inertial and elastic loads
In the presence of an elastic load varying at the frequency of movement (i.e., in the OBJECT condition), grip force and load force co-varied in a linear fashion, as revealed by the relatively high cross-correlation coefficients (0.88 in YG and 0.90 in EG). Under this condition the modulations in the GF signal preceded the variation in load by 30–40 ms in both the younger and older adults. With the load varying predominantly at the frequency of movement (F1), both younger and older participants were able to efficiently modulate their grip force with respect to the upcoming variations in load force. This view is corroborated by a high percentage of cycle being retained (98%), as well as high cross-correlation coefficients between grip and load forces (R > 0.88).
In the NO ELAST and ARM conditions, the grip-to-load force coupling was less efficient, as demonstrated by a lower percentage of cycle retained (64% for the young adults and 87% for the elderly) and by lower correlation coefficients between grip and load forces (on the order of 0.5 for the young adults and 0.4 for the elderly). Although this lesser coupling under inertial load conditions is a recurrent observation (Gilles and Wing 2003; Descoins et al. 2006), the characteristics of the grip force signal underlying this factually poor correlation have not been addressed in the literature. We think that our spectral analyses of GF of LFO signals provide salient information to address this issue.
Because load depends on the absolute value of acceleration in the NO ELAST and ARM conditions, load varied predominantly at twice the frequency of movement (F2) under those conditions. Although the spectral analysis revealed a clear F2 component in the GF profiles of both the younger and older adults, an important result of the present study is the persistence of an unnecessarily large F1 component. Moreover, elderly participants revealed an enhanced F1 component as compared to younger participants. Thus, we can conclude that the presence of a considerable F1 component in the grip force profiles underlies the low correlations between grip and load force in the condition with a pure inertial load. Because they demonstrate an even stronger F1 component, elderly participants have an even lower correlation between grip and load force than young adults, when manipulating an inertial object.
What mechanisms could be responsible for this particular pattern of variation in grip force? One possibility is that when humans perform a rhythmical movement of the arm, due to a coupling phenomenon between neural oscillators (for a review see Kelso 1995) grip force modulations are naturally encouraged at the same frequency. As a result, when the environmental constraints lead LFO to vary at twice frequency of movement frequency (ARM and NO ELAST), these spontaneous GF oscillations could perturb the GF–LFO coupling. However, when the environmental constraints lead LFO to vary predominantly at the movement frequency (OBJECT), these spontaneous GF oscillations are reinforced. Elderly participants have been reported to experience more difficulty in maintaining non-preferred patterns during two-limb coordination tasks (Serrien et al. 2000; Wishart et al. 2000). This scheme could therefore account for the stronger tendency of elderly to exhibit grip force modulation at F1 in our task. Obviously at this stage, our scheme is just a proposition, and needs to be further tested with other types of load varying at the twice the frequency of movement (ex: viscous load). Moreover, the influence of the mean total load at the object–finger interface should also be investigated because it was almost 10 times larger in OBJECT as compared to ARM and NO ELAST (see Fig. 4a).
Concluding comments
The goal of this experiment was to explore the contribution of neural factors that could account for a decline in the ability of elderly people to actively manipulate objects. Specifically, we investigated whether the ability of elderly to predict the mechanical consequences of their ongoing actions was preserved under various external force fields. We addressed this issue by monitoring the co-variation between the load force resulting from movement, and the grip force produced. Overall, the present set of data demonstrated that, although elderly participants on average apply an unnecessarily large amount of grip force, they continue to modulate their grip force in a feedforward manner (i.e., through a predictive control mechanism) as a function of the load force exerted at the object–finger interface (see also Gilles and Wing 2003). However, while this predictive control successfully accommodated different force fields acting on the arm and on the object, it appeared less able to accommodate an external load varying at twice the frequency of movement, as demonstrated by both weaker grip to load force coupling and delayed grip force modulations in the ARM and NO ELAST conditions. To account for this latter observation, we suggest that the contribution of spontaneous grip force modulations occurring at movement frequency increases with age. Although the origin of this phenomenon needs to be further addressed, the present contribution revealed that the processes involved in the predictive regulation of grip force are altered by aging (Cole and Rotella 2002).
References
Barry BK, Riek S, Carson RG. (2005) Muscle coordination in rapid force production by young and older adults. J Gerontology (med sci) 60A:232–240
Bemben MG, Massey BH, Bemben DA, Misner JE, Boileau RA (1996) Isometric intermittent endurance of four muscle groups in men aged 20–74 yr. Med Sci Sports Exerc 28:145–154
Blakemore SJ, Goodbody SJ, Wolpert DM (1998) Predicting the consequences of our own actions: The role of sensorimotor context estimation. J Neurosci 18:7511–7518
Blank R, Breitenbach A, Nitschke M, Heizer W, Letzgus S, Hermsdorfer J (2001) Human development of grip force modulation relating to cyclic movement-induced inertial loads. Exp Brain Res 138:193–199
Burstedt MKO, Flanagan RL, Johansson RS (1999) Control of grasp stability in humans under different frictional conditions during multi-digit manipulation. J Neurophysiol 82:2393–2405
Bleuse S, Cassim F, Blatt JL, Labyt E, Derambure P, Guieu JD, Defebvre L (2006) Effect of age on anticipatory postural adjustments in unilateral arm movement. Gait Posture 24:203–210
Cole KJ (1991) Grasp force control in older adults. J Mot Behav 23:251–258
Cole KJ, Abbs JH (1988) Grip force adjustments evoked by load force perturbations of a grasped object. J Neurophysiol 60:1513–1522
Cole KJ, Rotella DL (2001) Old age affects fingertip forces when restraining an unpredictably loaded object. Exp Brain Res 136:535–542
Cole KJ, Rotella DL (2002) Old age impairs the use of arbitrary visual cues for predictive control of fingertip forces during grasp. Exp Brain Res 143:35–41
Cole KJ, Rotella DL, Harper JG (1998) Tactile impairments cannot explain the effect of age on a grasp and lift task. Exp Brain Res 121:263–269
Cole KJ, Rotella DL, Harper JG (1999) Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J Neurosci 19:3238–3247
Danion F (2004) How dependent are grip force and arm actions during holding an object? Exp Brain Res 158:109–119
De Guzman GC, Kelso JA (1991) Multifrequency behavioral patterns and the phase attractive circle map. Biol Cybern 64:485–495
Descoins M, Danion F, Bootsma RJ (2006) Predictive control of grip force when moving object with an elastic load applied on the arm. Exp Brain Res 172:331–342
Falconer J, Hughes SL, Naughton BJ, Singer R, Chang RW, Sinacore JM (1991) Self report and performance-based hand function tests as correlates of dependency in the elderly. J Am Geriatr Soc 39:695–699
Flanagan JR, Wing AM (1995) The stability of precision grip forces during cyclic arm movements with a hand-held load. Exp Brain Res 105:455–464
Flanagan RJ, Wing AM (1997) The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17:1519–1528
Flanagan JR, Vetter P, Johansson RS, Wolpert DM (2003) Prediction precedes control in motor learning. Curr Biol 13:146–150
Gilles MA, Wing AM (2003) Age-related changes in grip force and dynamics of hand movement. J Mot Behav 35:79–85
Guiard Y (1997) Fitts’ law in the discrete versus continuous paradigm. Hum Mov Sci 16:97–131
Johansson RS, Cole KJ (1992) Sensory-motor coordination during grasping and manipulative actions. Curr Opin Neurobiol 2:815–823
Kallman DA, Plato CC, Tobin JD (1990) The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol 45:82–88
Kelso JAS (1995) Dynamic patterns: the self-organization of brain and behavior. MIT, Bradford
Kenshalo DR (1986) Somesthetic sensitivity in young and elderly humans. J Gerontol 41:732–742
Kinoshita H, Francis PR (1996) A comparison of prehension force control in young and elderly individuals. Eur J Appl Physiol Occup Physiol 74:450–460
Lowe BD (2001) Precision grip force control of older and younger adults, revisited. J Occup Rehabil 11:267–279
Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002) Neurophysiological correlates of age-related changes in human motor function. Neurology 58:630–635
Mottet D, Bootsma RJ (1999) The dynamics of goal-directed rhythmical aiming. Biol Cybern 80:235–245
Nowak DA, Hermsdorfer J (2003) Digit cooling influences grasp efficiency during manipulative tasks. Eur J Appl Physiol 89:127–33
Ohki Y, Edin BB, Johansson RS (2002) Predictions specify reactive control of individual digits in manipulation. J Neurosci 22:600–610
Ranganathan VK, Siemionow V, Sahgal V, Yue GH (2001) Effects of aging on hand function. J Am Geriatr Soc 49:1478–1484
Serrien DJ, Swinnen SP, Stelmach GE (2000) Age-related deterioration of coordinated interlimb behavior. J Gerontol B (Psychol Sci Soc Sci) 55:295–303
Shinohara M, Scholz JP, Zatsiorsky VM, Latash ML (2004) Finger interaction during accurate multi-finger force production tasks in young and elderly persons. Exp Brain Res 156:282–292
Voelcker-Rehage C, Alberts JL (2005) Age-related changes in grasping force modulation. Exp Brain Res 166:61–70
Ward NS, Frackowiak RSJ (2003) Age-related changes in the neural correlates of motor performance. Brain 123:873–888
White O, McIntyre J, Augurelle AS, Thonnard JL (2005) Do novel gravitational environments alter the grip-force/load-force coupling at the fingertips? Exp Brain Res 163:324–334
Wishart LR, Lee TD, Murdoch JE, Hodges NJ (2000) Effects of aging on automatic and effortful processes in bimanual coordination. J Gerontol B (Psychol Sci Soc Sci) 55:85–94
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danion, F., Descoins, M. & Bootsma, R.J. Aging affects the predictive control of grip force during object manipulation. Exp Brain Res 180, 123–137 (2007). https://doi.org/10.1007/s00221-006-0846-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0846-3