Abstract
Persistent motor deficits in the paretic arm present a major barrier to the recovery of the ability to perform bimanual tasks even in individuals who have recovered well after a stroke. Impaired performance may be related to deficits in bimanual temporal coordination due to stroke-related damage of specific brain motor structures as well as changed biomechanics of the paretic arm. To determine the extent of the deficit in bilateral temporal coordination after the stroke, we investigated how bilateral reciprocal coordination was regained after external perturbations of the arm in individuals with hemiparesis due to stroke. We used a bilateral task that would be minimally affected by the unilateral arm motor deficit. Nine non-disabled control subjects and 12 individuals with chronic hemiparesis performed reciprocal (anti-phase) arm swinging in the standing position for 15 s per trial. In each trial, movement of one arm was unexpectedly and transiently (~150–350 ms) arrested at the level of the wrist once in the forward and once in the backward phase of swinging. Perturbation was applied to the left and right arms in control subjects and to the paretic and non-paretic arms of individuals with hemiparesis. Kinematic data from endpoint markers on both hands and electromyographic activity of anterior and posterior deltoid muscles from both arms were recorded. The oscillatory period, the phase differences between arms and the mean EMG activity before, during and after perturbation were analyzed. In both groups the perturbation altered the period of the perturbed cycle in both the arrested and non-arrested arms and resulted in a change from anti-phase to in-phase coordination, following which anti-phase coordination was regained. Recovery of anti-phase swinging took significantly longer in patients with hemiparesis compared to control subjects. Stable pre-perturbed (anti-phase) reciprocal coordination was regained within one cycle following perturbation for the control subjects and within two cycles following perturbation for the patients with hemiparesis. Analysis of EMG activation levels showed that, compared to control subjects, there was significantly less activation of the shoulder muscles in response to perturbation in the patient group and the pattern of muscle activation in the paretic arm was opposite to that in the non-paretic and control arms. The finding that patients had a reduced capacity for maintaining and restoring the required reciprocal coordination when perturbation occurred suggests that stroke-related brain damage in our patients led to instability of bilateral temporal coordination for this rhythmical task.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following a stroke, individuals with upper limb paresis have difficulties coordinating the movement of both arms together in daily life activities and tend to use only the unaffected arm, even when the paretic arm is able to perform the task (Carr and Shepherd 1987; Taub and Wolf 1997). However it is unclear to what extent the “non-use” of the paretic arm in bimanual tasks may be the result of sensorimotor deficits such as muscle weakness (Bohannon and Andrews, 1998), alteration in inter-joint coordination (Levin 1996) or disrupted temporal coordination (Archambault et al. 1999; Dick et al. 1986; Wiesendanger et al. 1994a; Carson et al. 1999). Bimanual manipulative skills require appropriately timed coordinated movements of both arms. Examples of bimanual coordination range from simple arm swinging during walking or running to complex coordination during the playing of musical instruments. Moreover, the ability to appropriately coordinate movements of both arms may be task-related (Wiesendanger et al. 1994b; Cardoso de Oliveira 2002; Swinnen 2002). In particular, coordination may be considered in terms of synchronization determined as the coincidence in movement initiation and/or termination when reaching with both arms during, for example, picking up or manipulating an object (Kazennikov et al. 2002). Cyclical bimanual movement accompanying walking or running requires another kind of synchronization in which movement phases of each limb are opposite to each other in each cycle. Such reciprocal coordination can be described in terms of the phase relationship between movements of both limbs (Kelso et al. 1986; Swinnen et al. 1996). Behaviorally, perturbation of one limb during rhythmical bilateral movement disrupts intersegmental coupling and results in a phase change of the opposite limb. In the lower limb for example, perturbation of one leg during gait obstructed or prolonged both the ipsilateral swing phase and the contralateral stance phase (Dietz et al. 1986; Duysens and Van de Crommet 1998). These adaptations (or phase changes) in the lower limb are thought to be mediated by interlimb reflexes, and similar reflex mechanisms may also play a role in the coordination of bimanual arm movements (Grillner and Wallen 1985). For example, electrical stimulation of the median or ulnar nerve of one arm during rhythmic bimanual cranking resulted in prolongation of the movement phase of the contralateral arm (Zehr and Kido 2001). Further evidence of interlimb reflexes stems from the work of Wei et al. (2003), who showed that discrete elbow flexion of one arm in the horizontal plane changed the phase of an ongoing cyclical flexion-extension movement of the opposite arm.
Control of coordination is needed not only for bilateral movement initiation but also to maintain rhythmical synchrony that may vary during cyclical movement because of inter-limb asymmetry (Kelso et al. 1986). The preservation of synchrony during ongoing movement requires continuous phase corrections to avoid the accretion of synchronization errors (Repp 2002). Behaviorally, signals generated from one hemisphere would result in the contralateral arm leading the phase correction (referred to as the ‘leading arm’). Whether the leading arm is dominant or non-dominant appears to be task-dependent (Semjen et al. 1995; Swinnen et al. 1996; Franz et al. 2002). Alternatively signals emanating from both hemispheres would result in there being no arm leading preference.
Despite the research interest in bimanual coordination, little is known about the specific deficits in temporal coordination during bimanual tasks in individuals with hemiparesis due to stroke-related brain damage. Temporal coordination during attempts to bring both arms together to touch an object placed in the midline was described by Rose and Winstein (2002) for individuals with stroke. Movements of the hemiparetic arm were both delayed in onset and asynchronous with respect to the contralateral arm. However, the influence of sensorimotor deficits such as spasticity on the motor performance of the arm contralateral to the brain lesion during this type of goal-directed task is not trivial and cannot be separated from possible deficits in the temporal control of bimanual coordination. A preferable way to study temporal coupling in patients with hemiparesis would be to use a movement involving both the affected and less-affected arms in which the influence of the sensorimotor deficit would be minimized. Thus, Rice and Newell (2001) studied simple bilateral synchronous elbow flexion movements in the vertical plane in individuals with hemiparesis. Although patients adjusted the frequency and velocity of movement of the less affected arm to that of the affected arm, they showed a deficit in their ability to synchronize bimanual movements compared to healthy subjects.
Our goal was to determine the extent of the deficit in bilateral temporal coordination in patients with stroke-related brain damage. Since the motor deficit after stroke is more marked in distal than in proximal muscles (Colebatch et al. 1986; Bourbonnais and Vanden Noven 1989), we chose to investigate a cyclical bilateral movement performed primarily by the less-affected proximal muscles so as to minimize the influence of the motor deficit of the paretic arm on movement production. Except for the requirement to maintain a reciprocal pattern of swinging, our task was considered as non-goal-directed since it did not have a functional endpoint as in other goal-directed tasks such as pointing or reaching towards a target. We hypothesized that reciprocal coordination of the arms may be disrupted after stroke-related brain damage. Specifically we focused on the comparison of how the bilateral reciprocal movement pattern would be regained in control subjects and in adults with unilateral stroke following brief perturbations of the swinging arm. Some results have appeared in abstract form (Ustinova et al. 2004).
Methods
Subjects
Twenty-one adults including 9 non-disabled subjects (control group) and 12 adults with chronic hemiparesis participated in the study. All participants were informed of the experimental procedures and signed a consent form conforming to the requirements of the institutional ethics review board. Adults with hemiparesis had unilateral stroke-related brain damage in the right (n=6) or left hemisphere (n=6) in the territory of the middle cerebral artery. The group included nine men and three women with a mean age (±SD) of 62.7±14.8 years. Exclusion criteria were the presence of one or more of the following factors: cerebellar or brain stem lesions; significant verbal, visual, cognitive or perceptual deficits assessed by standard clinical tests; pain or orthopedic problems in the arms or legs; marked deficit in balance (less than 35/56 on the Berg balance scale described below); inability to extend the arm (less than 35/66 on the Arm and Hand section of Fugl–Meyer stroke assessment scale described below); marked deficit in proprioception (less than 6/8 on the sensory evaluation of the Fugl–Meyer scale); or an onset of stroke less than 6 months previously. All patients were right-hand dominant according to self-report. They were tested by experienced clinicians using a battery of tests. Upper limb impairment was evaluated with the Arm and Hand section of the valid (Berglund and Fugl–Meyer 1986) and reliable (Duncan et al. 1983) Fugl–Meyer stroke assessment scale (Fugl–Meyer et al. 1975), where a score of 66 corresponds to normal functioning. Participants in the study had a mild to moderate motor deficit (40–63/66). Spasticity was measured using the valid and reliable composite spasticity index (CSI, Levin and Hui-Chan 1992; Goulet et al. 1996; Nadeau et al. 1997). The CSI includes measures of biceps tendon jerks, resistance to full-range passive elbow extension performed at a moderate speed and wrist clonus for a total score of 16 points. Spasticity scores ranged from four (mild) to ten (moderate). Finally, the 56-point Berg balance scale (Berg et al., 1989) was used to evaluate postural stability when sitting, standing and stepping. Berg scores between 41 and 56, and between 21 and 40 correspond to good or fair balance respectively. All but one participant in the stroke group had good balance. Clinical and demographic data are presented in Table 1.
The control group included six men and three women without pain, neurological or orthopedic deficits involving the arms, legs or trunk and having a mean age (±SD) similar to that of the participants with stroke (57.2±8.2 years). They were not specifically age- or gender-matched. All control participants were right-handed according to self-report.
Experimental procedure
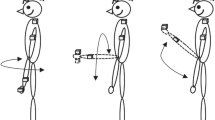
While standing, participants swung their arms reciprocally forwards and backwards in the sagittal plane (each arm moving in an opposite direction), simulating arm movement during walking (Fig. 1a). Subjects were asked to swing their arms as naturally as possible and not to attempt to stiffen or voluntarily move their elbows or wrists. Subjects produced a standard frequency of arm swinging in each trial throughout the whole experiment. To accomplish this, before beginning each trial, subjects matched their arm swinging frequency to the beat of a metronome signal set at 0.8 Hz. We chose 0.8 Hz (having a cycle period of 1.25 secs) since it was a frequency that was easily maintained in individuals with stroke according to preliminary experiments while being within the range of preferred frequencies for control subjects (Ustinova et al., 2004). When the frequency of movement stabilized for approximately 1 min, the metronome was turned off. This point marked the beginning of the trial and subjects were instructed to continue swinging their arms at the same frequency for an additional 15 s. Twelve trials were performed (six for each arm), during which one arm was unexpectedly perturbed (arrested) for 150–350 ms at the level of the wrist (Fig 1a). The perturbation was applied through a rigid plastic rod connected to a wrist bracelet on one arm by a clamp with a universal joint allowing movement in all directions. The clamp was activated by an electromagnet that produced adequate force to briefly interrupt the movement of the rod, after which the clamp was released and the arm continued swinging. The rod rolled on frictionless bearings and was attached by a universal joint to a bar of adjustable height situated behind the subject. The apparatus had negligible resistance.
Arm perturbations occurred randomly in two directions: once when the arm moved forward and once when it moved backward. The perturbation was applied when the arm reached –10 to –30° with respect to the middle of the arm trajectory (Fig. 1b) in each trial. The determination of mid-cycle was done automatically after performance of the first complete swinging cycle. As the amplitude of arm swinging could change during the trial, the mid-cycle position may have slightly varied throughout the trial. Perturbations were applied to two sides of the body: half of the perturbations (in six trials) were applied to the right arm and half to the left arm. The first arrest was applied 3–4 movement cycles after the beginning of the trial (defined above). The two arrests per trial were separated by a random interval of 3.5–4 cycles. This cycle length was used since in preliminary trials this interval was adequate for recovery of the reciprocal pattern to occur. We randomized the interval in order to avoid subject anticipation of the next perturbation. In addition, in order to minimize fatigue due to long trial times, we limited the trial length to 15 s. To ensure that the same time interval was maintained between arrests for all participants, the duration of the trial was extended to 17 sec for four subjects with hemiparesis, who were unable to maintain a frequency of 0.8 Hz. In these subjects, the frequencies ranged from 0.53 to 0.62 Hz throughout the whole experiment.
All subjects were asked to continue the anti-phase bilateral swinging despite the perturbation and to maintain approximately the same amplitude of arm swinging throughout each trial. However, after the metronome was turned off, they were not given any feedback about phasing or movement amplitude during swinging.
Data analysis
Three-dimensional kinematic data were collected with retro-reflective markers placed on the dorsum of both hands below the head of the second metacarpals (endpoints). Marker movement was recorded by a Vicon Motion Analysis 512 system (Oxford Metrics Ltd) at a sampling frequency of 120 Hz. After low-pass filtering at 10 Hz, the amplitude, cycle period and relative phase of arm swinging were computed from displacements of the endpoint markers. Movement parameters were analyzed for the left and right arms in control subjects, and for the paretic and non-paretic arms in patients with hemiparesis.
The amplitude of arm swinging was calculated as the peak-to-peak arm displacement in the sagittal (anterior–posterior) direction. Oscillatory periods were defined as the time between two minimum or maximum peaks of endpoint displacements for perturbations in the backward and forward directions, respectively (Fig. 1b). To evaluate how the cycle period of both arms was affected by the arrest of one arm, we computed the mean, T, of the two full periods (T −2 and T −1; see Fig. 1b) preceding the perturbation, the value of the transitional interval, T 0; and the values of the 3 periods (T 1, T 2, T 3) following the perturbation. Then we normalized the values of T 0, T 1, T 2, T 3 by dividing them by T:
The normalized values reflected both immediate, in terms of T 0, and remote effects of perturbation (in terms of T 1 , T 2 , T 3). The immediate effects of perturbation (arrested arm, free arm) in the transitional interval were analyzed for each arm, and the direction of swinging and the means and SEs were computed. We also computed the amount of time within the interval that both arms moved in the same direction (in-phase coordination), normalized with respect to the pre-perturbed cycles (T). The beginning of this interval was defined as the point at which the relative phase trace (see below) deviated more than 1 SD of the mean trace of the pre-perturbed cycle. The end of this interval was defined as the point when the relative phase trace returned to within 1 SD of the mean pre-perturbed trace.
Changes in cycle periods following the transitional interval in T 1 , T 2 , T 3 were determined. The pre-arrest cycle was given a value of 1. Cycle periods greater than 1 represented a prolongation of the cycle (or a decrease in frequency) and those less than 1 represented a shortening of the cycle (an increase in frequency). The stability of the baseline cycle periods was determined by the ratio of T−1/T−2.
The relative phase, reflecting the relative position of the two oscillating arms within an oscillatory cycle (phase difference), was calculated according to the following equation, proposed by Kelso et al. (1986), where the value of a perfectly out-of-phase movement is 180°.
where φ is relative phase, a and b are coefficients detecting the displacements of both arms.
The computation of the relative phase was done only for stable conditions and measured on a point-by-point basis. In the perturbed cycle, the relative phase was computed only after arm release. Thus, we calculated the mean value (φ) of the relative phase in the two cycles preceding (φ−1, φ−2) and in the three cycles following the arrest (φ1, φ2, φ3). The computed means of the relative phase deviated from 180° in both arms. The relative phase before and after the arrest was used to determine the leading arm as well as the degree of bilateral synchronization. The leading arm was determined by the value of the absolute means of the relative phase, where means exceeding or less than 180° by 3% corresponded to the right arm or left arm leading, respectively. A value of ±3% was used to estimate the system error since it corresponded to the SE of the mean (5.4°) of the relative phase in control subjects calculated throughout all trials (excluding the perturbed cycles). The magnitude of the phase difference between arms was used to determine the degree of bimanual synchronization. The analysis was done with respect to 180° indicating perfect synchronization.
The EMG activity of shoulder flexors (anterior deltoid, AD) and extensors (posterior deltoid, PD) was recorded from both arms with active bipolar surface electrodes and further amplified (× 5–10 k), band-pass filtered (75–500 Hz) and sampled at a rate of 1,080 Hz. AD of the arrested arm was considered as the agonist muscle when the arrest occurred during forward swinging and PD was the agonist during backward swinging.
To analyze the patterns of shoulder muscle activation during swinging, raw EMG was filtered and rectified. The root mean square EMG (rEMG) was computed in the two pre-arrest cycles. Then, the rEMG in the perturbed cycle for each of the four muscles was expressed as a percentage of their respective pre-arrest means. For this analysis, means were calculated over the entire cycle duration, which ranged from 1.0 to 1.8 s for non-perturbed cycles to 1.6–2.6 s for perturbed cycles. The mean rEMG was calculated over the entire cycle and not in specific epochs following the perturbation for several reasons. First, the duration of the perturbation (150–350 ms) encompassed both latencies for reflex and voluntary responses. Second, correction of arm synchronization could occur in varied ways at any time following the perturbation.
Statistical analysis
Independent t-tests were used to determine initial between-group differences in mean cycle periods, amplitudes of arm displacement and magnitudes of absolute phase differences in cycles preceding the perturbation.
Two-way ANOVAs were used to compare the time of in-phase coordination in the transitional interval between groups (control/hemi) and side of arrest (left/right or paretic/non-paretic). Data for each direction of arrest were pooled for this analysis following determination that there were no differences between groups or sides for this variable.
Two-way repeated measures ANOVAs with appropriate post-hoc tests (LSD) were used to detect the changes in cycle periods, magnitudes of phase differences and absolute relative phases within and between groups of subjects for each direction of arrest (forward/backward). For the analysis of cycle period, factors were perturbation (arrested arm, free arm) and side of arrest (left/right or paretic/non-paretic) repeated over five cycles (T, T 0, T 1, T 2, T 3) (df=4, 128 and 4, 176 for control and stroke groups respectively). For the analysis of magnitude, factors were cycles (4, excluding T 0) and side of arrest (left/right). These analyses were done separately for control subjects (df=3, 48) and for individuals with stroke (df=3, 66).
We also compared the period of the perturbed cycle in each subject group between arms with two-way ANOVAs with factors arm (arrested/free) and side of arrest (left/right or paretic/non-paretic) for each direction of swinging.
Absolute relative phase (leading arm) was computed separately for subgroups of patients with right- or left-sided lesions since dominance may have influenced the results. A 3×2 ANOVA with factors, group (control, left-hemi, right-hemi) and side of arrest (left/right or paretic/non-paretic) was computed for all four cycles described above.
To analyze EMG responses to the perturbations, first the consistency of the EMG responses was determined by comparing the root mean square (r) EMG areas of each muscle in the two cycles preceding the arrest with paired t-tests in each individual. Changes in the rEMG values in the perturbed cycle were expressed as percentages of the pre-arrest mean for each shoulder muscle separately and compared between groups with two-way ANOVAs and appropriate post-hoc tests (LSD). Factors were group (control/hemi) and muscle (left/paretic agonist and antagonist; right/non-paretic agonist and antagonist). Analyses were performed separately for the arrests in forward and backward directions. For all analyses, subject means were used and minimal significance levels were P<0.05.
Results
Arm movement prior to perturbation
Figure 2 shows endpoint displacements in the sagittal direction of four representative control subjects and four participants with stroke. Initial movement parameters from the two cycles preceding the perturbation (vertical arrows, Fig. 2) were compared within- and between- groups as a basis for comparison of subsequent changes in arrested cycles (Table 2). In control subjects, cycle periods, movement amplitudes and magnitudes of phase differences were similar for both arms. Synchronization between arms was approximately 20° less than ideal anti-phase movement (the magnitude of the phase difference between arms was 161° and 160° for right and left arms respectively, see Table 2). In participants with hemiparesis, movements were slower (cycle periods were longer; Table 2) for both the non-paretic and paretic arms compared to either arm of the control subjects (P<0.05; P<0.05, respectively). Arm swinging amplitudes of the paretic arm were significantly lower than those of the control subjects and the amplitude of swinging of the paretic arm was significantly less than that of the non-paretic arm (P< 0.05). In this group, bilateral swinging was not strictly reciprocal (magnitude of phase difference was 152° for both arms) in contrast to control subjects (P<0.01).
Trajectories of arm movements during swinging showing perturbations (arrows) and recovery of bilateral coordination. Representative trials are from four control subjects (left panels), and four patients with hemiparesis (right panels). Perturbations were applied to the right and left arms in control subjects and to the paretic, and non-paretic arms in patients, swinging their arms in the forward (top two panels) or backward (bottom two panels) directions. Vertical lines indicate cycle periods. Horizontal bars above each pair of traces indicate the amount of time that both arms moved in the same direction following the perturbation (in-phase coordination)
Effects of perturbation
In control subjects, arrest typically prolonged the oscillatory period of the transitional interval (in 78% of cases, i.e., 168/216 perturbations) for both the arrested and the contralateral free arms by a similar amount (vertical lines, Fig. 2). In the perturbed cycle, the free arm made an obvious adjustment to regain reciprocal coordination and complete the cycle simultaneously with the arrested arm. Reciprocal coordination was regained in the first cycle following the perturbation. This occurred regardless of the direction of arrest or arm arrested.
In contrast, in participants with hemiparesis, arrest resulted in the prolongation of the transitional interval of the arrested arm and shortening or no change of the interval of the free arm for both directions of arrest (Fig. 2). In the majority of patients (7 out of 12) for arrests in the forward direction, the free arm performed two small oscillations within one cycle. In general, the sum of the duration of the two small oscillations was less than the duration of the cycle of the perturbed arm.
In most cases (60%) in control subjects, the differences in oscillatory frequencies due to the arrest resulted in a brief (normalized time 0.120±0.001 of pre-perturbed period) transition from an anti-phase to an in-phase coordination, following which, reciprocal anti-phase swinging was regained within the same cycle (Fig. 2, horizontal bars).
In 70% of the cases (202/288 perturbations) in the stroke group, the normalized amount of time during which both arms moved in the same direction (Fig. 2, right panel, horizontal bars) was significantly greater than in control subjects (0.337±0.098, F1,19 =5.88, P<0.01). In addition, in one subject (Table 1, subject 1), one of the 12 perturbations resulted in the transition to in-phase coordination that persisted until the end of the trial. A perturbation could result in a continuation of the pre-perturbed anti-phase coordination or in a transition to an in-phase coordination in one and the same subject regardless of subject group or side and direction of arm arrest.
Normalized cycle periods were compared separately for each group between arms and sides of arrest (pre-arrest to post-arrest, T–T 3). The two cycles (T −2, T −1) used to determine the mean period of the pre-arrest cycle (T) were highly consistent (0.99 ± 0.01 in control subjects; 1.02 ± 0.02 in patients with hemiparesis). In control subjects (Fig. 3a, b), the perturbed cycles were characterized by similar increases in cycle periods of both the arrested and free arms for the forward direction (F4,128=8.71, P<0.001, post-hoc T–T 0; P<0.001) and for the backward direction (F4,128=12.93, P<0.001, post-hoc T–T 0; P<0.001). No overall effects of arm or side of arrest were detected for either direction. After the arrest, movements in control subjects regained their initial frequency by the first post-arrest cycle (T 1) and the arrest did not affect the magnitude of the phase difference for either direction (T–T 1: 161°±2.8° vs 159°±3°; F3,48=1.75, P>0.05 forward; 160°±2.3° vs 161°±2.4°; F3,48=1.07, P>0.05 backward).
Cycle periods (mean±SE) normalized to the initial means, during (T 0) and after (T 1, T 2, T 3) perturbation in control subjects (left panel) and in patients with hemiparesis (right panel). Initial means of cycle periods (T) are denoted by 1. Means were calculated for right and left arms in control subjects (black lines) and for paretic and non-paretic arms in patients with hemiparesis (grey lines). Arrested arms are indicated in all plots by solid lines and free arms are shown by dashed lines
In contrast to the control subjects, the period of the perturbed cycle (T 0) in participants with hemiparesis was increased only for the arrested arm while that of the free arm was either shortened (forward) or remained the same (backward; Fig. 3c, d). Overall, mean cycle periods were significantly different for each arm (arrested, free) during the perturbed cycle for the forward (F3,44=4.10, P<0.01) and backward directions (F3,44 = 5.90, P<0.01) while there were no differences between paretic and non-paretic arms. In the arrested cycle, there was a significant increase of the cycle period of the arrested arm for the backward direction (F4,176=15.39, P<0.001; post-hoc test for perturbed cycle P<0.001) but not for the forward direction since for this direction, the arrest had opposite effects on the free and the arrested arms (Fig. 3c). For the forward direction, the cycle period decreased for free arms and increased for the arrested arms (paired t-test, P<0.05).
In individuals with hemiparesis, after the arrest, both arms moved with significantly different frequencies. The free arm oscillated faster than the arrested arm resulting in asynchronous bilateral swinging. Recovery of the pre-arrest magnitude of the relative phase required more time than in control subjects. The difference in means between the pre- and the three post-arrest cycles was significant for the forward (F3,66=5.83, P<0.001) and for the backward directions (F3,66=16.7, P<0.001). Reciprocity was regained by the third post-arrest cycle for both the forward (T–T 3: 154°±3.7° vs. 151±4.8°, post-hoc test P>0.05) and the backward (150°±3.4° vs. 149°±5.1°, post-hoc test P>0.05) directions.
The levels of spasticity and paresis were not correlated with the initial response to perturbation (r=0.09 and r=0.23 P>0.05) nor with the ability to rapidly recover reciprocal arm movement (in the first or second post-arrest cycles; r=0.12 and r=0.19 P>0.05).
Leading arm
Means of relative phase before (φ) and in the three post-arrest cycles (φ1, φ2, φ3) were analyzed to determine which arm acted as the ‘leading arm’ in the control of bilateral coordination (Fig. 4). This analysis was done separately for each condition (right arm arrest and left arm arrest) in order to determine if the fact that one arm was attached to the rod affected lead arm preference. The control subjects showed no preference in the leading arm in the pre-arrest cycle (e.g., φ=178.8°±4.1° for right arm arrest and 184.6°±3.1° for left arm arrest, Fig. 4a,b). Determination of the leading arm in the stroke group was done separately for individuals with right- and left-sided hemiparesis. Participants with left-sided hemiparesis led slightly with the paretic left arm in the pre-arrest cycle when the right non-paretic arm was arrested (173.1°±4.6° φ1; Fig. 4d) but there was no preference in initial leading arm when the paretic arm was arrested (177.3°±5.1° φ1; Fig. 4c). Individuals with right-sided hemiparesis did not demonstrate a pre-arrest arm preference for either side (177.4°±2.1° for paretic and 178.4°±2.6° for non-paretic side, Fig. 4e, f) during bilateral swinging.
Arm leading during swinging in control subjects (a, b) and in patients with left-sided (c, d) and right-sided (e, f) hemiparesis. The leading arm is indicated for cycles before the arrest (φ) and for three cycles following the arrest (φ1, φ2, φ3) by solid circles. Data points greater than 180° indicate that the right (R) arm was leading, while those less than 180° indicate that the left (L) arm was leading. The schematic picture at the top of each panel shows which arm was arrested
Only participants with left hemiparesis had a preference for a leading arm (post-hoc test; P<0.01 for both paretic or non-paretic sides) but further inspection of the data revealed that the tendency to lead with the left arm was influenced by the behavior of a single subject (subject # 1; Table 1). When the data were re-analyzed after removal of this subject, this effect was no longer significant (F5,72 = 2.11, P>0.05). For all subject groups, the leading arm did not change due to the perturbation of either arm (2x2 ANOVA, F3,76 = 1.08, P>0.05).
Thus, overall, neither control subjects nor participants with hemiparesis showed any arm leading preference for this task of bilateral swinging.
EMG activity
The arrest altered the EMG activity of shoulder flexors and extensors for the arrested and free arms in the transitional intervals (vertical lines, Fig. 5) in both control subjects and in participants with hemiparesis. The data from a control subject as shown in Fig. 5a, perturbation of the right arm in the forward direction was accompanied by a larger increase of activity of the antagonist ( PD) than the agonist muscle of the arrested arm. In contrast, in the example of the individual with right-sided hemiparesis as shown in Fig. 5b, perturbation applied to the paretic arm in this direction was characterized by the opposite pattern in which the agonist muscle was more active than the antagonist in the perturbed cycle. These patterns were observed in all subjects. The example also shows that in this subject, following perturbation, both arms moved in an in-phase coordination for a short time within the transitional interval (Fig. 5b), and subsequently, the pre-perturbed anti-phase coordination was gradually regained.
Trajectories of arm movements and EMG signals recorded from both left (grey traces) and right (black traces) anterior deltoid (AD) and posterior deltoid (PD) muscles during swinging. Representative trials are from one control subject (a) and one individual with right hemiparesis (b). Perturbations shown by the pulse trace, were applied in the forward direction to the right arm in the control subject and to the paretic arm in the patient. Vertical dotted lines delineate the durations of the transitional interval in each panel
In order to determine the pattern of muscle activation used to regain bilateral coordination after the arrest, we first characterized the stability of the sizes of the agonist and antagonist muscle bursts in the two cycles preceding the perturbed cycle. For both muscles in all groups of subjects, no significant differences were found in mean rEMG for the two pre-arrest cycles. Thus, we were confident in expressing the changes in the rEMG of each muscle in the perturbed cycle as a percentage of their respective pre-arrest means.
The arrest resulted in an increase of muscle activity of both shoulder flexor and extensor muscles in both groups of subjects (Fig. 6). Overall, the increases in the rEMG areas for all muscles (agonist and antagonist for each arm) were significantly smaller in patients with hemiparesis (F1,19=55.41, P<0.001 for arrests in the forward direction; F1,19=7.45, P<0.02 for arrests in the backward direction) compared to the control subjects. For the control group, when the perturbation was applied to the right arm, the increase in antagonist muscle activation for each direction (PD for forward and AD for backward swinging) was significantly greater than the increase in the corresponding agonist. Thus, the antagonist PD had significantly greater activity (51%±18%) than the agonist AD for the forward direction (13±7%; P<0.0001). This was also true for the agonist PD compared to the antagonist AD for the backward direction (18±7% vs 4±6%; P<0.001 Fig. 6a,b). For the left arm however, both agonist and antagonist muscles showed a similar increase in activation with a tendency (P=0.06) for the increase in antagonist activity (27±12%) to be larger than that of the agonist (10±6%) only for the backward direction (Fig. 6b).
Changes in mean root mean square (rEMG) areas (mean±SE) in the transitional interval expressed as percentages of the areas of the pre-arrest cycles for the agonist muscles (white bars; AD for arrest in the forward direction, PD for arrest in the backward direction) and antagonist muscles (grey bars). Data is shown for the arrested arm in control (right/left) subjects (a, b) and in patients (paretic/non-paretic) with hemiparesis (c, d)
In patients with hemiparesis, arrest of the non-paretic arm resulted in similar shoulder muscle activation patterns as those in control subjects. Antagonist muscles had significantly higher activity than the agonist muscles (18±10% vs 4±7%, P<0.001 for arrests in the forward direction; 26±15% vs –3±8%, P<0.001 for the backward direction). However, when the paretic arm was arrested, the increase in antagonist muscle activation was significantly less than that in the agonist muscle (5±10% vs 20±7%, P<0.05 for the forward direction; 4±4% vs 14±8%, P<0.04 for the backward direction).
The pattern of muscle activation in the non-arrested arm tended to be opposite to that of the arrested arm in all groups of subjects, but responses were smaller and agonist-antagonist differences were not significant.
Discussion
Unexpected and transient (~150–350 ms) perturbations of each arm at the level of the wrist were applied in the forward and the backward phases of rhythmical reciprocal arm swinging in non-disabled control subjects and in individuals with hemiparesis due to stroke. Our results show that in control subjects, the perturbation altered the duration of the transitional interval in both the arrested and non-arrested arms. This behavior was stable over a series of repeated arrests without habituation. Perturbation resulted in a short-lasting change in coordination from anti-phase to in-phase following which pre-perturbed anti-phase coordination was regained, usually in the same cycle. In addition, in the arrested arm, activity in the antagonist shoulder muscle increased relatively more than that of the agonist muscle, regardless of the direction of arm movement. In contrast, in participants with hemiparesis, perturbation resulted in disruption of bilateral coordination such that both arms moved with different frequencies during the transitional interval and, in the majority of trials, the period of in-phase coordination lasted longer than in the control subjects. As a result, recovery of the pre-perturbed reciprocal (anti-phase) coordination was delayed, taking up to two additional cycles compared to the control subjects, and did not depend on whether the paretic or the non-paretic arm was arrested. In the paretic arm, the level of shoulder muscle activation in the agonist and antagonist muscles was lower and the pattern of activation was opposite to that of the non-paretic and non-disabled arms.
Response to perturbation and recovery of reciprocal coordination
In individuals with hemiparesis, the perturbation led to both arms moving at different frequencies during a transitional period and a delayed recovery of pre-perturbed bilateral coordination. Several mechanisms may have contributed to this delay. For example, the between-arm coupling prior to perturbation could be weaker in the patients with hemiparesis. This suggestion is supported by findings that, in non-disabled subjects, perturbations occurring near a critical transition frequency when hand coupling is weaker or less stable, caused transitions from anti-phase to in-phase coupling and increased the time of recovery of anti-phase coordination during rhythmical hand movements (Scholz and Kelso 1989). The lower interlimb coupling in the patient group might also have resulted from the interlimb asymmetry, i.e., differences in the amplitude of swinging between the paretic and non-paretic arms. The difference in the amplitude could lead to the arms being in different places in the cycle over time, which, if uncorrected, would result in an unstable coordination.
Another mechanism contributing to the delay in recovery of reciprocal coordination may have been a disruption in the ability of the paretic arm and less affected non-paretic arm to execute movement. This is suggested by the altered pattern of shoulder muscle activation (EMG areas) in the perturbed cycle. Since the EMG areas of agonist and antagonist muscles were computed in the entire cycle, they encompass both reflex and voluntary responses. The shoulder muscles in patients with hemiparesis had smaller responses following perturbation than those in the control subjects, especially in the antagonist muscles of the paretic arm. Our data suggest that the shoulder muscle co-activation was decreased in the less-affected non-paretic arm and was substantially altered in the paretic arm. Lower co-activation in patients with hemiparesis is consistent with previous findings for the single and double-joint arm systems (Levin and Dimov 1997; Chae et al. 2002; Mihaltchev et al. 2005). As revealed by unexpected unloading of preloaded muscles, participants with hemiparesis had smaller zones of agonist and antagonist co-activation than non-disabled subjects, resulting in a loss of arm stability and excessive oscillations around the final arm or hand position in response to unloading. Thus, lower shoulder muscle co-activation may also have contributed to the delay in recovery of bilateral coordination in the present study.
Coordination of bimanual swinging
One way to control coordination of limb movements is to produce an appropriate phase change of oscillations when movement is disrupted due to internal or external perturbations, a mechanism known as ‘phase resetting’ (Andersson and Grillner 1981). We observed an appropriate phase change in the control subjects when the perturbation applied to one arm prolonged the oscillatory periods of both arms simultaneously. Physiologically this is likely to be produced by intersegmentally-linked groups of spinal neurons in lateralized central pattern generators.
The spinal networks involved in bilateral coordination are also modulated from higher motor centers (supplementary and primary motor areas; Brinkman 1984; Donchin et al. 1998; Kazennikov et al. 1999; Gribova et al. 2002) via lateral and ventral descending corticospinal pathways (Lawrence and Kuypers 1968). Our results show that the stroke-related damage led to both arms moving at different frequencies in the perturbed cycle and that recovery of the original coordination was delayed. In the healthy nervous system, control signals correct movement when the phase difference between motions of both arms reaches a critical level that may threaten bilateral stability. If such a correction does not occur, as for example in the case of a coordinated finger flexion-extension movements with increasing speed (Kelso et al. 1986), a de-synchronization or even a change from out-of-phase to in-phase movement can occur. Damage to higher motor centers may result in delays in processing of afferent signals leading to temporal disruption of bilateral coordination. In part, this might explain why individuals with hemiparesis in our experiment had a diminished degree of interlimb coupling in the cycles prior to the perturbation. In contrast to non-disabled subjects (Repp 2002), participants with hemiparesis may be less able to constantly minimize small phase differences in order to sustain coordinated bilateral movement for prolonged periods of time. This minimization may occur via an adjustment in the interaction between two separate central pattern generators (Dietz et al. 1994) controlled by a common central signal to both arms. Supporting this hypothesis is our finding that the recovery of coordination was delayed following the arrest of either arm in patients despite the fact that the altered pattern of shoulder muscle activation was mainly evident only in the paretic arm.
Previous studies have shown that motor centers in both hemispheres are active during the performance of bilateral movement (Tanji et al., 1988) such that both hemispheres work as a common functional unit often referred as a coordinative structure (Bernstein 1967; Kugler 1980) or “generalized motor program” (Schmidt 1979). Two models of inter-hemispheric interaction during bilateral movement have been proposed to explain this cooperation. In one model, at least some components of a general program of bilateral coordination are thought to be generated in one hemisphere, which are then transmitted to the contralateral one (Ivry et al. 2004). For example, based on the finding that the drive of electroencephalographic signals from the dominant to the non-dominant primary sensorimotor cortex prevailed during rhythmical bimanual wrist movements, Serrien et al (2003) suggested that the coordinated bilateral hand movements may be controlled from the dominant left hemisphere. This hypothesis is also supported by the observation of a temporal delay between the activity of the two arms (leading arm) during figure drawing tasks. In such tasks that require visual guidance, either the dominant or non-dominant arm may lead depending on movement direction or task difficulty (Stucchi and Viviani 1993; Franz et al. 2002). In our study, if control of bilateral swinging were lateralized, then the behavior of the right (dominant) arm should have been different from that of the left arm and a leading arm would have been identified. However, our data showed that, at least for tasks such as arm swinging, both arms reacted similarly to the perturbation. Indeed, cycle periods were increased in both arms regardless of the side of arrest and a similar number of cycles were required to regain bilateral coordination following the perturbation. Thus, our data support the alternative hypothesis that signals from both hemispheres are integrated into one common timing process controlling the activity of lateralized central pattern generators (Wiesendanger et al. 1994a).
Sub-cortical structures are the most likely anatomical substrate for the generation of control signals emanating from both hemispheres. Sub-cortical bilateral interaction is suggested by studies demonstrating that split-brain patients preserve temporal, in contrast to spatial, coordination (Ivry and Hazeltine 1998) and findings that TMS-evoked responses from the motor cortex in the ipsilateral arm were facilitated by voluntary contractions of the contralateral hand in subjects with abnormalities of the corpus callosum (Meyer et al. 1995).
Limitations of the study
The results of this study may be generalized only to those patients with mild hemiparesis and little variability in movement parameters. The experimental paradigm required patients to stand for long periods of time while simultaneously moving both arms, which for some post-stroke patients with more severe hemiparesis, may pose a threat to balance. Further studies of the disruption of bilateral temporal coordination due to unilateral stroke-related brain damage may also investigate differences between patients with different amounts of initial arm coupling strengths (strong vs. weak) and the critical frequencies at which phase transitions may occur during forward and backward arm swinging.
References
Andersson O, Grillner S (1981) Peripheral control of the cat’s step cycle. I. Phase dependent effects of ramp-movements of the hip during “fictive locomotion”. Acta Physiol Scand 113:89–101
Archambault P, Pigeon P, Feldman AG, Levin MF (1999) Recruitment and sequencing of different degrees of freedom during pointing movements involving the trunk in healthy and hemiparetic subjects. Exp Brain Res 126:55–67
Berg KO, Wood-Dauphinee SL, Williams JI, Gayton D (1989) Measuring balance in the elderly: preliminary development of an instrument. Physiother Can 41:304–311
Berglund K, Fugl-Meyer AR (1986) Upper extremity function in hemiplegia. A cross validation study of two assessment methods. Scand J Rehab Med 18:155–157
Bernstein NA (1967) The coordination and regulation of movements. Pergamon Press, London
Bohannon RW, Andrews AW (1998) Relationships between impairments in strength of limb muscle actions following stroke. Percept Mot Skills 87:1327–1330
Bourbonnais D, Vanden Noven S (1989) Weakness in patients with hemiparesis. Am J Occup Ther 43:313–319
Brinkman C (1984) Supplementary motor area of the monkey’s cerebral cortex: short and long-time deficit after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4:918–929
Cardoso de Oliveira S (2002) The neuronal basis of bimanual coordination: recent neurophysiological evidence and functional models. Acta Psychol 110:139–159
Carr JH, Shepherd RB (1987) A motor relearning program after stroke, 2nd edn. William Heinemann, London
Carson RG, Rick S, Byblow WD, Abernethy BA, Summers JJ (1999) The timing of intralimb coordination. J Mot Behav 31:113–118
Chae J, Yang G, Park BK, Labatia I (2002). Muscle weakness and cocontraction in upper limb hemiparesis: relationship to motor impairment and physical disability. Neurorehab Neural Repair 16:241–248
Colebatch JG, Gandevia SC, Spira PJ (1986) Voluntary muscle strength in hemiparesis: Distribution of weakness at the elbow. J Neurol Neurosurg Psych 49:1019–1024
Dick JP, Benecke R, Rothwell JC, Day BL (1986) Simple and complex movements in a patient with infarction of the suplementary motor area. Mov Disord 1:255–266
Dietz V, Quintern J, Boos G, Berger W (1986) Obstruction of the swing phase during gait: phase-dependent bilateral leg muscle coordination. Brain Res 384:166–169
Dietz V, Zijlstra W, Duysens J (1994) Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 10:513–520
Donchin O, Gribova A, Steinberg O, Bergman H, Vaaida E (1998) Primary motor cortex is involved in bimanual coordination. Nature 395:274–278
Duncan PW, Propst M, Nelson SG (1983) Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 63:1606–1610
Duysens J, Van de Crommet H (1998) Neural control of locomotion; Part 1: the central pattern generator from cats to humans. Gait Posture 7:131–141
Franz EA, Rowse A, Ballantine B (2002) Does handedness determine which hand leads in a bimanual task? J Mot Behav 34:402–412
Fugl-Meyer AR, Jääsko L, Leyman L, Olsson S, Steglind S (1975) The post-stroke hemiparetic patient. I. A method for evaluation of physical performance. Scand J Rehab Med 7:13–31
Goulet C, Arsenault AB, Bourbonnais D, Laramée MT, Lepage Y. (1996). Effects of transcutaneous electrical nerve stimulation on the H-reflex in spinal spasticity. Scand J Rehab Med 28:169–176
Gribova A, Donchin O, Bergman H, Vaaida E, Cardoso de Oliveira S (2002) Timing of bimanual movements in human and non-human primates in relation to neuronal activity in primary motor cortex and supplementary motor area. Exp Brain Res 146:322–355
Grillner S, Wallen P (1985) Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8:233–261
Ivry RB, Diedrichsen J, Spencer R, Hazeltine E, Semjein A (2004) A cognitive neuroscience perspective on bimanual coordination and interference. In: Swinnen S (ed) Neuro-behavioral determinants of interlimb coordination.. Kluwer, Boston, pp 259–295
Ivry RB, Hazeltine E (1998) Subcortical locus of temporal coupling in the bimanual movements of a callosotomy patients. Hum Mov Sci 18:345–375
Kazennikov O, Hyland B, Corboz M, Babalian A, Rouiller EM, Wiesendanger M (1999) Neural activity of supplementary and primary motor areas in monkeys and its relation to bimanual and unimanual movement sequences. Neuroscience 6:203–210
Kazennikov O, Perrig S., Wiesendanger M (2002) Kinematics of a coordinated goal-directed bimanual task. Behav Brain Res 134:83–91
Kelso JA, Scholz JP, Schoner G (1986) Non-equilibrium phase transitions in coordinated biological motion: critical fluctuations. Phys Lett 118:279–284
Kugler PN, Kelso JAS, Turvey MT (1980) On the control and coordinative structure as dissapative structures. I. Theoretical lines of convergence. In: Stelmach GE, Requin J (eds) Tutorials in motor behavior. North Holland, Amsterdam, pp 3–47
Lawrence DG, Kuypers NGJM (1968) The functional organisation of the motor system in the monkey. Brain 91:1–14
Levin MF (1996) Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain 119:281–293
Levin MF, Dimov M (1997) Spatial zones for muscle coactivation and the control of postural stability. Brain Res 757:43–59
Levin MF, Hui-Chan CWY (1992) Relief of hemiparetic spasticity by TENS is associated with improvements in reflex voluntary functions. Electroenceph Clin Neurophysiol 85:131–142
Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A (1995) Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118:429–440
Mihaltchev P, Archambault PS, Feldman AG, Levin MF (2005) Control of double-joint arm posture in patients with unilateral brain damage. Exp Brain Res 163:468–486
Nadeau S, Gravel D, Arsenault AB, Bourbonnais D, Goyette M (1997). Dynamometric assessment of the plantarflexors in hemiparetic individual: relations between muscular, gait and clinical parameters. Scand J Rehab Med 29:137–146
Repp B (2002) Phase correction in sensorimotor synchronisation: Nonlinearities in voluntary and involuntary responses to perturbations. Hum Mov Sci 21:1–37
Rice MS, Newell KM (2001) Interlimb coupling and left hemiplegia because of right cerebral vascular accident. Occup Ther J Res 21:12–28
Rose DK, Winstein CJ (2002) Temporal control of bimanual reaching following unilateral hemispheric brain damage. Soc Neurosci (Abstract 666.3)
Schmidt RA, Zelaznik H, Hawkins B, Frank JS, Quinn JT (1979) Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 47:415–451
Scholz JP, Kelso JAS, (1989) A quantitative approach to understanding the formations and change of coordinated movement patterns. J Mot Behav 21:122–144
Semjen A, Summers JJ, Cattaert D (1995) Hand coordination in bimanual circle drawing. J Exp Psychol Hum Percept Perform 21:1139–1157
Serrien DJ, Cassidy MJ, Brown P (2003) The importance of the dominant hemisphere in the organisation of bimanual movements. Hum Brain Mapp 18:296–305
Stucchi N, Viviani P (1993) Cerebral dominance and asynchrony between bimanual two-dimensional movements. J Exp Psychol Hum Percept Perform 19:1200–1220
Swinnen SP, Jardin K, Meulenbroek R (1996) Between limb asynchronies during bimanual coordination: effects of manual dominance and attentional cueing. Neuropsychology 34:1203–1213
Swinnen SP (2002). Intermanual coordination: from behavioral principles to neural-network interactions. Nature Rev Neurosci 3:350–361
Tanji J, Okano K, Sato KC (1988) Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol 60:325–343
Taub E, Wolf SL (1997) Constraint induction techniques to facilitate upper extremity use in stroke patients. Topics in Stroke Rehabil 3:1–24
Ustinova KI, Balasubramaniam R, Goussev V, Fung J, Levin MF (2004) Impairment of coordination during bimanual arm swinging in adults with hemiparesis.p46 (ISEK Abstract)
Wei K, Wertman G, Sternad D (2003) Interactions between rhythmic and discrete components in a bimanual task. Mot Cont 7:134–154
Wiesendanger M, Wicki U, Rouiller E (1994a) Neural, dynamical and cognitive constraints. In: Swinnen SP, Heuer H, Massion J, Casaer P (eds) Interlimb coordination. Academic, San Diego, pp 179–207
Wiesendanger M, Kaluzny P, Kazennikov O, Palmeri A, Perrig S (1994b) Temporal coordination in bimanual actions. Can J Physiol Pharmacol 72:591–594
Zehr P, Kido A (2001) Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol (Lond) 537:1103–1045
Acknowledgements
We wish to thank the patients who volunteered for this study as well as Valery Goussev, Ramesh Balasubramaniam, Laura Galiana, Caroline Paquette and Anatol Feldman. KU was supported by a Focus-on-Stroke Training Program grant from the Heart and Stroke Foundation of Canada/Canadian Institutes of Health Research (CIHR)/Canadian Stroke Network/Astra Zeneca. The project was partially supported by NSERC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ustinova, K., Fung, J. & Levin, M. Disruption of bilateral temporal coordination during arm swinging in patients with hemiparesis. Exp Brain Res 169, 194–207 (2006). https://doi.org/10.1007/s00221-005-0136-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0136-5