Abstract
Rapid grip force responses to unexpected pulling loads on the fingertips are deteriorated in older adults due to, in part, age-related declines in somatosensory function. Such reports are limited to one-hand conditions despite the higher frequency of using two hands together in daily living activities of older adults. Unexpected perturbations during bimanual movements elicit goal-oriented and cortically-meditated bilateral rapid motor responses. Since aging is associated with declined somatosensory and cognitive functions, we hypothesized that bilateral rapid motor responses differ between young and older adults, such that older adults exert stronger grip forces following perturbation and the unperturbed hand is more involved in stabilizing the object in older adults. We tested our hypothesis by comparing the rapid grip force responses of both hands in young and older adults. A total of 13 right-handed young individuals (24.2 ± 4.0 years old, 5 men) and 13 right-handed older individuals (68.7 ± 7.1 years old, 5 men) were recruited. Tactile detection threshold, fingertip friction, and the rapid grip force responses of both hands triggered by unpredicted pulling loads during grip-lift movements were assessed. Older adults had higher tactile detection thresholds and lower fingertip friction compared to young adults. Regardless of age, rapid motor responses were found in both the perturbed (right) hand and the indirectly perturbed (left) hand at 73 ms and 135 ms after the perturbation, respectively, while magnitudes of the responses depended on perturbation magnitudes. Higher values in maximum grip force and maximum grip force rate were found in older adults as compared to young adults. In older adults, the indirectly perturbed (left) hand was more involved in stabilizing the object as compared to young healthy adults. The current study suggests that age-related changes in the peripheral and central nervous systems contribute to the greater involvement of the indirectly perturbed hand in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of physiological changes occur across the body as one ages. Generally, both sensory and motor function decline in parallel with aging. Specifically, declined somatosensory function, such as deteriorated tactile acuity (Stevens and Patterson 1995), is reported in older adults (Shaffer and Harrison 2007). As afferent feedback plays a critical role in successful object manipulation (Johansson and Flanagan 2009), it is not surprising that deteriorated tactile acuity coincides with declined fine motor skills in older adults (Tremblay et al. 2003).
The evidence base also indicates that motor responses such as rapid pinch force responses, which correspond to short-latency reflex responses, are highly reliant on afferent input and that these responses degrade with advanced age (Cole and Johansson 1993; Crevecoeur et al. 2017; Johansson et al. 1992a; Macefield and Johansson 2003). Grip force adaptations in response to unexpected load force are known to be affected when afferent input from cutaneous mechanoreceptors is blocked (Johansson et al. 1992b). Degraded rapid pinch force responses were seen in older adults, which may be associated with degraded afferent input and declined information processing speed due to age-related changes in cutaneous mechanoreceptors and cognitive function, respectively (Cole and Rotella 2001).
Effects of aging on fine motor skills have been investigated by evaluating control of precision grip using “passive” and “active” objects. “Passive” objects refer to objects that have stable physical properties and, therefore, predictable; “active” objects refer to objects that produce unexpected load force (Johansson and Cole 1994). Using “passive” objects, age-related changes in precision grip, such as higher grasping forces, larger safety margins, greater skin slipperiness (Cole 1991; Cole and Beck 1994; Gilles and Wing 2003), delayed object lifting (Kinoshita and Francis 1996), digit misalignment causing object roll (Parikh and Cole 2012), and larger digit-object contact areas (Gorniak and Alberts 2013) have been reported. Considering all of these age-related changes in precision grip, Gorniak and Alberts (2013) concluded that older adults have a higher risk of accidently dropping objects.
Some studies have examined the effects of aging on object manipulation of separate objects (Cole and Rotella 2001; Gorniak and Alberts 2013). Unfortunately, knowledge of the rapid pinch force responses in older adults is limited to one-hand conditions, which does not reflect the daily life of older adults (Clark et al. 1990; Kilbreath and Heard 2005). With respect to bimanual function, sudden load force increases in one finger elicits rapid twitch-like responses in the contralateral non-loaded finger (Ohki and Johansson 1999). However, recent investigations suggested that non-perturbed fingers can execute more sophisticated responses based on the goal of motor tasks. For example during motor tasks requiring coordination of the two arms, mechanical perturbations to one arm elicit rapid motor responses in both arms, suggesting motor responses are based on integration of sensory information from both arms (Diedrichsen 2007; Dimitriou et al. 2012; Mutha and Sainburg 2009). Thus, rapid bilateral motor responses appear to be goal-oriented and cortically-mediated (Mutha and Sainburg 2009).
As sensory feedback and its processing are reduced in older adults, rapid bimanual motor responses may differ from those in young adults. The purpose of the current study was to investigate the effects of age on motor responses elicited by an unexpected perturbation during a motor task requiring a coordination of two hands. We expected higher pinch forces and pinch force rates in older adults in the directly perturbed hand, in the line with the evidence base (Hypothesis #1) (Cole and Rotella 2001). We expected rapid motor responses in the indirectly perturbed hand as reported by other studies, with higher pinch forces and pinch force rates in older adults, possibly due to a greater mis-estimation of perturbation caused by deteriorated sensory feedback and poor processing information of sensory feedback as a result of declining cognitive function (Hypothesis #2) (Diedrichsen 2007; Dimitriou et al. 2012; Mutha and Sainburg 2009). Lastly, as the bilateral motor response is a cortically meditated response, we expected age-related differences in phases broadly corresponding to long-latency (60–120 ms post-perturbation) and volitional responses (120–180 ms post-perturbation) since a cortical contribution is reported in these responses (Hypothesis #3) (Crevecoeur et al. 2017; Macefield and Johansson 2003; Pruszynski 2014).
Methods
Subjects
Thirteen (13) healthy young individuals (24.2 ± 4.0 years old: five men and eight women) and 13 older individual (68.7 ± 7.1 years old: five men and eight women) were recruited. Handedness was determined using Edinburgh Handedness Inventory (Oldfield 1971), ranging from a laterality quotient (LQ) of − 100 (very strong left-hand dominance) to + 100 (very strong right-hand dominance). All subjects were strongly right-handed individuals (Young LQ average: 86.2, Older LQ average: 94.4) and had no previous history of health conditions or traumas affecting manual sensorimotor function such as: hand arthritis, carpal tunnel syndrome, cubital tunnel syndrome, muscle wasting, stroke, cerebral palsy, Parkinson’s disease, Huntington’s disease, multiple sclerosis, muscle dystrophy, brachial plexus injury, dementia, Type 1 or Type 2 diabetes, amputation, Alzheimer’s disease, or peripheral neuropathy. All participants gave informed consent according to the procedures approved by the Institutional Review Board of the University of Houston.
Experimental apparatuses
Grip device
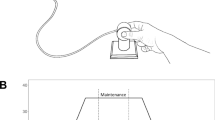
A grip device shown in Fig. 1 was created to determine force characteristics of rapid bimanual motor responses following an unexpected perturbation. The device was designed to have a symmetrical mass distribution with the center of mass located at the mid-point of the device. The device’s length, height, and width were 30 cm, 8.5 cm, and 10 cm, respectively. Four identical six-component force-moment transducers (nano25 Transducers: ATI Industrial Automation, Garner, NC, USA) were attached firmly on the device to record grip force (aka: normal force), load force, and torque exerted by thumb and index finger of both hands. The contact surfaces for the fingers were covered with 320-grit sandpaper to increase the coefficient of friction between the surfaces of the transducers and the fingers. The distance between the two contact surfaces for thumb and index finger was 5.5 cm. A bubble level was placed in the middle of device. A string was attached on each semicircular loop. The string attached on the right semicircular loop was connected to a perturbation system. The left-side string had a weight to equalize mass distribution when the object was not perturbed. The total weight of the system was 550 g. The device was placed on the top of a container incorporating the perturbation system.
Perturbation system
A motorized perturbation system was developed to deliver pulling loads to the right side of the grip device, as viewed by study participants. The perturbation system consisted of a 12 V DC motor (OT366: Kohree, Charlotte, NC, USA) controlled by a microcontroller (Arduino Uno: Arduino, Somerville, MA, USA) via a motor driver module (BTS7960: HiLetgo, Shenzhen, Guangdong, China). The DC motor had a stall torque of 0.6 N·m and a no-load speed of 120 rpm, and the motor driver module had a maximum continuous output current of 43 A continuous and maximum supply voltage of 12 V. The DC motor was secured on a metallic base affixed to a table. The perturbation system delivered via two different step-shaped pulling loads: 12 N and 8 N. The pulling loads were modulated by controlling the duty cycle of pulse width modulation (100% and 75% duty cycles for 12 N load and 8 N load, respectively). The pulling loads lasted for 500 ms (Crevecoeur et al. 2017). The perturbation system was hidden by a containment unit; this containment unit served as the resting surface for the grip device.
Experimental procedures
Tactile sensory evaluation
The threshold of tactile sensation detection of the hands was measured using Semmes–Weinstein Monofilament Test (Touch Test Sensory Evaluator: North Coast Medical Inc, Morgan Hill, CA, USA). The measuring sites included: the distal pad of right-hand index finger (RI), the distal pad of right-hand thumb (RT), the distal pad of left-hand index finger (LI), and the distal pad of left-hand thumb (LT). Subjects were instructed to respond when they registered a stimulus on the finger pads. The threshold of tactile registration was determined using a descending procedure where the force of the monofilament becomes smaller as subjects successfully recognized the stimulus three consecutive times. The smallest force that was recognized three consecutive times was recorded.
Object slip task
An object slip task was performed to measure the coefficient of friction (COF) between fingertips of the right hand and the transducers (Johansson and Westling 1984; Khan and Gorniak 2016; Thames and Gorniak 2017). Subjects were instructed to pinch and lift an object which consisted of two identical six-component force-moment transducers. Two reflective markers (6.4 mm Reflective Markers: B&L Engineering, Santa Ana, CA, USA) were attached on the top of the object to track the object position with a 12-camera motion capture system (VICON, Yarnton, Oxford, UK). Subjects were asked to slowly separate their fingers and let the object slip from their hand. The COF was determined by quantifying the ratio of load force and grip force at the moment of object slip onset. The moment of object slip onset was defined as the moment that the acceleration of the object exceeded 3% of maximal acceleration. Subjects performed the object slip task three times and the average of COF was quantified.
Manual evaluation
Subjects sat on a height-adjustable chair in front of the desk supporting the perturbation system with containment unit and the grip device atop the containment unit. The postural configuration during the seated task included: shoulders abducted 15°, flexed 45°, elbows flexed 90°, and hands rested on starting positions marked on the container. The experimental apparatus was placed as the bubble level on the grip device was aligned with the subject’s midline. Subjects were instructed to perform a grip-lift movement using thumbs and index fingers of both hands. The main objective of this movement was to keep the device level while holding. Subjects were instructed to grasp the surfaces of the transducers using pinch grips (thumbs and index fingers only) after the first auditory cue and lift the device approximately 8 cm above from the top of the container at a self-determined lifting speed. Subjects were instructed to maintain the device level during the holding phase by monitoring the bubble level. The device was held in the same position until the second auditory cue, which occurred 15 s after the first auditory cue. Although grip and load forces can reach stable values (a static phase) one second after the onset of movement in young adults (Johansson and Westling 1984), we set the holding duration for 15 s to provide adequate time for older adults to stabilize their grip and load forces. Subjects repositioned their hands to the starting position once the device was rested back on top of the container. No contact was permitted prior to the trial onset. Recruiting the other three fingers of either hand during the task was prohibited. Subjects performed 10 sets of 20 trials each (200 trials in total). There was a minimum of 5 s rest intervals between trials and minimum 3 min rest intervals between sets. Participants were reminded of the main objective of the movement at the beginning of each set.
On random trials, a mechanical perturbation was applied to the grip device by activating the perturbation system in a time window corresponding to 5th–13th second of holding period. The puling load inducing the perturbation was arbitrary chosen. There were three randomly assigned perturbation trials in each set; there was a total of 30 perturbation trials (15 trials for 12 N load and 15 trials for 8 N load). The total perturbation trials were limited to 30 trials in order to prevent anticipation. The mechanical perturbation was not delivered in the first 5 s of 15-s holding period to allow subjects to stabilize the grip and load forces and the object. In order to eliminate anticipation effects, we delivered the mechanical perturbation between 5 to 13th seconds of the trial rather than at a certain time.
Data processing and analyses
The transducer signals were amplified and multiplexed using ATI hardware before routed to analog-to-digital converter (via two cDAQ-9174 chassis and four NI-9205 input modules: National Instruments, Austin, Texas, USA). Using a customized LabVIEW (National Instruments) program, grip forces, load forces, and torques were acquired at 1000 Hz. Data were processed with a customized MATLAB (Natick, Massachusetts, USA) program. The data were low-pass filtered at 10 Hz using a 2nd order zero-lag Butterworth filter (Ochoa et al. 2016; Ochoa and Gorniak 2014). The magnitude of grip forces exerted from each hand were defined as the average of normal forces applied to each transducer with thumb and index finger ([|Zi| +|Zth|]/2). The load force (LF) on each hand was calculated as the sum of the tangential force measured at each fingertip (Li + Lth). The tangential force to each grasping surface was quantified as follow: ([F2x + F2y]1/2), where Fx and Fy are the force component of x axis and y axis, respectively. The torque force (TF) on each hand was computed by summing the torque force measured at each fingertip (Ti + Tth). The torque force at the fingertip was determined using the following equation: Tz – (Fy × Px – Fx × Py), where Tz is a torque component of z axis, Px and Py are the center of normal force application such that Px = − Ty/Fz and Py = Tx/Fz, respectively (Kinoshita, Backstrom, Flanagan, and Johansson, 1997). Fz is a force component of z axis, and Tx and Ty are torque components of the x and y axes, respectively. Slip force (SF), which is considered as the minimal normal force required to prevent object slip, of each hand was quantified by summing the estimated SF of each fingertip (SFi + SFth). SF was estimated using the regression model proposed by Kinoshita et al. (1997), which estimate a reasonable SF without measuring torsional slips. The equation estimating the SF was as follows: SF = LF + a−1 × |TF|+ b × Ft × |TF|, where a = 0.133/mm and b = − 0.011/Nmm. The estimated SFs from each fingertip was summed to quantify the SF of each hand.
Kinetic data from the perturbation trials were analyzed. Specifically, the region of interest was set as 100 ms before and 1300 ms after the onset of perturbation. The onset of perturbation was defined as the time that the stabilized SF of the right hand increased by 5%. The following SF data were quantified from both hands: (1) pre-perturbation SF (mean SF before the onset of perturbation; Pre SF), (2) post-perturbation SF (maximal SF after the onset of perturbation; Post SF), and (3) peak SF rate (the maximal value of the first derivative of SF; PSF rate). As SF is an estimated value based on measured load and torque forces and the two forces interact with each other, the following LF and TF data were also determined from both hands: (1) pre-perturbation LF (mean LF before the onset of perturbation; Pre LF), (2) post-perturbation LF (the LF corresponding to the time of the maximal SF; Post LF), (3) pre-perturbation TF (mean TF before the onset of perturbation; Pre TF), and (4) post-perturbation TF (the TF corresponding to the time of the maximal SF; Post TF). The following grip force data were quantified from both hands: (1) maximal grip force (MGF), (2) ΔGF (maximal grip force subtracted by the mean stabilized grip force prior to the onset of perturbation), (3) peak grip force rate (the maximal value of the first derivative of grip force; PGF rate), and (4) maximal grip/slip forces ratio (G/S ratio).
Additionally, the onset of rapid grip force (latency) was determined as a temporal analysis of grip force by subtracting the time of rapid grip force onset from the time of perturbation onset. The onset of rapid grip force was defined as the time that the stabilized grip force of each hand increased by 5%. For a better understanding of equivalence between the right hand and left hand, the ratio between the left and right hands from the kinetic data was calculated to highlight to what extent left hand was recruited relative to right hand (L/R ratio). In order to characterize the grip force during the rapid motor responses, we determined maximal grip force and PGF rate at the following time windows: (1) 30 ms prior to perturbation (T1), (2) 1–30 ms after the onset of perturbation (T2), (3) 31–60 ms after the onset of perturbation (T3), (4) 61–90 ms after the onset of perturbation (T4), (5) 91–120 ms after the onset of perturbation (T5), (6) 121–150 ms after the onset of perturbation (T6), and (7) 151–180 ms after the onset of perturbation (T7) (Crevecoeur et al. 2017). The T3 interval corresponds to the period of short-latency response, while T4 and T5 correspond to the period of long-latency response and T6 and T7 correspond to the period of volitional movement, respectively (Macefield and Johansson 2003).
Statistical analyses
Data are shown as mean ± standard deviation unless otherwise stated. Normality was examined using the Shapiro–Wilk test. Due to the non-normal distribution data in the tactile threshold evaluation, Mann–Whitney U test was performed to determine age differences in each evaluation site (RT, RI, LT, LI). An independent t test was performed to evaluate effects of aging on COF. Three-way mixed-design ANOVAs (MD ANOVA) with AGE (young and older adults), PERTURABTION (12 N and 8 N), TIME (Pre SF and Post SF; Pre LF and Post LF; Pre TF and Post TF) were performed to evaluate (1) SF, (2) LF, and (3) TF before and after the perturbation. Three-way MD ANOVAs were performed to evaluate the effects of AGE, HAND (Right Hand and Left Hand), and PERTURBATION on (1) PSF rate, (2) Latency, (3) MGF, (4) ΔGF, (5) PGF rate, (6) G/S ratio. A two-way MD ANOVA was performed to evaluate the effects of AGE and PERTURBATION on L/R ratio. Four-way MD ANOVAs were performed to evaluate the effects of AGE, HAND, PERTURBATION, and TIME (T1, T2, T3, T4, T5, T6, and T7) on (1) MGF and (2) PGF rate. Mauchly’s test of sphericity was used to test the assumption of sphericity, and the Huynh–Feldt correction was used if the sphericity was violated. If a statistically significant interaction was found in MD ANOVAs, a Bonferroni correction was applied for follow-up analyses of main and interaction effects.
Results
Tactile sensory evaluation
Tactile sensory function was found to differ significantly between groups for the RT (U = 38.0, z = − 2.75, p < 0.01), RI (U = 45.5, z = − 2.32, p < 0.05), and LT locations (U = 45.0, z = − 2.51, p < 0.05), but not the LI location. Graphical representation of these data are in Fig. 2a.
Tactile sensory function and slip force data. a Group median tactile sensory function data for all 4 tested sites (RT, RI, LT, and LI). b Slip force (SF) of the right hand before perturbation and post perturbation; data shown for both groups, both perturbation conditions, and pre- and post-perturbation. c SF of the left hand before perturbation and post perturbation; data shown for both groups, both perturbation conditions, and pre- and post-perturbation. d Peak slip force rate (PSF rate) during the task; data shown for both groups, both hands, and both perturbation conditions
Coefficient of friction
The coefficient of friction was higher in young adults (1.12 ± 0.39) compared to older adults (0.71 ± 0.37), a statistically significant age difference of 0.42, t24 = 2.77, p < 0.05.
Overall kinetics
To evaluate kinetic characteristics before and after the perturbation, we specifically focused on grip forces at 100 ms before and 1300 ms after the onset of perturbation. As Fig. 3 shows, we observed increased grip force in both hands regardless of age after perturbation. This observation suggested that both hands were used in order to stabilize the active objects. While substantial SF increase was seen in the right hand, a subtle SF increase was also observed in the left hand. We first analyzed whether the SF after perturbation changed after perturbation in each hand. Additionally, we analyzed LF and TF before and after the perturbation since the SF is estimated value based on the measured LF and TF. For further analysis of rapid motor response following the perturbation, we analyzed the effects of age, hand, and perturbation magnitude on the kinetics.
Averaged force data showing the transition of right-hand grip force and left-hand grip force in young (solid gray) and older (solid black) adults. The transition of averaged slip force in young (dash gray) and older (dash black) adults is also shown. The grip force and slip force transitions are also separated based on the magnitude of delivered perturbation: 12 N (1st row) and 8 N (2nd row). The period delivering perturbation is indicated by two vertical light gray dotted lines
Slip force (SF) changes following perturbation and PSF rate
Overall, SFs following perturbations were higher compared to the SFs before the onset of perturbation. While profound SF increases were observed in the right hand, regardless of age and perturbation condition, the SF of the left hand also increased after the onset of perturbation. Although the magnitude of perturbation was higher on the right (direct) side, these outcomes indicated that the perturbation system induced perturbations to both hands.
We performed a three-way MD ANOVA with AGE, PERTURBATION, and TIME (Pre SF and Post SF) on SFs of each hand. We performed a separated statistical analysis for the right-hand SF and the left-hand SF because the primary interest of this statistical analysis was to determine whether the SFs increased after the perturbation in each hand rather than whether the magnitude of the changes in SFs were different between hands.
In the right hand, SF increased significantly after perturbation regardless of age. While no difference was found between young and older adults before the onset of perturbation, older adults had higher SF as compared to young adults after the onset of perturbation. A three-way MD ANOVA on the right-hand SF showed a significant TIME × AGE interaction (F1,24 = 9.4, p < 0.01) and a significant PERTURBATION × TIME interaction (F1,24 = 101.1, p < 0.001). The main effects of AGE, PERTURBATION, and TIME were significant (AGE: F1,24 = 9.3, p < 0.01, PERTURBATION: F1,24 = 120.3, p < 0.001, TIME: F1,24 = 159.7, p < 0.001). Given the significant TIME × AGE interaction and significant main effects of TIME and AGE, simple main effect analyses of TIME and AGE were performed. Simple main effects of TIME were found in both groups, with greater differences in older adults. The right-hand SF increased by ~ 200% in young adults (4.25 ± 0.59 to 12.62 ± 4.40) and ~ 326% in older adults (4.21 ± 0.59 to 17.94 ± 4.40). Simple main effect analyses of AGE demonstrated age differences only at post-perturbation (young vs. older: 12.62 ± 4.40 vs. 17.94 ± 4.40). Figure 2b shows the mean ± SD values of SF obtained from the right hand.
A three-way MD ANOVA on the left-hand SF demonstrated a significant TIME × AGE interaction (F1,24 = 17.1, p < 0.001) and a significant PERTURBATION × TIME interaction (F1,24 = 52.5, p < 0.001). The main effects of AGE, PERTURBATION, and TIME were significant (AGE: F1,24 = 14.0, p < 0.001, PERTURBATION: F1,24 = 46.9, p < 0.001, TIME: F1,24 = 92.3, p < 0.001). Since the three-way MD ANOVA found a significant TIME × AGE interaction and main effects of TIME and AGE, simple main effect analyses of TIME and AGE were performed. Similar to the finding of right-hand SF, we found a significant simple main effect of TIME in both groups, with a greater difference in older adults. The left-hand SF increased by ~ 22% in young adults (4.12 ± 0.35 to 5.03 ± 0.83) and ~ 56% in older adults (4.14 ± 0.35 to 6.42 ± 0.83). A simple main effect of AGE was found only after the perturbation (young vs. older: 5.03 ± 0.83 vs. 6.42 ± 0.83). Figure 2c shows the mean ± SD values of SF obtained from the left hand.
The analyses of SF changes before and after perturbation suggested that, regardless of age, SF increased in both hands. To better understanding of the effects of perturbation on SF, we analyzed the peak slip force rate (PSF rate). Our analysis found that the right-hand PSF rate is higher than the left-hand PSF rate, and the 12 N perturbation condition had higher PSF rate compared 8 N perturbation condition. These observations were independent from aging. A three-way MD ANOVA with AGE, PETURBATION, and HAND found a significant PERTURBATION × HAND interaction (F1,24 = 17.8, p < 0.001) and significant main effects of AGE, PERTURBATION, and HAND (AGE: F1,24 = 6.2, p < 0.05, PERTURBATION: F1,24 = 47.6, p < 0.001, HAND: F1,24 = 67.7, p < 0.001). As the three-way MD ANOVA found the significant PERTURBATION × HAND interaction and the main effects of PERTURBATION and HAND, simple main effect analyses of PERTURBATION and HAND were performed. The simple main effect analysis of PERTURBATION found significant differences between 12 and 8 N perturbation conditions regardless of hand (right hand 12 N vs. 8 N: 90.98 ± 37.62 vs. 69.48 ± 28.31, left hand 12 N vs. 8 N: 23.38 ± 8.58 vs. 17.74 ± 7.86). The simple main effect analysis of HAND found higher PSF rate in the right hand compared to the left hand regardless of perturbation conditions (12 N right vs. left: 90.98 ± 37.62 vs. 23.38 ± 8.58, 8 N right vs. left: 69.48 ± 28.31 vs. 17.74 ± 7.86). Figure 2d shows the mean ± SD values of PSF rate.
Load force (LF) and torque force (TF) changes following perturbation
Our statistical analyses revealed that SF changes after the perturbation. Since the SF is an estimated value based on the measured load forces and torque forces, we also evaluated how LF and TF changed following the perturbation in each hand. We performed separate three-way MD ANOVAs with AGE as a between-subject factor and PERTURBATION and TIME (Pre LF and Post LF, Pre TF and Post TF) as within-subject factors for each hand. In short, our analyses demonstrated that LFs and TFs following perturbation were higher than to the LFs and TFs before the onset of perturbation.
Three-way MD ANOVA with AGE, PERTURBATION, and TIME (Pre LF and Post LF) on LFs of each hand found only a significant main effect of TIME (right-hand LF: F1,24 = 46.3, p < 0.001, left-hand LF: F1,24 = 7.1, p < 0.05). No other main effects and interactions were significant in both hands. Figure 4a and b show the mean ± SD values of LF obtained from the right hand and the left hand, respectively.
Load force and torque force data. a Load force (LF) of the right hand before perturbation and post perturbation; data shown for both groups, both perturbation conditions, and pre- and post-perturbation. b LF of the left hand before perturbation and post perturbation; data shown for both groups, both perturbation conditions, and pre- and post-perturbation. c Torque force (TF) of the right hand before perturbation and post perturbation; data shown for both groups, both perturbation conditions, and pre- and post-perturbation. d TF of the left hand before perturbation and post perturbation; data shown for both groups, both perturbation conditions, and pre- and post-perturbation
Although small differences, three-way MD ANOVAs with AGE, PERTURBATION, and TIME (Pre TF and Post TF) on TFs of each hand found a significant main effect of TIME in both hands (right-hand TF change: F1,24 = 39.0, p < 0.001, left-hand TF change, F1,24 = 5.6, p < 0.05). No other main effects and interactions were significant in both hands. Figure 4c and d show the mean ± SD values of TF obtained from the right hand and the left hand, respectively.
Latency
Temporal analysis demonstrated that the latency was different between two hands, while this difference depends on the magnitude of perturbation force. A three-way MD ANOVA found a significant PERTURBATION × HAND interaction (F1,24 = 4.8, p < 0.05) and significant main effects of AGE, PERTURBATION, and HAND (AGE: F1,24 = 4.6, p < 0.05, PERURBATION: F1,24 = 24.8, p < 0.001, HAND: F1,24 = 219.5, p < 0.001). The significant PERTURBATION × HAND interaction suggests that latency difference between hands depends on the perturbation force. A post-hoc analysis of two-way interaction revealed significant simple main effects of HAND and PERTURBATION. A significant simple main effect of HAND was found in both perturbation conditions, while 12 N condition had slightly greater difference between the right hand and left hand (12 N Right Hand vs. Left Hand: 66.38 ± 18.61 vs. 132.13 ± 9.66, 8 N right hand vs. left hand: 80.22 ± 15.74 vs. 138.44 ± 9.80). A significant simple main effect of PERTURBATION was found in both hands while right hand had greater perturbation difference (right hand 12 N vs. 8 N: 66.38 ± 18.61 vs. 80.22 ± 15.74, left hand 12 N vs. 8 N: 132.13 ± 9.66 vs. 138.44 ± 9.80). Figure 5a shows the mean ± SD values of Latency; the main and interaction statistical results can be found in Table 1.
Latency and primary grip force data. a Latency of rapid grip force response; data shown for both groups, both hands, and both perturbation conditions. b Maximal grip force (MGF) produced during the task; data shown for both groups, both hands, and both perturbation conditions. c The change in grip force (ΔGF) produced after the perturbation; data shown for both groups, both hands, and both perturbation conditions. d PGF rate during the task; data shown for both groups, both hands, and both perturbation conditions
Maximal grip force (MGF), ΔGF, and peak grip force (PGF) rate
Overall, three-way MD ANOVAs indicate that the magnitude of the difference between the right hand and left hand becomes greater as the force of perturbation becomes stronger, but only in young adults. A significant AGE × HAND × PERTURBATION interaction was found along with a significant main effect of AGE, HAND, and PERTURBATION in MGF, ΔGF, and PGF rate (Table 1). Since there was a significant three-way interaction and our primary interest in the study was how bimanual rapid motor response differs between young and older adults, we performed post-hoc analyses in which we separated the young and older groups. The post-hoc analyses for the three-way interaction of all three measures demonstrated a significant simple HAND × PERTURBATION interaction only in young adults (MGF: F1,24 = 26.9, p < 0.001, ΔGF: F1,24 = 26.2, p < 0.001, PGF rate: F1,24 = 15.2, p < 0.001). These post-hoc analyses suggested that, in the young group, the magnitude of the difference between right and left hand in MGF, ΔGF, and PGF rate values became greater as the perturbation force became stronger [(MGF) 12 N right vs. left: 20.42 ± 5.59 vs. 10.86 ± 3.99, 8 N right vs. left: 16.66 ± 5.85 vs. 9.04 ± 3.48, (ΔGF) 12 N right vs. left: 15.02 ± 5.78 vs. 5.90 ± 3.80, 8 N right vs. left: 11.40 ± 5.86 vs. 4.18 ± 3.26, (PGF) 12 N right vs. left: 107.64 ± 43.15 vs. 45.84 ± 33.68, 8 N right vs. left: 76.09 ± 35.13 vs. 29.03 ± 19.58]. Figure 5b–d shows the mean ± SD values of MGF, ΔGF, and PGF rate; the main and interaction statistical results can be found in Table 1.
MGF and PGF rate L/R ratio
In order to better understand the equivalence of MGF and PGF rate between the right hand and the left hand, a ratio of left to right hand for MGF and PGF rate was calculated (L/R ratio). Values of L/R ratio ~ 1 indicate that the MGF and PGF rates are equivalent between the hands while values < 1 indicate higher values in the right hand.
In general, the analysis of the MGF L/R ratio suggested that the MGF generated by the left hand became more equivalent to the grip force generated by the right hand as the perturbation force became stronger in older adults. A two-way MD ANOVA for MGF L/R ratio showed a significant main effect of AGE and a significant AGE × PERTURBATION interaction. The statistical analysis implied that older adults demonstrated higher overall MGF L/R ratios; however, the magnitude of difference between young and older adults was unequal across the two perturbation forces. A post-hoc analysis of the two-way interaction revealed a simple main effect of AGE only in the 12 N condition (F1,24 = 15.8, p < 0.001). Figure 6a shows the mean ± SD values of MGF L/R ratio; the main and interaction statistical results can be found in Table 1.
Force ratio data. a MGF evaluated via a between hand ratio (left to right ratio, L/R ratio) during the task; data shown for both groups and both perturbation conditions. b PGF rate evaluated via a between hand ratio (left to right ratio, L/R ratio) during the task; data shown for both groups and both perturbation conditions. c The ratio between grip and slip forces produced; data shown for both groups, both hands, and both perturbation conditions. d The L/R ratio of grip and slip forces ratio during the task; data shown for both groups and both perturbation conditions
Similar to the description of MGF, the analysis of PGF rate L/R ratio suggested that the PGF rate of the left hand becomes more equivalent to the PGF rate of the right hand as the perturbation force became stronger in older adults. A two-way MD ANOVA demonstrated a significant main effect of AGE, PERTUBATION, and a significant AGE × PERTURBATION interaction. The significant two-way interaction suggested that the magnitude of difference between young and older adults in PGF rate L/R ratio were different between 12 and 8 N perturbation conditions. A post-hoc analysis of the two-way interaction revealed a simple main effect of AGE in both the 12 N and 8 N perturbation conditions with a different significance (12 N: F1,24 = 23.6, p < 0.001, 8 N: F1,24 = 14.8, p < 0.01). Figure 6b shows the mean ± SD values of PGF rate L/R ratio; the main and interaction statistical results can be found in Table 1.
Grip force to slip force (G/S) ratio and G/S L/R ratio
To understand to what extent grip force was generated relative to the slip force, the ratio of grip force and slip force (G/S ratio) within each hand was calculated. Additionally, to better understand the equivalence of G/S ratio between hands, the ratio of left and right hands of G/S ratio (G/S L/R ratio) was calculated.
The analysis of G/S ratio revealed an equivalent G/S ratio between right and left hands in older adults, while younger adults had higher G/S ratio in the right hand (Fig. 6c). This outcome did not change with perturbation force. A three-way MD ANOVA demonstrated significant interactions of AGE × HAND and HAND × PERTURBATION, as well as significant main effects of HAND and PERTURBATION (Table 1). As our interest was to highlight the age differences, we performed a post-hoc analysis of the AGE × HAND interaction separating data by age group. The post-hoc analysis of the AGE × HAND interaction revealed a simple main effect of HAND only in young adults (F1,24 = 16.7, p < 0.001).
For the L/R ratio in the Grip/Slip force assessment, values > 1 indicate larger G/S values in the left hand whereas values < 1 indicate larger G/S values in the right hand, and values ~ indicate similar values between the hands. Overall, older adults had higher equivalent G/S ratio values between hands, while young adults had a consistent bias towards right-hand G/S values (Fig. 6d). A two-way MD ANOVA demonstrated significant main effects of AGE and PERTURBATION were found, while there was no significant AGE × PERTURBATION interaction (Table 1).
Rapid motor response analyses
MGF
In order to assess the time course of MGF changes, 30 ms time windows were set to identify the latencies of MGF changes. The analysis revealed that the timing of hand difference is different between young and older adults. A four-way MD ANOVA with AGE, HAND, PERTURABTION, and TIME with MGF as a dependent variable showed a significant AGE × PERTURBATION × HAND × TIME interaction, as well as all four main effects (Table 2). This outcome suggested that the magnitude of the difference between right hand and left hand depends on AGE, PERTURBATION, and TIME. Since there was a four-way interaction and all four main effects, we performed a series of post-hoc analyses for a better understand of the main effect of HAND. Post-hoc analyses revealed a significant simple PERTURBATION × HAND × TIME interaction in both age groups (Young: F1.3,15.5 = 22.9, p < 0.001, Older: F1.8,21.5 = 18.4, p < 0.001). Further post-hoc analyses of simple three-way interaction demonstrated significant a simple HAND × TIME interaction in both perturbation conditions regardless of age (Young 12 N: F1.2,14.3 = 67.6, p < 0.001, Young 8 N: F1.3,15.2 = 63.0, p < 0.001, Older 12 N: F1.4,16.3 = 55.6, p < 0.001, Older 8 N: F1.2,14.4 = 42.9, p < 0.001). We conducted a simple main effect analysis of HAND at each time window to better understand when the MGF of the right hand become greater than the MGF of the left hand. In young adults, the simple main effect of HAND was observed at T5, T6, and T7 regardless of perturbation conditions (12 N T5 right vs. left: 6.74 ± 1.28 vs. 5.12 ± 1.10, 12 N T6 right vs. left: 8.75 ± 1.61 vs. 5.51 ± 1.11, 12 N T7 right vs. left: 11.24 ± 1.94 vs. 6.37 ± 1.22, 8 N T5 right vs. left: 6.27 ± 1.08 vs. 5.01 ± 1.10, 8 N T6 right vs. left: 7.66 ± 1.19 vs. 5.30 ± 1.11, 8 N T7 right vs. left: 9.35 ± 1.51 vs. 5.87 ± 1.15). In older adults, the simple main effect of HAND was found at T6 and T7 regardless of perturbation (12 N T6 right vs. left: 10.44 ± 3.06 vs. 7.12 ± 1.81 12 N T7 right vs. left: 13.51 ± 3.63 vs. 8.39 ± 2.16, 8 N T6 right vs. left: 9.75 ± 2.76 vs. 6.88 ± 1.72 8 N T7 right vs. left: 12.29 ± 3.39 vs. 7.83 ± 2.04). Figure 7a shows the mean ± SD of MGF at each time window.
PGF rate
With respect to PGF rate during different time windows of the task, similar results to MGF data were found. The time windows demonstrating hand difference were different between young and older adults. In older adults, the time windows showing hand differences were not the same between 12 and 8 N perturbation conditions. A four-way MD ANOVA found significant main effects of HAND, PERTURBATION, and TIME, as well as a significant AGE × PERTURBATION × HAND × TIME interaction (see Table 2). Given the four-way interaction, we performed a series of post-hoc analyses for a better interpretation of the main effect of HAND. Post-hoc analyses revealed simple PERTURBATION × HAND × TIME in both age groups (Young: F2.7,32.2 = 17.0, p < 0.001, Older: F2.2,26.9 = 5.8, p < 0.01), as well as a simple HAND × TIME interaction in both perturbation conditions regardless of age (Young 12 N: F2.2,26.7 = 42.3, p < 0.001, Young 8 N: F1.8,21.6 = 30.2, p < 0.001, Older 12 N: F2.4,28.7 = 36.8, p < 0.001, Older 8 N: F2.1,25.3 = 35.3, p < 0.001). A series of simple main effect analyses of HAND at each time window found the hand difference at in T1, T2, T4, T5, T6, and T7 in young adults regardless of perturbation (12 N T1 right vs. left: 3.20 ± 1.05 vs. 0.07 ± 0.21, 12 N T2 right vs. left: 6.89 ± 3.22 vs. 0.61 ± 0.54, 12 N T4 right vs. left: 14.80 ± 10.34 vs. 2.58 ± 1.03, 12 N T5 right vs. left: 52.25 ± 23.44 vs. 6.31 ± 2.48, 12 N T6 right vs. left: 81.13 ± 29.72 vs. 21.09 ± 12.48, 12 N T7 right vs. left: 94.79 ± 40.25 vs. 36.62 ± 28.10). In older adults, the simple main effect of HAND was found at T1, T2, T3, T5, T6, T7 and T1, T2, T5, T6, T7 in 12 N and 8 N perturbation conditions, respectively (12 N T1 right vs. left: 2.83 ± 0.89 vs. 0.12 ± 0.25, 12 N T2 right vs. left: 7.15 ± 3.16 vs. 0.66 ± 0.65, 12 N T3 right vs. left: 6.76 ± 3.66 vs. 2.43 ± 1.54, 12 N T5 right vs. left: 55.76 ± 38.71 vs. 6.72 ± 6.75, 12 N T6 right vs. left: 96.83 ± 44.14 vs. 27.70 ± 22.92, 12 N T7: 120.22 ± 44.18 vs. 58.76 ± 31.47, 8 N T1 right vs. left: 2.47 ± 0.93 vs. 0.21 ± 0.37, 8 N T2 right vs. left: 4.96 ± 2.02 vs. 0.62 ± 0.76, 8 N T5 right vs. left: 46.26 ± 33.93 vs. 5.51 ± 5.41, 8 N T6 right vs. left: 79.74 ± 41.79 vs. 21.45 ± 18.42, 8 N T7 right vs. left: 99.22 ± 45.59 vs. 42.83 ± 27.08). Figure 7b displays these data graphically.
Discussion
The purpose of the current study was to investigate the effects of age on motor responses elicited by an unexpected perturbation during a motor task requiring coordination of the two hands. The kinetic data supported our first hypothesis, where older adults demonstrate higher values in maximum grip force and maximum grip force rate. Our second hypothesis was also supported as the value of ΔGF from the left hand was non-zero. In addition, age-related differences in the L/R ratio in MGF and PGF rate supported our second hypothesis. Our third hypothesis was partially supported. While hand-based differences in MGF corresponds to long-latency responses and volitional response phases (supporting our third hypothesis), the hand differences in PGF rate contradicted our hypothesis. In the following paragraphs, we will discuss how our findings are associated with reports from the evidence base.
Slip force changes after perturbation
Our slip force analyses revealed increases in the right-hand and left-hand slip forces following perturbation regardless of the magnitude of delivered perturbation and age. While the right-hand slip force increased dramatically, we also observed slip force increase in the left hand. Changes in load forces in both hands after an unexpected perturbation were reported by Bracewell et al. (2003). They reported that load force produced by each hand depends on the size of weight causing unexpected perturbation and the location of the perturbation. In their study, a profound load force increase was observed in the hand that corresponds to the side where weight was added, while load force changes–although smaller–were also found in the contralateral hand. Since the perturbation was delivered to the right side of the object in the current study, the right-hand slip force increase is mostly due to the perturbation. The increase in the left-hand slip force may be due to the rigidity of the object, similar to the Bracewell study. In support of this, a previous study demonstrated a redistribution of load force when a slip on one finger holding a manipulandum increased the tangential force on a non-slipping finger that was holding the same manipulandum (Birznieks et al. 1998).
Regardless of hand, older adults had higher slip force increase after the perturbation. A higher slip force in older adults is reported by other investigations, and age-related changes in skin property are considered as a contributing factor to increasing slip force (Cole 1991; Kinoshita and Francis 1996). Our analysis of the coefficient friction demonstrated that older adults have lower digit friction compared to young adults in the right hand. Although we did not assess the coefficient of friction of the left hand, we assume the older adults have lower digit friction in their left hand as well. The declining friction of skin may associate with declining sweat and oil secretion systems, such as a decreasing number of sweat glands (Mackinnon 1954).
While a greater increase of slip force was seen in older adults after the perturbation, changes in load force and torque force were comparable between age groups. These observations, particularly the left hand which may have served as a pivot point, are not in line with findings by Kinoshita et al. (1997), who reported that the increase in slip force for a given increment in torque decreases as a function of tangential force. Several methodological differences may account for these conflicting findings. The first difference is with respect to the mechanism inducing the perturbation. In the present study, we built and used a perturbation system that pulls down the right side of the object, while Kinoshita et al. (1997) used an object that was subject to a rotation. A torsional slip might happen at the onset of perturbation since the pulling load is approximately perpendicular to the object. However, since our perturbation system is based on pulling, the characteristic of slip might transition from a torsional slip to a tangential slip. The second factor that may contribute to these contradicting findings is that the contact point of the fingertip relative to the device surface may have moved. As mentioned above, redistribution of the load force might occur due to the nature of the task. Such redistribution of the load force may induce a tangential slip at the fingertip and shift the center of the contact point. In addition, the left hand was free to move in the space in our study. The contacting surface was covered with a sandpaper in order to increase the fingertip friction at the contact site. Because of the higher friction, the left hand might move from the original position when the perturbation system was pulling the object.
Rapid grip force responses in perturbed and indirectly perturbed hands
We demonstrated rapid grip force responses in the perturbed hand, regardless of age. Maintaining grip force slightly higher than load force is critical in successful object manipulation (Johansson and Westling 1984). In response to unexpected increase in load force, the grip force rapidly increases to restore the relationship with the load force where the grip force is higher than the increased load force (catch-up response) (Johansson and Westling 1988). The post-perturbation catch-up response is scaled based on the magnitude of load force increase (Johansson et al. 1992a, b). After the post-perturbation catch-up response, the grip force changes in proportion to the changes in load force (Johansson et al. 1992c). These responses are disrupted when afferent feedback is diminished, suggesting the significance of sensory feedback in restraining “active” objects (Johansson et al. 1992a, b). As both groups exhibited intact tactile sensory function (Fig. 2a), the rapid grip force responses in the perturbed hand may be triggered by afferent feedback conveying information related to load force.
We also demonstrated similar results in the indirectly perturbed (left) hand, although the responses were smaller compared to the perturbed (right) hand, consistent with prior work in this area (Bracewell et al. 2003; Ohki and Johansson 1999). Ohki and Johansson (1999) observed twitch-like responses in the non-perturbed hand approximately 15 ms after the grip force response in the perturbed hand. They argued that the responses in the non-perturbed hand were triggered by sensory information originated from the perturbed hand, suggesting that sensory input crossed the midline at a certain level of central nervous system. In our study, the responses in the indirectly perturbed (left) hand were more than a muscle twitch as higher grip forces than grip forces before perturbation were maintained during the period of load force delivery. Motor task context may attribute to the different responses in the indirectly perturbed hand, since Ohki and Johansson (1999) conducted a motor task that did not require coordination of the two hands. Several upper-limb investigations examining rapid motor responses to unexpected perturbations have demonstrated that the responses in the non-perturbed extremity depends on the goal of motor task (Diedrichsen 2007; Dimitriou et al. 2012; Mutha and Sainburg 2009). Specifically, perturbations while manipulating two independent objects did not elicit responses in the non-perturbed arm; however, perturbations during the single shared object condition elicited responses in the non-perturbed arm, such as contributing to compensate the perturbation and bring the object/cursor to the target (Diedrichsen 2007). Similar investigations also reported contributive responses in the non-perturbed hand at a time corresponding to the long-latency responses and argued that such responses meditate transcallosal circuits (Mutha and Sainburg 2009).
The responses observed in the left hand may be triggered only by the increase slip force in the left hand, as our slip force analysis found slip force changes in the left hand. However, we decline that possibility and argue that the left hand responses are cortically meditated responses as the latency of left hand corresponded to volitional responses (Fig. 4a) (Macefield and Johansson 2003). Several studies examined the effects of load force rate on the latency and demonstrated that the onset of grip force response is inversely related to the load force rate (Cole and Abbs 1988; Cole and Rotella 2001; Johansson et al. 1992a, b). The latency of the left hand in 8 N perturbation condition was approximately 140 ms while peak slip force rate 10 N/s. The latency following a unilateral 8 N/s pulling loads applied to tangential to the skin of the digits was 108 ± 13 ms (Johansson et al. 1992). The latency of the left hand should be slightly faster than the latency reported by Johansson et al. (1992a; b) if the rapid response of the left hand was triggered by the left-hand slip force change.
Underlying causes of age-related differences in bimanual rapid grip force response
Age-related changes in rapid grip force responses were demonstrated previously (Cole and Rotella 2001); however, the examination of effects of aging on rapid grip force responses was limited to a one-hand condition. Our study extends this investigation by employing a bimanual motor task requiring a coordination of two hands. Older adults in our study showed higher values in the maximal grip force and maximal grip force rate regardless of hand.
A recent investigation demonstrated that the initial rapid motor responses are triggered by proprioceptive feedback, and the contribution of tactile feedback in the rapid motor responses began later (Crevecoeur et al. 2017). Age-related declines in proprioception and tactile resolution in hands are reported (Kalisch et al. 2012; Stevens 1992). The decline in somatosensory function may attribute to age-related changes in mechanoreceptors. Regarding to proprioception, older adults demonstrated less accurate proprioception in proximal and distal joints (Adamo et al. 2009, 2007; Wright et al. 2011). Such age differences in proprioception may be attributed to an increase in muscle spindle capsular thickness, a decreased muscle spindle diameter, a decreased muscle spindle sensitivity, and a declined joint mechanoreceptors density (Aydoğ et al. 2006; Kararizouet al. 2005; Kim et al. 2007; Morisawa 1998; Swash and Fox 1972). The declines in tactile sensation may be caused by a density reduction of the Meissner’s corpuscles and Merkel cells, changes in the placement, size, and morphology of these mechanoreceptors (Bruce 1980; García-Piqueras et al. 2019). Since the endings of rapidly adapting type 1 and slowly adapting type 1 units are Meissner’s corpuscles and Merkel cells, respectively, and these two units respond to localized slips, age-related alteration in these mechanoreceptors may lead to impaired and misestimated slip detection (Johansson and Westling 1987). Although the assessment of proprioception was not done in the current study, we demonstrated higher tactile sensory threshold in older adults compared to young adults (Fig. 2a). Thus, it is possible that age-related low resolution of somatosensory information may reduce response gain (Johansson et al. 1992a, b).
Beyond the peripheral changes, central nervous system alteration, such as sensory processing impairment, may lead to the age-related differences in the rapid grip force responses. Sensory processing speed and accuracy decline as people age (Master et al. 2010). These declines in sensory processing in older adults may be associated with functional remodeling in brain regions responsible in somatosensory information processing. Higher excitability of primary somatosensory cortex and an enlarged somatosensory cortex area representing hand are reported in older adults along with impaired tactile acuity (Kalisch et al. 2009; Lenz et al. 2012). Intercortical inhibition may also be declined in older adults as they had decreased cortical activation in contralateral secondary somatosensory cortex and cingulate cortex during simple tactile stimulation, while increased cortical activation was found in ipsilateral primary somatosensory cortex (Brodoehl et al. 2013).
Decline in attentional capacity is another potential underlying mechanism leading to age differences in bimanual motor responses. A strong link between cognitive and sensorimotor functions in older adults are reported using a dual task paradigms (Li and Lindenberger 2002). Dual-tasking tasks have been demonstrating the demand of attention in voluntary movements including compensatory motor responses following an unexpected perturbation. Lower extremity investigations suggested attentional demands in compensatory stepping response following unexpected perturbations (Brauer et al. 2002). Although it is unclear to what extent the rapid grip force responses require attentional resources, it is plausible that the rapid grip force responses in older adults were influenced by impairment in sensory processing and attentional capacity.
L/R Ratio data suggested that older adults recruited the indirectly perturbed hand more than young adults to stabilize the object. Dimitriou et al. (2012) suggested that bilateral rapid motor responses are the sophisticated responses based on integrating sensory information arising from two extremities. As sensory information transfers to the contralateral hemisphere via corpus callosum, alteration in corpus callosum associated with aging may also contribute in the differences in bimanual rapid motor response between young and older adults. For instance, impairment in utilizing sensory information to guide the contralateral limb is reported in older (Adamo et al. 2007, 2009). It has been argued that age-related morphological changes in corpus callosum are responsible in such the impairment in an interhemispheric transformation of sensory information (Schaap et al. 2015). Central and posterior regions of corpus callosum are critical in sensory information transfer since axons from primary and sensory somatosensory cortices cross the two regions. (Fabri et al. 2014; Fabri and Polonara 2013). A recent investigation found microstructural changes along with aging in posterior corpus callosum (Fan et al. 2019). These changes might add noises to the crossed sensory information and lead to a miscalculation of the perturbation magnitude.
Different timing of hand differences in early rapid motor response
In contrast to our hypothesis #3, the analysis of PGF rate demonstrated that the value of right hand was higher than left hand at the stable phase and 30 ms after the perturbation in both age groups. Greater fatigue in the right hand due to the right-side biased perturbation may be attributed to higher maximum grip force rate in the right hand. Fatigue disrupts the coupling of grip force and load force (Todd et al. 2010). The declined ability to produce constant submaximal grip force due to fatigue is also reported by other investigations (Maluf et al. 2005; Tarkeshwar Singh et al. 2010). The effects of fatigue on a precise grip force control using multiple fingers have been found to be independent from aging (Singh et al. 2013).
The perturbations were limited to the right hand, hence subjects produced higher grip force with the right hand when perturbations were delivered (Fig. 3). Such bias on the perturbation might induce greater fatigue in the right hand than left hand, eventually enhancing grip force fluctuation during the stable phase. The greater grip force fluctuation in the right hand can lead to higher values of PGF rate in the right hand since the PGF rate is the first derivative of grip force. We included 5 s and 3 min rest intervals between trials and between sets, respectively, to minimize the effects of fatigue on the performance. However, the interval might not be adequate to minimize the effects of fatigue on constant force production.
Although preliminary investigations suggest that the fatigue can changes reflex responses, it is questionable whether those reports are applicable to our study. Hallmarks of fatigue are observed at peripheral and central level (Allen et al. 2008; Gandevia 2001). For instance, fatigue attributes to disruptions of excitation–contraction coupling in neuromuscular junction. The disruption of excitation–contraction coupling are due, in part, to changes in extracellular and cytoplasmic sides of K+ concentration (Juel et al. 2000; Sjogaard et al. 1985). Such changes suppress the excitability of muscles and ultimately lead to recruitment of motor units that were not initially recruited. This also leads to a decrease in motor unit firing rate (Enoka and Stuart 1992; Gandevia 2001; Overgaard and Nielsen 2001; Overgaard et al. 1999). Decreased firing rate of motor units may be due to central fatigue at spinal level associated with changes in afferent input from muscles (Gandevia 2001). Changes in short- and long-latency responses were reported following fatigue in muscle spindles; however, it should be noted that such changes in reflex responses were observed after a minimum 30-s of maximum voluntary contraction (Balestra et al. 1992; Hagbarth et al. 1995). As perturbations lasted only 500 ms in our study, we assume that our subjects exerted the highest pinch force less than 1 s as young and older adults seem to terminate force production immediately after the end of increasing load forces (Cole and Rotella 2001, from visual inspectino of Fig. 3 in this reference).
The analysis of rapid motor response at different time windows revealed that the timing of hand differences is different between young and older adults. The analysis of maximum grip force between right hand and left hand in prior to the perturbation were comparable regardless of age in both perturbation conditions. However, the hand differences became prominent in 91–120 ms and 121–150 ms intervals in young and older adults, respectively, suggesting that older adults responded to the perturbation slower than young adults. This result is in line with the previous investigation demonstrating age differences in the onset of rapid motor response following unexpected perturbation (Cole and Rotella 2001). The onset of rapid motor response is affected by the rate of load force and sensory input (Johansson et al. 1992a, b). Since same pulling load forces are delivered to both age groups, the age differences in the onset of rapid motor response may be due to a higher threshold of the sensory registration due to age-related changes in the somatosensory system (Shaffer and Harrison 2007).
Conclusion
Although the rapid grip force responses were scaled based on the magnitude of perturbation, we demonstrate that older adults recruit the indirectly perturbed (left) hand more than young adults and might be due to age-related changes in peripheral and central levels. However, it is unknown to what extent each level contributes to the more recruitment of the indirectly perturbed (left) hand. Such questions may be interesting to address in the future studies.
References
Adamo DE, Martin BJ, Brown SH (2007) Age-related differences in upper limb proprioceptive acuity. Percept Mot Skills 104(3 Pt 2):1297–1309. https://doi.org/10.2466/pms.104.4.1297-1309
Adamo DE, Alexander NB, Brown SH (2009) The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act 17(3):272–293
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88(1):287–332. https://doi.org/10.1152/physrev.00015.2007
Aydoğ ST, Korkusuz P, Doral MN, Tetik O, Demirel HA (2006) Decrease in the numbers of mechanoreceptors in rabbit ACL: the effects of ageing. Knee Surg Sports Traumatol 14(4):325–329. https://doi.org/10.1007/s00167-005-0673-2
Balestra C, Duchateau J, Hainaut K (1992) Effects of fatigue on the stretch reflex in a human muscle. Electroencephalogr Clin Neurophysiol 85(1):46–52. https://doi.org/10.1016/0168-5597(92)90101-g
Birznieks I, Burstedt MK, Edin BB, Johansson RS (1998) Mechanisms for force adjustments to unpredictable frictional changes at individual digits during two-fingered manipulation. J Neurophysiol 80(4):1989–2002. https://doi.org/10.1152/jn.1998.80.4.1989
Bracewell RM, Wing AM, Soper HM, Clark KG (2003) Predictive and reactive co-ordination of grip and load forces in bimanual lifting in man. Eur J Neurosci 18(8):2396–2402
Brauer SG, Woollacott M, Shumway-Cook A (2002) The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait Posture 15(1):83–93. https://doi.org/10.1016/s0966-6362(01)00163-1
Brodoehl S, Klingner C, Stieglitz K, Witte OW (2013) Age-related changes in the somatosensory processing of tactile stimulation—an fMRI study. Behav Brain Res 238:259–264. https://doi.org/10.1016/j.bbr.2012.10.038
Bruce MF (1980) The relation of tactile thresholds to histology in the fingers of elderly people. J Neurol Neurosurg Psychiatry 43(8):730–734
Clark MC, Czaja SJ, Weber RA (1990) Older adults and daily living task profiles. Hum Factors 32(5):537–549
Cole KJ (1991) Grasp force control in older adults. J Mot Behav 23(4):251–258. https://doi.org/10.1080/00222895.1991.9942036
Cole KJ, Abbs JH (1988) Grip force adjustments evoked by load force perturbations of a grasped object. J Neurophysiol 60(4):1513–1522. https://doi.org/10.1152/jn.1988.60.4.1513
Cole KJ, Beck CL (1994) The stability of precision grip force in older adults. J Mot Behav 26(2):171–177. https://doi.org/10.1080/00222895.1994.9941671
Cole KJ, Johansson RS (1993) Friction at the digit-object interface scales the sensorimotor transformation for grip responses to pulling loads. Exp Brain Res 95(3):523–532
Cole KJ, Rotella DL (2001) Old age affects fingertip forces when restraining an unpredictably loaded object. Exp Brain Res 136(4):535–542
Crevecoeur F, Barrea A, Libouton X, Thonnard JL, Lefevre P (2017) Multisensory components of rapid motor responses to fingertip loading. J Neurophysiol 118(1):331–343. https://doi.org/10.1152/jn.00091.2017
Diedrichsen J (2007) Optimal task-dependent changes of bimanual feedback control and adaptation. Curr Biol 17(19):1675–1679. https://doi.org/10.1016/j.cub.2007.08.051
Dimitriou M, Franklin DW, Wolpert DM (2012) Task-dependent coordination of rapid bimanual motor responses. J Neurophysiol 107(3):890–901. https://doi.org/10.1152/jn.00787.2011
Enoka RM, Stuart DG (1992) Neurobiology of muscle fatigue. J Appl Physiol 72(5):1631–1648. https://doi.org/10.1152/jappl.1992.72.5.1631
Fabri M, Polonara G (2013) Functional topography of human corpus callosum: an FMRI mapping study. Neural Plast 2013:251308–251308. https://doi.org/10.1155/2013/251308
Fabri M, Pierpaoli C, Barbaresi P, Polonara G (2014) Functional topography of the corpus callosum investigated by DTI and fMRI. World J Radiol 6(12):895–906. https://doi.org/10.4329/wjr.v6.i12.895
Fan Q, Tian Q, Ohringer NA, Nummenmaa A, Witzel T, Tobyne SM, Huang SY (2019) Age-related alterations in axonal microstructure in the corpus callosum measured by high-gradient diffusion MRI. Neuroimage 191:325–336. https://doi.org/10.1016/j.neuroimage.2019.02.036
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789. https://doi.org/10.1152/physrev.2001.81.4.1725
García-Piqueras J, García-Mesa Y, Cárcaba L, Feito J, Torres-Parejo I, Martín-Biedma B, Vega JA (2019) Ageing of the somatosensory system at the periphery: age-related changes in cutaneous mechanoreceptors. J Anat 234(6):839–852. https://doi.org/10.1111/joa.12983
Gilles MA, Wing AM (2003) Age-related changes in grip force and dynamics of hand movement. J Mot Behav 35(1):79–85. https://doi.org/10.1080/00222890309602123
Gorniak SL, Alberts JL (2013) Effects of aging on force coordination in bimanual task performance. Exp Brain Res 229(2):273–284. https://doi.org/10.1007/s00221-013-3644-8
Hagbarth KE, Bongiovanni LG, Nordin M (1995) Reduced servo-control of fatigued human finger extensor and flexor muscles. J Physiol 485(Pt 3):865–872. https://doi.org/10.1113/jphysiol.1995.sp020776
Johansson RS, Cole KJ (1994) Grasp stability during manipulative actions. Can J Physiol Pharmacol 72(5):511–524. https://doi.org/10.1139/y94-075
Johansson RS, Flanagan JR (2009) Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10(5):345–359. https://doi.org/10.1038/nrn2621
Johansson RS, Westling G (1984) Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56(3):550–564
Johansson RS, Westling G (1987) Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res 66(1):141–154. https://doi.org/10.1007/bf00236210
Johansson RS, Westling G (1988) Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res 71(1):72–86
Johansson RS, Hager C, Riso R (1992a) Somatosensory control of precision grip during unpredictable pulling loads. II. Changes in load force rate. Exp Brain Res 89(1):192–203
Johansson RS, Hger C, Backstrom L (1992b) Somatosensory control of precision grip during unpredictable pulling loads. III. Impairments during digital anesthesia. Exp Brain Res 89(1):204–213
Johansson RS, Riso R, Hager C, Backstrom L (1992c) Somatosensory control of precision grip during unpredictable pulling loads. I. Changes in load force amplitude. Exp Brain Res 89(1):181–191
Juel C, Pilegaard H, Nielsen JJ, Bangsbo J (2000) Interstitial K(+) in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol 278(2):R400–406. https://doi.org/10.1152/ajpregu.2000.278.2.R400
Kalisch T, Ragert P, Schwenkreis P, Dinse HR, Tegenthoff M (2009) Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex 19(7):1530–1538. https://doi.org/10.1093/cercor/bhn190
Kalisch T, Kattenstroth JC, Kowalewski R, Tegenthoff M, Dinse HR (2012) Age-related changes in the joint position sense of the human hand. Clin Interv Aging 7:499–507. https://doi.org/10.2147/cia.s37573
Kararizou E, Manta P, Kalfakis N, Vassilopoulos D (2005) Morphometric study of the human muscle spindle. Anal Quant Cytol Histol 27(1):1–4
Khan A, Gorniak SL (2016) Effects of force requirements on pinch force production in healthy adults. Mot Control 20(3):299–315. https://doi.org/10.1123/mc.2014-0081
Kilbreath SL, Heard RC (2005) Frequency of hand use in healthy older persons. Aust J Physiother 51(2):119–122
Kim GH, Suzuki S, Kanda K (2007) Age-related physiological and morphological changes of muscle spindles in rats. J Physiol 582(Pt 2):525–538. https://doi.org/10.1113/jphysiol.2007.130120
Kinoshita H, Francis PR (1996) A comparison of prehension force control in young and elderly individuals. Eur J Appl Physiol Occup Physiol 74(5):450–460. https://doi.org/10.1007/bf02337726
Kinoshita H, Backstrom L, Flanagan JR, Johansson RS (1997) Tangential torque effects on the control of grip forces when holding objects with a precision grip. J Neurophysiol 78(3):1619–1630. https://doi.org/10.1152/jn.1997.78.3.1619
Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Hoffken O, Gatica Tossi MA, Dinse HR (2012) Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci 32(5):1811–1816. https://doi.org/10.1523/jneurosci.2722-11.2012
Li KZ, Lindenberger U (2002) Relations between aging sensory/sensorimotor and cognitive functions. Neurosci Biobehav Rev 26(7):777–783
Macefield VG, Johansson RS (2003) Loads applied tangential to a fingertip during an object restraint task can trigger short-latency as well as long-latency EMG responses in hand muscles. Exp Brain Res 152(2):143–149. https://doi.org/10.1007/s00221-003-1421-9
Mackinnon PC (1954) Variations with age in the number of active palmar digital sweat glands. J Neurol Neurosurg Psychiatry 17(2):124–126. https://doi.org/10.1136/jnnp.17.2.124
Maluf KS, Shinohara M, Stephenson JL, Enoka RM (2005) Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res 167(2):165–177. https://doi.org/10.1007/s00221-005-0017-y
Master S, Larue M, Tremblay F (2010) Characterization of human tactile pattern recognition performance at different ages. Somatosens Mot Res 27(2):60–67. https://doi.org/10.3109/08990220.2010.485959
Morisawa Y (1998) Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci 3(2):102–110. https://doi.org/10.1007/s007760050029
Mutha PK, Sainburg RL (2009) Shared bimanual tasks elicit bimanual reflexes during movement. J Neurophysiol 102(6):3142–3155. https://doi.org/10.1152/jn.91335.2008
Ochoa N, Gorniak SL (2014) Changes in sensory function and force production in adults with type II diabetes. Muscle Nerve 50(6):984–990. https://doi.org/10.1002/mus.24261
Ochoa N, Gogola GR, Gorniak SL (2016) Contribution of tactile dysfunction to manual motor dysfunction in type II diabetes. Muscle Nerve 54(5):895–902. https://doi.org/10.1002/mus.25137
Ohki Y, Johansson RS (1999) Sensorimotor interactions between pairs of fingers in bimanual and unimanual manipulative tasks. Exp Brain Res 127(1):43–53. https://doi.org/10.1007/s002210050772
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Overgaard K, Nielsen OB (2001) Activity-induced recovery of excitability in K(+)-depressed rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 280(1):R48–55. https://doi.org/10.1152/ajpregu.2001.280.1.R48
Overgaard K, Nielsen OB, Flatman JA, Clausen T (1999) Relations between excitability and contractility in rat soleus muscle: role of the Na+–K+ pump and Na+/K+ gradients. J Physiol 518(Pt 1):215–225. https://doi.org/10.1111/j.1469-7793.1999.0215r.x
Parikh PJ, Cole KJ (2012) Handling objects in old age: forces and moments acting on the object. J Appl Physiol 112(7):1095–1104. https://doi.org/10.1152/japplphysiol.01385.2011
Pruszynski JA (2014) Primary motor cortex and fast feedback responses to mechanical perturbations: a primer on what we know now and some suggestions on what we should find out next. Front Integr Neurosci 8:72–72. https://doi.org/10.3389/fnint.2014.00072
Schaap TS, Gonzales TI, Janssen TW, Brown SH (2015) Proprioceptively guided reaching movements in 3D space: effects of age, task complexity and handedness. Exp Brain Res 233(2):631–639. https://doi.org/10.1007/s00221-014-4142-3
Shaffer SW, Harrison AL (2007) Aging of the somatosensory system: a translational perspective. Phys Ther 87(2):193–207. https://doi.org/10.2522/ptj.20060083
Singh T, Varadhan SKM, Zatsiorsky VM, Latash ML (2010) Fatigue and motor redundancy: adaptive increase in finger force variance in multi-finger tasks. J Neurophysiol 103(6):2990–3000. https://doi.org/10.1152/jn.00077.2010
Singh T, Zatsiorsky VM, Latash ML (2013) Contrasting effects of fatigue on multifinger coordination in young and older adults. J Appl Physiol 115(4):456–467. https://doi.org/10.1152/japplphysiol.00375.2013
Sjogaard G, Adams RP, Saltin B (1985) Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol 248(2 Pt 2):R190–196. https://doi.org/10.1152/ajpregu.1985.248.2.R190
Stevens JC (1992) Aging and spatial acuity of touch. J Gerontol 47(1):P35–40
Stevens JC, Patterson MQ (1995) Dimensions of spatial acuity in the touch sense: changes over the life span. Somatosens Mot Res 12(1):29–47
Swash M, Fox KP (1972) The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci 16(4):417–432
Thames BH, Gorniak SL (2017) Coefficient of friction at the fingertips in type II diabetics compared to healthy adults. J Appl Biomech 33(3):185–188. https://doi.org/10.1123/jab.2016-0147
Todd G, Gandevia SC, Taylor JL (2010) Change in manipulation with muscle fatigue. Eur J Neurosci 32(10):1686–1694. https://doi.org/10.1111/j.1460-9568.2010.07444.x
Tremblay F, Wong K, Sanderson R, Cote L (2003) Tactile spatial acuity in elderly persons: assessment with grating domes and relationship with manual dexterity. Somatosens Mot Res 20(2):127–132. https://doi.org/10.1080/0899022031000105154
Wright ML, Adamo DE, Brown SH (2011) Age-related declines in the detection of passive wrist movement. Neurosci Lett 500(2):108–112. https://doi.org/10.1016/j.neulet.2011.06.015
Acknowledgements
We thank Nikita Watson for her assist in data collection, processing, and analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest to disclose.
Additional information
Communicated by Winston D. Byblow.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hibino, H., Gorniak, S.L. Effects of aging on rapid grip force responses during bimanual manipulation of an active object. Exp Brain Res 238, 2161–2178 (2020). https://doi.org/10.1007/s00221-020-05865-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05865-0