Abstract
Seaweeds are considered a promising ingredient to use in developing novel food products, due to their nutritional composition and richness in bioactive compounds. The aim of this work is to investigate the effect of four seaweeds (Chlorella vulgaris CH, Laminaria ochroleuca KO, Ulva spp. SL, Arthrospira platensis SP) enrichment (1%, 2.5%, 4%, flour basis) on technological performance and nutraceutical prospective of soft wheat bread. To do this, dough rheological properties measured by means of farinograph and consistograph parameters, baking response (loaf volume, colour, and porosity), and nutraceutical potential of bread (total soluble phenolic compounds, pigments, and antioxidant activity) were evaluated. Algae addition significantly decreased water absorption and development time, except to SL, and increased stability up to 2.5%, and tenacity for KO. In terms of bread properties, the specific volume was positively affected by SP addition, while negatively by KO enrichment; the porosity enhanced especially for SP and CH. Then, bread crumbs and crusts showed a darker and greener colour, especially for green algae. All seaweeds enriched breads showed significant higher phenolic compounds, chlorophylls a and b, and yellow pigment. At last, SP, SL, and CH at 4% level pointed out the highest antioxidant capacity. Our results suggested that the four algal biomasses could be a suitable ingredient in bread making, also in a perspective of a healthier and sustainable diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the stronger awareness towards health benefits associated to the consumption of specific foods or food ingredients, such as macro- and micro-algae, has been influencing the choices of customers and, as a consequent, the global demand for seaweeds has been growing rapidly. According to the report by Allied Market Research [1], the global seaweed market size was valued at $4097.93 million in 2017 and is projected to reach $9075.65 million by 2024. The human food segment accounts for the highest share in seaweed market, due to the increasing utilization of seaweed for human consumption. In fact, seaweeds have traditionally been forming part of the Oriental diet, but, recently, consumption of these sea vegetables has also been increasing in Western countries, as consequence of globalization, migration, multiculturalism, various end-use for industries, and the growing awareness towards health benefits [2]. To encourage greater consumption of algae in Western countries as an important alternative source of protein for a sustainable global food system and food security, the European Commission has provided a well-targeted support for the industry of algae in the Blue Growth initiative and the Farm to Fork Strategy. In support to these initiatives, the European researchers are increasing studies on the possible applications of seaweeds across various end-use industries.
The relevance of seaweeds is due to their richness in bioactive compounds and nutrients such as polysaccharides, proteins, minerals, vitamins, and phenols, and their low lipid content with high concentrations of certain long-chain polyunsaturated fatty acids [3], although chemical composition strongly depends on species, origin, season of harvest, and environmental conditions [2]. Furthermore, a regular seaweeds consumption is associated with health benefits, including anti-inflammatories, antimicrobial, antihypertensive, antihyperlipidemic, immunomodulatory, antidiabetic, and anticoagulant activities, weight management, and disease prevention [4,5,6,7,8]. Therefore, algae can be used to extract compounds for pharmaceutical, cosmetics, and food industries as source of phycocolloids, thickening, and gelling agents [3]. Furthermore, palatable and healthy algae-based foods, such as pasta, bread, biscuits, snacks, vegetable soups, candy bars or gums, yoghurts, ice creams, drinks, etc., were developed [9,10,11,12,13,14,15]. Seaweeds are also interesting for their technological properties: they can influence textural properties, colour stability, solubility, water/oil absorption capacity, foaming capacity, and emulsification when added to other ingredients in food making [16]. For all these reasons, they could be promoted as functional ingredients or nutraceuticals [4, 17].
Nowadays, many studies have focused attention on two micro-algae, Spirulina and Chlorella, being the most cultivated worldwide today [18], as ingredients for functional foods. On the contrary, few researchers have evaluated the effect of enrichment of other strains into algae-based foods. In light of these considerations, the aim of this work was to investigate the influence of four seaweeds [Chlorella vulgaris, Laminaria ochroleuca (Kombu), Ulva spp. (Sea lettuce), Arthrospira platensis (Spirulina)] at different integration level (1%, 2.5%, and 4%) on dough rheological properties, and final quality and relative antioxidant potential of enriched breads. To the best of our knowledge, there is only one study dealing with the effect of Kombu addition on cereal-based baked products [19], while there are no literature data on the use of Sea lettuce for the same purpose. The choice to investigate Kombu and Sea lettuce is due to the fact that they are algae commonly consumed and readily available in markets, but currently underutilised in food industry.
Materials and methods
Materials
Commercial refined soft wheat flour and four seaweeds, Kombu (brown alga), Chlorella (green alga), Sea lettuce (green alga), and Spirulina (blue-green alga), were considered in this study. Algae were produced and purchased from the following companies: Chlorella from Cibocrudo srl [Ciciliano (RM), Italy], Kombu from La finestra sul cielo S.p.A. [Villareggia (TO), Italy], Sea lettuce from Consonni bioalghe srl (Milan, Italy), and Spirulina from Ellegi frutta secca Sas (Rome, Italy). Chlorella, Kombu, and Spirulina were bought powdered, whereas Sea lettuce was ground by a Bühler MLI 203 sifter (Milan, Italy) and sieved to obtain a fine flour with particle sizes from 400 to 500 μm. Seaweeds, flours, fresh compressed yeast, and salt were bought from local markets.
Reagents used to perform the analyses
Analytical reagent grade hydrochloric acid and methanol were obtained from Carlo Erba Reagents (Milan, Italy). Standards of gallic acid, Folin–Ciocalteu reagent, was purchased from Sigma–Aldrich (Milan, Italy), as well as 2,2-diphenyl-1-picrylhydrazyl radical (DPPH), potassium persulfate and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox). K-TDFR reagent kit, were by Megazyme Int., (Wicklow, Ireland). Deionized water was produced by a Milli-Q unit (Millipore, Bedford, MA, USA).
Proximate composition
The determination of the proximate composition of the matrices under study was performed in triplicate and the data were expressed as weight percentage on a dry weight basis (%dw). Moisture, proteins, lipids, and ash were determined by the ICC standard methods 110/1, 105/2, 136, 104/1, respectively [20]. Protein content was estimated using the conversion factor 5.70 for wheat flour and 6.25 for seaweeds. Total dietary fiber (TDF) content was measured according to Lee et al. [21].
FTIR analysis
FTIR-ATR spectrometer (iS 10 FT-IR Nicolet Thermo Fisher Scientific Inc., USA) equipped with a diamond crystal cell (ATR) was used for MIR spectra acquisition. The spectra were acquired at 4 cm−1 resolution, 32 scans of each milled sample, an interval between spectra data points of 0.482 cm−1 CO2 atmospheric correction, in the wavenumber range of 4000–650 cm−1. A background spectrum of air was collected to correct the sample spectra and after scanning each sample, the ATR crystal was cleaned with propan-2-ol and ethanol and the cleanliness of ATR crystal was verified. Measurements were repeated 10 times and spectra were acquired and then processed with the OMNIC™ software (Thermo Fisher Scientific Inc., USA).
Rheological tests
Water absorption (WA) at 14% moisture content, dough stability (DS), and development time (DT) were carried out with a Brabender farinograph, according to Standard Method No. 54‐21 [22]. The dough strength (W), dough tenacity (P), dough extensibility (L), configuration ratio (P/L), and elasticity index (Ie) of wheat flour were determined by means of a Chopin Alveograph (Method 54‐30 A) (AACC, 2003). The elasticity index is defined as the percentage ratio between pressure P200 after 200 mL volume of air has been blown into the dough test piece or bubble and the maximum pressure (P). Determination of alveoconsistograph properties of dough at constant and adapted hydration was obtained using a Chopin Consistograph (Method 54‐50) (AACC, 2003). These properties were: maximum pressure recorded during kneading (PrMax); water absorption capacity of sample at a target pressure of 2200 ± 100 mm H2O (HYD2200); time to reach maximum pressure (TprMax); tolerance (Tol), i.e. time elapsed since dough consistency reaches its maximum until it decreases down to a 20%; drop in pressure at 250 s from PrMax minus 20% (D250); drop in pressure at 450 s from PrMax minus 20% (D450). Decay values at 250 s and 450 s are negatively correlated with dough mixing stability.
Since wheat flour/seaweeds blends did not have the chemical–physical characteristics for the alveographic test (excessive viscosity and low extensibility), especially for those with Kombu, the consistograph tests were considered to measure the water absorption capacity of blends, and to follow dough behaviour during mixing.
Bread making procedure
Different types of bread were considered: one of soft wheat flour, used as a reference (the Control bread, indicated by CO), and others with wheat flour/seaweeds blends at three levels (99/1%, 97.5/2.5%, and 96/4%). Therefore, bread loaves enriched with 1% Chlorella (CH1), 2.5% Chlorella (CH2.5), 4% Chlorella (CH4), 1% Kombu (KO1), 2.5% Kombu (KO2.5), 4% Kombu (KO4), 1% Sea lettuce (SL1), 2.5% Sea lettuce (SL2.5), 4% Sea lettuce (SL4), 1% Spirulina (SP1), 2.5% Spirulina (SP2.5), and 4% Spirulina (SP4) were produced. The choice of enriching soft wheat flour with maximum algae level at 4% was determined considering that some authors found that higher levels could negatively affect the qualitative and sensory characteristics of foods [23,24,25].
The dough was made with 500 g flour (14% moisture basis), water (the optimum quantity was determined by means of the Farinograph), 20 g of compressed baker’s yeast, and salt (4% on flour weight). The ingredients were mixed for 6 min in a planetary bread mixer (Quick 20 by Sottoriva, Marano, Italy). Once the dough was formed, it was fermented for 30 min in a fermentation cabinet at 30 °C. Then, the dough was scaled into three equal pieces, which were placed in baking tins and proofed for 1.50 min at 30 °C. At the end, they were baked for 28 min at 220 °C in a convection/steam oven. Baking tests were performed on each blend using three replicates.
Volume measurements
The volume of bread loaf was measured 24 h after removal from the oven using method 10–05.01 [22], based on rapeseed displacement. The specific volume (three replicates) was determined through the volume/weight ratio and expressed in cm3 g−1.
Colour measurement and image analysis
Colour measurements were taken on crumb of bread loaves, using a Chroma Meter CR‐200 (Konica Minolta, Tokyo, Japan) and according to CIE-Lab system of lightness (L), redness (a*), and yellowness (b*). The tests took place on day 1, and the results are the average of measurements of four different points per sample.
To assess crumb porosity, images of central slices (20 mm thickness) of each loaf were acquired twice using a digital camera Nikon D850 at a high resolution and a colour depth of 16 bits, saving the captured images in uncompressed RAW format. A region of interest (ROI) of 650 × 650 pixels, representative of the whole sample surface, was extracted from each image using the Adobe Photoshop. Then, the collected images were processed by Image Pro 10 software (Media Cybernetics Inc., USA). Morphological characterization of the bubble area (mm2) and porosity (%), i.e. the area of pores over the total area, were calculated. Furthermore, pores were classified into five classes CAi based on their area (CA1: bubbles with area < 0.49 mm2; CA2: bubbles with area between 0.50 and 0.99 mm2; CA3: bubbles with area between 1.00 and 4.99 mm2; CA4: bubbles with area between 5.00 and 49.99 mm2; CA5: bubbles with area ≥ 50.00 mm2). The CAi classes were calculated as the ratio between the sub-area and total pore area.
Bioactive compounds and antioxidant capacity determination
Total soluble phenolic compounds (TSPC) and antioxidant activity (AA) were determined on methanolic extracts. Briefly, 2 g of milled freeze dry bread were extract with 25 ml of a hydroalcoholic solution (methanol: water = 80:20, v/v) acidified with 0.1% HCl (v/v) under magnetic stirring (300 rpm), for 30 min, at room temperature and in the dark. The mixture was then submitted to an ultrasound-assisted extraction for another 30 min (40 kHz, 10 °C). The resulting extracts were then centrifuged at 6792 g, for 15 min at 4 °C. Pellets were extracted once again in the same manner. Then, the supernatants were collected and immediately analysed. TSPC and AA were determinate according to Ciccoritti et al. [26] and the data were expressed as mg gallic acid equivalents (GAE) and mg of Trolox equivalent antioxidant capacity per kilogram (mg TEAC/kg dw) using a gallic acid and Trolox dose–response curve.
Pigments, i.e. chlorophyll a (ChA), chlorophyll b (ChB), and b carotenoids (yellow pigment YPG), were determined using methanol as a solvent accordingly to Dere et al. [27]. The results were expressed as micrograms of chlorophyll a, chlorophyll b, and b carotenoids per grams of dry weight (μg/g dw).
All determination was performed in triplicate.
Statistical analysis
Differences in all measured properties were determined using a one‐way analysis of variance (ANOVA) and the non‐parametric Dunn’s post hoc test at a significance level of 5%. Data were processed using SPSS statistical software (version 22, SPSS, Chicago, IL, USA).
Results and discussion
Wheat flour and seaweeds characteristics
Biochemical composition of the soft wheat flour and four commercial seaweeds (Chlorella, Kombu, Sea lettuce, and Spirulina) is shown in Table 1. The wheat flour proximal composition showed a moisture level of 14.3%, ash 0.56 g/100 g dw, total protein of 12.5 g/100 g dw, lipids of 0.7 g/100 g dw, and total dietary fiber of 2.5% g/100 g dw. These values were typical of the flours used for bread making.
Regarding the seaweeds, it is well known that protein content mainly depends on algal species, their geographic area, year, season, and environmental conditions [28]. Microalgae as Spirulina and Chlorella are rich in protein, containing up to 70% dry weight protein. For this reason, they are sold as supplements or used to enrich many foods. Although to a lesser extent than micro-algae, green macro-algae, such as Sea lettuce, have high level of protein. On the contrary, brown algae, such as Kombu, show the lowest protein content (below 15 g/100 g dw), in comparison to all the various groups of algae [2, 28]. Our results confirm this trend. Spirulina showed the highest protein contents (63.5 g/100 g dw), followed by Chlorella (58.1 g/100 g dw), Sea lettuce (15.9 g/100 g dw), and Kombu (7.1 g/100 g dw). Seaweeds typically contain low lipid content, mainly made up of polyunsaturated fatty acids, most noteworthy as functional foods [28]. Lipid content ranged from 2.0 g/100 g dw for Sea lettuce to 8.2 g/100 g dw to Spirulina. Carbohydrates varied from 9.1 g/100 g dw for Spirulina to 16.0 g/100 g dw for Kombu. Edible macro-algae usually contain higher amounts of dietary fiber than micro-algae [28]. Kombu exhibited the highest value (35.0 g/100 g dw), similarly to Sea lettuce (34.4 g/100 g dw). Chlorella and Spirulina values were lower (12.1 g/100 g dw and 7.0 g/100 g dw, respectively). The enrichment of foods with seaweeds flours with low or null fiber content could help to reach the recommended daily fiber intake of 30–35 g/day for men and 25–32 g/day for women in most Western countries [29].
Infrared spectra of seaweeds
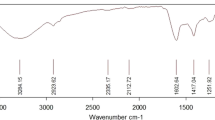
FTIR-ATR spectra of seaweeds samples (Chlorella, Kombu, Sea lettuce, and Spirulina) in the range 4000–650 cm−1 revealed the presence of hydroxyl, amino, carboxylic, and carbonyl groups (Fig. 1). In particular, a variable number of characteristic absorption peaks in relation to the different algae (from 8 to 10) was well noticeable (Fig. 1, Table 2) and revealed a different chemical composition of samples. In detail, the first peak (around 3280–3230 cm−1) could be assigned to the OH and NH stretching vibrations, while the bands observed between 3000 and 2800 cm−1 were due to CH and CH2 stretching vibrations. Carbonyl (C double bond O) stretching of carboxylic acids was found around 1750–1580 cm−1, while bands involving the bending vibrations of (N–H) bending (C–N) stretching groups were centred at 1570–1400 cm−1 [30,31,32].
Absorption band maxima in the mid-infrared region between 1390 and 1280 cm−1 were associated with the typical absorptions due to the stretching of C–O bonds and O–H bending vibration like those of alcohols moieties found in carbohydrates, celluloses, and hemicelluloses present in seaweeds [30]. Moreover, a number of signals was found at 1200–970 cm−1 and 950–800 cm−1, due to stretching vibration of glycosidic bonds [33].
According to Ponnuswamy et al. [30], the region from 3000 to 3600 cm−1 was characterized by the typical bands of water and protein such as O–H and N–H stretching, whereas the spectral regions between 3000 and 2800 cm−1 typical for CH2 stretching could be associated to lipid and carbohydrate (Table 2). Furthermore, Ponnuswamy et al. [30] reported that the region between 1750 and 1470 cm−1 was characterized by the C=O stretching, (N–H) bending and (C–N) stretching strongly correlated to the proteins. The peak around 1480–1400 cm−1 was assigned to the CH2 and CH3 binding with methyl and indicated a representative feature of proteins and lipid. The region from 1240 to 1200 cm−1 was peculiar for P=O of the nucleic acid and compounds containing phosphate, while the signals between 1150 and 800 cm−1, assigned (–O–C) (C–O–C) and planar C–H bending, referred to carbohydrates such as polysaccharides [30, 33]. As depicted in Fig. 1, significant differences in these spectral regions were found highlighting a significant quality quantitative composition variation among the different seaweeds samples. In particular, the highest protein content samples (Spirulina and Chlorella) showed the highest peak height in the spectral regions between 1580 and 1400 cm−1, differently from Kombu and Sea lettuce, which were significantly lower at around 1650–1600 cm−1 and completely absent at around 1540 cm−1. Differences in the proteins content were also observed by chemical determination reported in Table 1. Other important spectral variations among the samples were found in region 3000–2800 cm−1, characterized by lowest signal for Kombu and Sea lettuce in comparison with Chlorella and Spirulina. As previously reported, this spectral region was generally referred to lipids (Table 2), which were low in Kombu and Sea lettuce respect to Chlorella and Spirulina. The spectral region between 1150 and 800 cm−1, generally referred to algae polysaccharides, revealed significantly differences, showing highest signal for Kombu and Sea Lettuce, which were characterized by highest carbohydrates and total dietary fiber content (Fig. 1). Other minor spectral signal variations were reported in the regions at around 1390–1280 cm−1 (C–O stretching and OH bending of alcohols). As reported in Fig. 1, Chlorella and Spirulina showed high peak compared to Kombu and Sea Lettuce.
Rheological characteristics of doughs
The rheological characteristics of control sample showed dough strength W = 232 × 10−4 J; dough tenacity P = 64 mm; dough extensibility L = 110 mm; configuration ratio P/L = 0.58; elasticity index Ie = 58.2%. Blending the wheat flour with seaweeds influenced dough technological properties, as showed in Table 3, because only wheat proteins (gliadin and glutenin) are able to form gluten network. As well known, wheat flour protein quantity and quality affected dough viscoelasticity [34].
The water absorption ranged between 51.6 and 53.6% for Chlorella; between 50.1 and 52.7% for Kombu; between 53.7 and 58.7% for Sea lettuce; between 50.9 and 53.9% for Spirulina. WA values of blends were lower than CO except for SL2.5 and SL4. It could depend on the great number of hydroxyl groups existing in fiber structure, which allow more hydrogen bonding with water than gluten and starch [35]. The incorporation of algae strongly reduced dough development time for all samples, going from 11.9 to about 1.0 min for Chlorella, Kombu, and Spirulina. Sea lettuce had a less markable decrease in DT (from 9.8 min for SL1 to 2.6 min for SL2.5). It might be a consequence of the combined action of fat, fiber, and proteins [36]. Furthermore, weaker gluten-protein network can lead to a greater water diffusion into dough, reducing mixing time [13]. Dough stability noticeably increased for all blends at 1% seaweed and CH2.5 and KO2.5, due to the presence of hydrocolloids (mainly alginate, agar, and carrageenan [28]), whereas it strongly decreased for KO4 and SL4. This decrease is probably due to an increase of viscosity of hydrated algae fibers, especially for Kombu and Sea lettuce, which have high fiber values.
Consistograph was employed to measure the water absorption capacity of flours, by following dough behaviour during mixing and by monitoring the pressure applied by the dough. Higher pressure corresponds to a tougher dough, i.e. under-hydrated dough. Maximum pressure (PrMax) decreases when hydration level increases. Maximum pressure is measured at a target consistency of 2200 mb and the water absorption needed to reach the target consistency (HYD2200) was calculated. The hydration measured by consistograph can be compared to the farinograph water absorption, although could be lower than farinograph values (because a firmer consistency is sought) [37]. Our results indicated that HYD2200 was increased by fiber and hydrocolloids addition (Table 3), according to results previously obtained [36, 38, 39]. Regarding PrMax, Chlorella and Spirulina showed a decrease in pressure with increasing algae content (from 3231 to 2590 mb, and from 3196 to 2775 mb, respectively), whereas Kombu and Sea lettuce showed an opposite behaviour (from 2741 to 3070 mb, and from 2715 to 3706 mb, respectively) (Table 3). High values of non-gluten proteins could make weaker the gluten network, reducing the number of intra/inter chain disulfide bonds, and decreased tenacity [40]. On the contrary, the presence of high fiber values increased the resistance to mixing for dough with Kombu and Sea lettuce; therefore, PrMax increased with increasing algae content. For the same reasons, time to reach maximum pressure (TprMax) increased as a consequence of higher levels of fiber incorporated with these seaweed flours for macro-algae (from 245 to 330 s for Kombu, and from 193 to 233 s for Sea lettuce), whereas decreased for micro-algae (from 249 to 139 s for Chlorella, and from 223 to 166 s for Sea lettuce). Studies have shown similar behaviour, highlighting that a different extent depends on fiber origin and its composition [36, 38]. In particular, fibers and microcrystalline cellulose with high values of insoluble fiber contents yielded low increase of TprMax, while high values of soluble fiber contents produced high increase of TprMax. In general, high non-gluten proteins content has a positive effect on tolerance, whereas high fat and fiber contents decrease tolerance [36, 41,42,43]. The interaction of these three parameters caused a masked and unclear effect on all doughs, also in comparison with CO. At last, decay values at 250 s (D250) and 450 s (D450) of blends were higher than those of CO, with the exception of Kombu. It means that the algae addition makes doughs less stable. Furthermore, stability decreased with increasing micro-algae, SL1 and SL2.5 content. On the contrary, it increased as increase Kombu levels. These results are in accordance with previous studies. Stability is increased by fibers and/or small amounts of fats, and it is diminished by non-gluten proteins and/or high amounts of fats [36, 38, 41,42,43,44].
Physical characteristics of breads
The specific volume of bread loaves is generally considered a measure of the bread expansion. The effects of algae incorporation on specific volumes are shown in Table 4. The enrichment with Spirulina positively affected specific volume (on average increase about 5%) whereas an opposite behaviour was observed for Kombu, Sea lettuce, and CH4. The different performance could be due to dilution and disruption effects on wheat proteins, water holding, and interaction with fibers and non‐gluten proteins, which implies a reduction of the extensibility and weakening of the gluten network [38, 45, 46]. In addition, as previously reported by Russel et al. [39], the presence of algae hydrocolloids could positively affect specific volume with the exception of alginate. In fact, alginate, the major polysaccharide of brown algae [28], could be the cause of the reduction in the bread volume, even at low Kombu content.
Colour parameters of bread crust and crumb are reported in Table 4. They were significantly influenced by algae addition. Due to their pigmentation, seaweeds caused a darkening effect for almost all samples (L* was significantly lower than CO), and a shift towards green and blue for the samples enriched with green algae (a* and b* were significantly lower than CO, respectively). The high chlorophyll content in blue-green and green algae [47] also influenced bread crumb colour. As algae concentration increased, lightness decreased in relation to CO, especially for Chlorella and Spirulina. At the same time, greenness increased for all the samples with the exception of KO4. In fact, the presence of fucoxanthin, various xanthophylls and β-carotene as dominant pigments in brown algae masks effect of chlorophylls a [48]. At last, yellowness decreased as the algae content increased, especially for SP4. It could be due to the presence of phycocyanin in Spirulina, a natural pigment often used as food colorant [10].
Image analysis was applied to assess crumb porosity. Representative images of the whole surface of slices of each loaf bread are shown in Fig. 2. The addition of seaweeds significantly influenced the area of porosity from 18.9% of CO to 56.5% of SP2.5 (Table 5). High porosity was observed especially for Spirulina and Chlorella, whereas the increase for Kombu and Sea lettuce was lower. A similar behaviour was also reflected in the trend of the five classes of pores (Table 5). Although the percentages of each class were similar for almost all the samples, differences in cell area were detected in particular for SP2.5, with 85% of pores in CA4 and CA5, and for KO4 and CO, with 25.5% and 44.0% of pores in CA4 and CA5, respectively. Correspondingly, small cell areas (< 1 mm2) accounted around 27.3% of the total pore area for KO4 and 15.6% for CO, while 4.7% for SP2.5 and 8.3% for SL2.5. Furthermore, almost all samples have the highest proportion of alveoli in class CA4, with the exception of SP2.5 (54% of pores in CA5), and CO and KO4 (with 40.4% and 47.2% of pores in CA3, respectively). Adding different amounts of Kombu to blends strongly affected the bread porosity. In fact, as the Kombu flour increases, the porosity in the higher classes has decreased, until it is zero in CA5 for KO4, and increased in the lower classes. Different behaviour from other algae was recorded for blends with Sea lettuce. In fact, the highest porosity occurred for SP2.5 and not for the composite with 1% of algae, as was the case for blends with the other three seaweeds. These results are in accordance with previous studies. The use of brown macro-algae as bread ingredient can affect crumb texture, reducing its porosity especially for an algae content above 4% [24]. It can be due to high dietary fiber content and interaction with fibers and non‐gluten proteins, which diminish the gas‐retention ability [49]. On the contrary, the high content of non-gluten proteins for micro-algae (from 63.5 g/100 g for Spirulina and 58.1 g/100 g for Chlorella) and the low fiber content (7.0 g/100 g for Spirulina and 12.1 g/100 g for Chlorella) decreased dough stability, favoured gas cell coalescence, i.e. a phenomenon that deteriorates the homogeneity of the cellular structure of bread crumb, and produced a less homogeneous structure with large pores.
Bioactive compounds and antioxidant capacity
The enrichment of wheat bread with algae significantly increased the amount of bioactive compounds with an increase directly proportional to the seaweeds content and depending on genotype (Table 6). The highest amount of total soluble phenols was recorded for Sea lettuce with an increase of 3.8% (SL1), 10.2% (SL2.5), and 25.2% (SL4) respect to CO, whereas the lowest value was observed for bread enriched with Kombu. Unlike Mabeau and Fleurence [50], green seaweed showed the highest phenols concentrations compared to brown seaweeds. This difference could be due to seasonal changes of polyphenols concentrations, to environmental factors (light, saltiness or temperature), and to variation within the different parts of thalli, such as old versus new thalli, basal part or frond [2, 51]. The TSPC values of bread with Spirulina were similar to those found in previous studies. Fradinho et al. [10] obtained a value of 0.24 mg GAE/g dw for pasta enriched with Spirulina at 2% level, whereas Rodriguez De Marco et al. [13] found a value of 0.55 mg GAE/g dw for pasta enriched with Spirulina at 5% level. Batista et al. [52] achieved total phenolic compounds values of 0.42 mg GAE/g dw and 0.08 mg GAE/g dw for cookies enhanced with Spirulina and Chlorella at 2% level, respectively. Regarding the YPG, expressed as β-carotene equivalent, all samples showed a significant increase in comparison with CO, except for KO1. Furthermore, Chlorella and Spirulina at 4% level showed the maximum values (213.2 ± 5.0 µg/g dw and 191.3 ± 5.1 µg/g dw of β-carotene), while the lowest was found for Kombu. Among the enriched breads, the highest amount of chlorophylls was found for SL4 (ChA 1.71 ± 0.10 µg/g dw and ChB 3.04 ± 0.18 µg/g dw), whereas the lowest for KO1 (ChA 0.14 ± 0.01 µg/g dw and ChB 0.27 ± 0.02 µg/g dw). Pigment variation could be strongly related to the solar radiation; higher content is often observed in summer and lower in winter [53]. Compared to control bread, seaweeds incorporation at 4% level significantly improved the antioxidant capacity of all samples. SL4 and SP4 showed the highest increase, followed by CH4 and KO4. In light of this, seaweeds can be considered as a potential source of antioxidants and phenols [54].
Conclusions
The results indicated that the dough rheological properties were strongly influenced by the algal species and the enrichment amount. In terms of technological performance, the combined action of non-gluten proteins, fats, and fibers from algae modified dough rheological properties, basically by reducing water absorption and mixing time, except for doughs enriched with Sea lettuce, by increasing stability up to 2.5% addition, and by increasing tenacity for Kombu. Moreover, bread showed an increase of loaves specific volume only for different wheat-to-Sea lettuce ratios. Furthermore, bread loaves porosity was positively affected by algae enrichment, albeit to a lesser extent for Kombu. Then, high content of chlorophyll and carotenoids changed the colour of crust and crumb, by producing a darkening effect, and a shift towards green and blue for breads enriched with green algae. In terms of nutraceutical potential, the seaweeds addition produced a strong and increasing effect on total soluble phenolic compounds, pigments, and antioxidant activity, especially for blends with Sea lettuce and Spirulina at 4% level. Our results demonstrated that seaweeds supplementation could represent a practical way of formulating functional bread with attractive qualitative and nutraceutical characteristics.
References
Allied Market Research (2018) Seaweed market overview. https://www.alliedmarketresearch.com/seaweed-market. Accessed 30 Mar 2021
Fernandez-Segovia I, Lerma-Garcia MJ, Fuentes A, Barat JM (2018) Characterization of Spanish powdered seaweeds: composition, antioxidant capacity and technological properties. Food Res Int 111:212–219
Gómez Ordóñez E, Jiménez Escrig A, Rupérez P (2010) Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res Int 43:2289–2294
Bishop WM, Zubeck HM (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci 2(5):147–152
Cardoso SM, Carvalho L, Silva PJ, Rodrigues MS, Pereira OR, Pereira L (2014) Bioproducts from seaweeds: a review with special focus on the Iberian Peninsula. Curr Org Chem 18(7):896–917
Garcia-Segovia P, Pagan-moreno MJ, Lara IF, Martínez-Monzó J (2017) Effect of microalgae incorporation on physicochemical and textural properties in wheat bread formulation. Food Sci Tech Int 23:437–447
Ovando CH, Carvalho JC, de Melo-Pereira VG, Jacques P, Soccol VT, Soccol CR (2016) Functional properties and health benefits of bioactive peptides derived from Spirulina: a review. Food Rev Int 34(1):34–51
Willcox DC, Willcox BJ, Todoriki H, Suzuki M (2009) The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr 28:500S-516S
Batista AP, Nunes MC, Fradinho P, Gouveia L, Sousa I, Raymundo A, Franco JM (2012) Novel foods with microalgal ingredients—effect of gel setting conditions on the linear viscoelasticity of Spirulina and Haematococcus gels. J Food Eng 110:182–189
Fradinho P, Niccolai A, Soares R, Rodolfi L, Biondi N, Tredici MR, Sousa I, Raymundo A (2020) Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res 45:101743
Lafarga T, Mayre E, Echeverría G, Viñas I, Villaró S, Acién Fernández FG, Castellari M, Aguiló-Aguayo I (2019) Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. LWT Food Sci and Tech 115:108439
Lucas BF, de Morais GM, Duarte Santos T, Costa JAV (2018) Spirulina for snack enrichment: nutritional, physical and sensory evaluations. LWT Food Sci and Tech 90:270–276
Rodríguez De Marco E, Steffolani ME, Martínez CS, León AE (2014) Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT Food Sci Technol 58(1):102–108
Şahin OI (2020) Functional and sensorial properties of cookies enriched with spirulina and dunaliella biomass. J Food Sci Technol 57:3639–3646
Tańska M, Konopka I, Ruszkowska M (2017) Sensory, physico-chemical and water sorption properties of corn extrudates enriched with spirulina. Plant Foods Hum Nutr 72(3):250–257
Chen Y, Chen J, Chang C, Cao F, Zhao J, Zhenh Y, Zhu J (2019) Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll 96:510–517
Plaza M, Cifuentes A, Ibanez E (2008) In the search of new functional food ingredients from algae. Trends Food Sci Technol 19:31–39
Garrido-Cardenas JA, Manzano-Agugliaro F, Acien-Fernandez FG, Molina-Grima E (2018) Microalgae research worldwide. Algal Res 35:50–60
Rico D, Alonso de Linaje A, Herrero A, Asensio-Vegas C, Miranda J, Martínez-Villaluenga C, de Luis DA, Martin-Diana AB (2018) Carob by-products and seaweeds for the development of functional bread. J Food Process Preserv 42:e13700
International Association for Cereal Science and Technology (2003) ICC standard methods (methods no. 104/1, 105/2, 110/1, 136). ICC, Vienna, Austria
Lee SC, Prosky L, DeVries JW (1992) Determination of total, soluble, and insoluble, dietary fibre in foods enzymatic-gravimetric method, MES-TRIS buffer: collaborative study. J Assoc Off Anal Chem 75:395–416
American Association of Cereal Chemistry (AACC) (2003) Approved methods of analysis, 10th edn. AACC, St. Paul, MN, USA
Amoriello T, Amoriello M (2021) L’alga Chlorella vulgaris come nuovo ingrediente nella panificazione: effetti sulla reologia dell’impasto e sulle proprietà del pane. Industrie Alimentari 621:3–9
Arufe S, Della Valle G, Chiron H, Chenlo F, Sineiro J, Moreira R (2018) Effect of brown seaweed powder on physical and textural properties of wheat bread. Eur Food Res Technol 244(1):1–10
Selmo MS, Salas-Mellado MM (2014) Technological quality of bread from rice flour with spirulina. Int Food Res J 21(4):1523–1528
Ciccoritti R, Taddei F, Gazza L, Nocente F (2021) Influence of kernel thermal pre-treatments on 5-n-alkylresorcinols, polyphenols and antioxidant activity of durum and einkorn wheat. Eur Food Res Technol 247:353–362
Dere S, Güneş T, Sivaci R (1998) Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22(1):13–18
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food resources: revisiting our understanding. J Appl Phycol 29:949–982
Barber M, Kabisch S, Pfeiffer AFH, Weickert MO (2020) The health benefits of dietary fibre. Nutrients 12:3209
Ponnuswamy I, Madhavan S, Shabudeen S (2013) Isolation and characterization of green microalgae for carbon sequestration, waste water treatment and bio-fuel production. Int J Biosci Biotech 5(2):17–26
Kannan S (2014) FT-IR and EDS analysis of the seaweeds Sargassum wightii (brown algae) and Gracilaria corticata (red algae). Int J Curr Microbiol App Sci 3(4):341–351
Bataller BG, Capareda SC (2018) A rapid and non-destructive method for quantifying biomolecules in Spirulina platensis via Fourier transform infrared–attenuated total reflectance spectroscopy. Algal Res 32:341–352
Gómez-Ordóñez E, Rupérez P (2011) FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll 25(6):1514–1520
Dobraszczyk BJ, Morgenstern MP (2003) Rheology and the bread-making process. J Cereal Sci 38:229–245
Collar C, Santos E, Rosell CM (2007) Assessment of the rheological profile of fibre-enriched bread doughs by response surface methodology. J Food Eng 78:820–826
Gómez M, Oliete B, Caballero PA, Ronda F, Blanco CA (2008) Effect of nut paste enrichment on wheat dough rheology and bread volume. Food Sci Tech Int 14(1):57–65
Dubois M, Dubat A, Launay B (2008) The Alveo consistograph handbook. AACC International, St. Paul, MN, USA
Gómez M, Ronda F, Blanco CA, Caballero PA, Apesteguia A (2003) Effect of dietary fiber on dough reology and bread quality. Eur Food Res Technol 216:51–56
Rosell CM, Rojas JA, Benedito de Barber C (2001) Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll 15:75
Junqueira RM, Castro IA, Areas JAG, Silva ACC, Scholz S, Mendes S, Oliveira KC (2007) Application of response surface methodology for the optimization of oxidants in wheat flour. Food Chem 101:131–139
Anil M (2007) Using of hazelnut testa as a source of dietary fiber in breadmaking. J Food Eng 80:61–67
Doxastakis G, Zafiriadis I, Irakli M, Marlani H, Tananaki C (2002) Lupin, soya and triticale addition to wheat flour doughs and their effect on rheological properties. Food Chem 77:219–227
Rehman S, Paterson A, Hussain S, Murtaza MA, Mehmood S (2007) Influence of partial substitution of wheat flour with vetch (Lathyrus sativus L) flour on quality characteristics of doughnuts. LWT Food Sci Technol 40:73–82
Sluimer P (2005) Principles of breadmaking: functionality of raw materials and process steps. American Association of Cereal Chemistry, St. Paul, MN, USA
Dhingra D, Michael M, Rajput H, Patil RT (2012) Dietary fibre in foods: a review. J Food Sci Technol 49:255–266
Sivam AS, Sun-Waterhouse D, Quek SY, Perera CO (2010) Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: a review. J Food Sci 75:163–174
Plaza M, Herrero M, Cifuentes A, Ibanez E (2009) Innovative natural functional ingredients from microalgae. J Agric Food Chem 57:7159–7170
Pangestuti R, Kim SK (2011) Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods 3:255–266
Amoriello T, Mellara F, Galli V, Amoriello M, Ciccoritti R (2020) Technological properties and consumer acceptability of bakery products enriched with brewers’ spent grains. Foods 9:1492
Mabeau S, Fleurence J (1993) Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci Technol 4:103–107
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23(3):543–597
Batista AP, Niccolai A, Fradinho P, Fragoso S, Bursic I, Rodolfi L, Biondi N, Tredici MR, Sousa I, Raymundo A (2017) Microalgae biomass as an alternative ingredient in cookies: sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res 26:161–171
Yuan YV (2008) Marine algal constituents. In: Barrow C, Shahidi F (eds) Marine nutraceuticals and functional foods. CRC, Boca Raton, pp 297–320
Huang HL, Wang BG (2004) Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J Agric Food Chem 52:4993–4997
Acknowledgements
Grateful acknowledgements are given to Dr. Roberto Ciorba and Mr. Luigi Bartoli for technical assistance. The authors also thank Paola Manzi (CREA Research Centre for Food and Nutrition) for providing the FT-IR instrument.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Compliance with ethics requirement
This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amoriello, T., Mellara, F., Amoriello, M. et al. Powdered seaweeds as a valuable ingredient for functional breads. Eur Food Res Technol 247, 2431–2443 (2021). https://doi.org/10.1007/s00217-021-03804-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03804-z