Abstract
This study was to evaluate the effect of antifreeze peptides from pigskin collagen hydrolysates (CoAPPs) on the fermentation properties, texture properties, water distribution and water mobility of dough during freezing and freeze–thaw cycles. The fermentation properties of the frozen dough were more stable in the CoAPPs groups than those of control groups. The texture profile analysis showed that the hardness, gumminess and chewiness of the CoAPPs breads were significantly smaller (P < 0.05) than controls. The NMR showed that addition of CoAPPs weakened the influence of the freeze–thaw treatment on water mobility and influenced the water distribution in frozen dough. The results suggest that CoAPPs could potentially serve as a food-origin cryoprotectant in the conventional dough products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antifreeze proteins (AFPs), known as a family of proteins with thermal hysteresis ability (THA), can depress freezing point and modify the morphology of ice crystals. They also have the recrystallization inhibition (RI) ability, which inhibits the growth of ice in an adsorption–inhibition mechanism by binding to the surface of ice crystals [1]. These unique functions of AFPs have attracted significant interest for their potential applications in additives for frozen foods. However, AFPs are mostly isolated from plants, polar fish, fungi and insects. Because of the high cost and low product yield, these applications of AFPs on a large scale have been limited. In recent study, antifreeze peptides (APPs) have been reported for the retardation of ice crystal growth. Damodaran [2] has reported that gelatin or collagen hydrolysates produced from papain hydrolysis were able to inhibit ice recrystallization in ice cream mix. It also confirmed that peptides obtained from pigskin collagen hydrolysates, with molecular mass distribution among 150–2000 Da, have the ability to protect S. thermophilus from hypothermic damage [3].
As the bakery business is being concentrated and rationalized, there has been a growing demand for frozen dough [4]. The freezing dough technology has served as a suitable preservation approach to make fresh products available for retail stores. Although the purpose of freezing technology is to keep the dough products in a fresh state, more experimental evidence shows that a number of physical and chemical phenomena affect the qualities of the final product in a mostly negative way occur [5], such as an increasing in fermentation time and decreasing in specific volume when compared to fresh dough products [6]. The deteriorated quality of frozen dough was mainly related to the loss of gluten network integrity and yeast fermentative capacity [7] mainly caused by ice crystallization and recrystallization [8].
Water is an integral part of wheat flour dough: the amount, physical state and location of water are crucial to the quality of a frozen dough, because it is closely related to the formation of dough, specifically how it will hold gas and produce an open, aerated crumb structure in the final product [9]. Related researches have indicated that it is not the water activity but the dynamic molecular mobility state of the water in foods that directly affects the food stability [10].
The objective of this study was to investigate the effect of antifreeze peptides from pigskin collagen hydrolysates (CoAPPs) on characteristics of frozen dough including the fermentation properties and the texture properties of steamed bread. In order to illustrate how the water state plays an important role in the dough quality and stability, water state and water distribution of samples with CoAPPs were further investigated.
Materials and methods
Materials
Wheat flour and instant dry yeast were purchased from the local supermarket. Antifreeze peptides (CoAPPs) were prepared from pig skin collagen based on the method described before [3].
Dough preparation and frozen dough freeze–thaw treatments
The steamed bread dough was prepared according to the following formula: 100 g of wheat flour, 1 g of instant dry yeast, 50 g of distilled water and 1 g of CoAPPs. The control groups had the same formula except for 1 g of CoAPPs. Yeast and CoAPPs were stirred in water before being added to the flour. All dough components were mixed in a bread machine (Moulinex Ow 5000, Nancy, France) in two steps. The ingredients were mixed during 10 min at a low speed (40 rpm), and then the remaining water was added at a high speed (80 rpm) during 10 min. The resulting dough (20 g) was molded, covered with polyethylene bags and frozen by air-blast freezer (BCD-649WE, Haier, China) at −20 °C [11]. Freeze–thaw treatments were applied to frozen steamed bread dough samples to mimic the temperature fluctuations that occur during frozen storage. A freeze–thaw cycle consisted of partially thawing the frozen dough at 30 °C until the center temperature was 4 °C and then subjected to frozen storage again at −20 °C for 24 h. Frozen dough samples after freeze–thaw cycles including 0, 1, 2, 3, 4, 5 and 6 cycles were named C0, C1, C2, C3, C4, C5 and C6, respectively, in which the number represents freeze–thaw times that dough samples endured [12].
Steamed bread preparation
Samples were removed from the air-blast freezer at the specified time and were placed in an environmental chamber (Model SPX-150C, Environmental Chamber, China) at 30 °C to thaw, for 30 min. Thawed dough was proofed in an environmental chamber at 35 °C and 85% relative humidity for 90 min. Proofed dough was baked at 150 °C for 20 min (Induction Cooker, Galanz). After baking, steamed bread samples were allowed to cool for 30 min in an environmental chamber.
Fermentation properties of frozen dough
The fermentation properties of fresh and frozen dough were evaluated in triplicate for each freeze–thaw treatment.
Specific steamed bread volume
In order to estimate the effect of CoAPPs supplementation on steamed bread quality, the steamed bread quality was evaluated by bread volume. Steamed bread volume was measured by rapeseed displacement, according to AACC method [13]. Specific volume was calculated from the steamed bread volume divided by the steamed bread weight. The variational rate R evaluates the changes of steamed bread volume between the bread from the fresh dough and the bread from the dough with freeze–thaw cycles, calculated by Eq. (1):
where VS is the bread from the fresh dough (mL/g) and VE is the bread from the dough with frozen storage (mL/g).
Fermentation times of dough
A dough sample of 20 g was placed in a sterilized graduated test tube (5 cm in diameter and 10 cm in height). The test tube was left for fermentation at 35 °C and 85% relative humidity in an environmental chamber. The dough heights were monitored as duplicate from the same dough that was divided into three samples of 20 g dough. The dough height was measured as the highest point of the dough, as the dough height has previously been found to correlate well with the volume expansions of the dough [14]. The height for optimally developed dough was 100 mm for fermentation at 35 °C [15]. Fermentation time was recorded when reaching the optimally height.
Texture profile analysis (TPA) of steamed bread
Texture profile analysis (TPA) of bread simulates the chewing movements and has been accepted universally [16]. The analysis was done by a Texture Analyzer equipped with a P36 probe (Stable Microsystems TA-XT2i, Scarsdale, NY, USA). The results include hardness, resilience, chewiness, gumminess, adhesiveness, cohesiveness, springiness and fracture ability, as defined by Fiszman et al. [16]. The steamed bread was sliced horizontally, and a bottom piece with a cubic dimension (L × W × H = 1 × 1 × 1) was compressed to 50% of its height. The compression test parameters were set as follows: pretest speed 3.0 mm/s; test speed 1 mm/s; posttest speed 5.0 mm/s; time 5.00 s and trigger force 5 g. The TPA of each sample was run in non-duplicate [17].
Water mobility determination
NMR spin–spin relaxation (T2) measurements were performed on fresh and frozen dough subjected to freeze–thaw cycles storage. The water mobility of dough samples was determined using NMR Analyzer (Niumag Corporation, Shanghai, China) with a corresponding resonance frequency for protons of 23 MHz which was used for measurement. Approximately 3 g of samples was packed into a 18-mm-inner NMR tube and inserted in the NMR probe. The spin–spin relaxation time (T2) was performed using Carr–Purcell–Meiboom–Gill (CPMG) sequences [18], and typical pulse test parameters were set as follows: P90 = 20 μs, P180 = 37 μs, TD = 87424, NS = 8, NECH = 1000, RG1 = 20, DRG1 = 3, SW = 200 kHz, TR = 1000 ms. The Multi-Exp Inv Analysis Software (Niumag Co., Ltd., Shanghai, China) was used to analyze the T2 relaxation time. This analysis resulted in a plot of relaxation amplitude for individual relaxation processes versus relaxation time. The time constants for each process were determined from the peak position, and the area under each peak (corresponding to the proportion of water molecules exhibiting that relaxation time) was calculated by cumulative integration.
Water distribution detection
The water distribution of dough samples (with or without CoAPPs) was measured in fresh and frozen dough samples using the NMR Analyzer mentioned above. The 2D proton density images were acquired using the multiple spin-echo (MSE) sequence. Approximately 5 g of cylindrical dough samples was packed into a 18-mm-inner NMR tube and inserted into the NMR probe. Typical pulse test parameters were set as follows: P90 = 1200 μs, P180 = 1200 μs, D0 = 1000 ms, TD = 1024, SF1 = 23 MHz. In the proton density images, the bright areas represent free water in the tissues.
Statistical analysis
All experiments were run in triplicate unless specified and the experimental data were expressed as means (standard deviations, SDs) of three independent experiments. All statistical analyses were performed by using SPSS 17.0 (SPSS, Chicago, IL, USA). The significance level of P < 0.05 was used.
Results and discussion
Effect of CoAPPs on specific volume
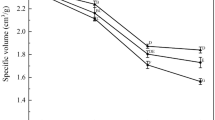
The effect of CoAPPs on specific volume of the steamed bread after freeze–thaw cycles is shown in Fig. 1. As freeze–thaw times increased, the steamed bread with lower specific volume for both doughs was obtained (Fig. 1). Specifically, the steamed bread specific volume of the control was from 2.58 to 1.86 mL/g, and CoAPPs groups from 2.58 to 2.34 mL/g. The variational rate R evaluates the changes of steamed bread specific volume between the bread from the fresh dough and the bread from the dough with freeze–thaw cycles. The R of the control groups and CoAPPs groups was determined to be 28.06 and 10.57%, respectively (Fig. 1). The addition of CoAPPs steamed bread with significantly higher specific volume at the same freeze–thaw times (P < 0.05). The R of the CoAPPs groups was significantly smaller than the control groups (P < 0.05), proving that the steamed bread volume of the CoAPPs groups was more steady than the control groups. The reduction in specific volume was probably due to the ice crystals pierce into the yeast and decrease the yeast survival during frozen storage, lowering the fermentation capacity and reducing ability of the dough gluten network to retain CO2 during fermentation [11]. These observations suggested that CoAPPs significantly reduced the negative effect of frozen storage on specific volume and increased the ability in holding steamed bread volume among the tested samples after freeze–thaw cycles.
Effect of CoAPPs on fermentation time
The effect of CoAPPs on fermentation time after six freeze–thaw cycles is shown in Fig. 2. As freeze–thaw times increased, the fermentation time of the dough correspondingly increased [19]. Compared with the fresh dough, frozen storage increased fermentation time for all doughs with or without CoAPPs. However, the dough with CoAPPs caused a small but significant (P < 0.05) decrease in fermentation time compared with control groups when they were stored frozen for the same times of freeze–thaw cycles. Specifically, fermentation time of control groups increased from 72 to 138.7 min, whereas the dough with CoAPPs increased from 72.2 to 110.2 min after six freeze–thaw cycles. The fermentation time of the CoAPPs groups was significantly smaller by 28.5 min than the control groups. The increase in fermentation time could be related to the decrease in the yeast survival count and in the gluten network structure in dough caused by freeze–thaw cycles, and the improvement of CoAPPs groups in fermentation time might be attributed to inhibition of ice growth and recrystallization by addition of CoAPPs. These observations were similar with the results reported by Jia et al. [20], who reported that the addition of thermostable ice structuring proteins extract from Chinese privet (Ligustrum vulgare) leaves into doughs could improve the fermentation time.
Texture profile analysis (TPA) of steamed bread
Texture profile analysis shows the effect of freeze–thaw on texture profile after six freeze–thaw cycles. Gumminess was calculated by hardness multiplied by cohesiveness, and chewiness was calculated by gumminess multiplied by springiness. The chewiness represents the amount of energy needed to disintegrate a food for swallowing [21]. The gumminess and chewiness changes of the steamed bread are shown in Figs. 3 and 4, respectively. Texture profile analysis showed that both gumminess and chewiness of CoAPPs groups were smaller significantly (P < 0.05) than those of the control groups.
Crumb hardness was an important characteristic of bread quality, and the change in hardness was commonly accompanied with the loss of resilience during storage. The hardness change of the steamed bread is shown in Fig. 5. After six freeze–thaw cycles, the hardness of the control dough increased to 625.9 g, whereas the CoAPPs dough only increased to 475.9 g. The hardness of the CoAPPs group was significantly smaller than that of the control groups at the same freeze–thaw cycle (Fig. 5), proving that the addition of CoAPPs resulted in significantly softer and steadier than that of the control bread during freeze–thaw cycles.
All in all, the hardness, gumminess and chewiness of the CoAPPs groups were significantly different with those of the control groups, and the results of hardness, gumminess and chewiness of the CoAPPs groups were all smaller significantly (P < 0.05) than the controls (Fig. 5). The crumb softening effect of CoAPPs agreed with the significantly increase in specific volume of CoAPPs bread described previously and was presumed to be related to its inhibition of ice formation and recrystallization.
Effect of CoAPPs on the water mobility of dough
To confirm water migration of the dough during frozen storage period, we studied the relaxation time of dough with or without CoAPPs. A typical T2 relaxation time distribution curve is shown in Fig. 6a. In our system, the T2 relaxation time distributions of dough system showed three peaks: T21 (0.02–3.35 ms), T22 (3.35–48.63 ms) and T23 (48.63–68.93 ms), which represented bound water, immobilized water and free water, respectively [22]. The results were closer to those detected in dough previously [17]. T21 and T22 represent about 98% of the water present in the dough, so these two main population groups are further discussed. The Y-axis in T2 graph represents the signal amplitude of protons. The X-axis of the T2 relaxation time represents the water activity in the food system. A shorter T2 relaxation time indicates a less water mobility. The peak area of T2 represents the water absorption by hydrophilic components [23]. According to previous studies, T21 and T22 represent bound water and immobilized water in food, respectively [24]. The peak area proportions of T21 and T22 after freeze–thaw cycles are shown in Fig. 6b, c, respectively. The ice recrystallization during freeze–thaw cycles reduces the cross-linking of gluten and consequently weakens the water binding capacity of dough gluten [25]. After six times of freeze–thaw cycles, the bound water proportions of the control groups decreased from 20.18 to 17.75% (P < 0.05), whereas the immobilized water proportions increased from 79.41 to 82.25% (P < 0.05). In contrast, increase in bound water and decrease in immobilized water were observed in the dough with the addition of CoAPPs. Similar results have been obtained in frozen dough after freeze–thaw cycles [12].
T21 can be divided into firmly bound water and loosely bound water, based on the bonding strength of the water molecules with the food macromolecules [26], and the latter is relatively lower and more likely to transform into free water. The decrease in T21 and the increase in T22 in the control dough indicated that the freeze–thaw treatment drove the water shift from T21 to T22. Phimolsiripol [27] suggested that relating the increased rate of the deterioration of frozen dough to the increased mobility of water with storage temperature fluctuations was a reasonable mechanistic explanation. T22 may represent the water trapped in the gluten network (immobilized water), which is the major existing form of water.
The increase in T21 and the decrease in T22 in the CoAPPs dough indicated that the addition of CoAPPs affected the water mobility and weakened the influence of the freeze–thaw treatment on the water mobility. This result is agreed with the report that antifreeze protein BaAFP-1 extracted from barley had a strong hydrophilicity, when it was added in the dough, and water tended to shift from T22 to T21 and weakened the influence of the freeze–thaw treatment on the water mobility [12]. Both antifreeze protein BaAFP-1 and CoAPPs had a strong hydrophilicity, which bond with water molecules through hydrogen bonds. So, it could infer that the CoAPPs inhibit the ice recrystallization probably by adsorption on ice surface by hydrogen bonding so as to reduce the damage of dough’s gluten network and decrease the content of immobilized water, resulting the relative content increase in bound water.
Effect of CoAPPs on water distribution of dough
The NMR is usually used to measure the water distribution of dough samples. Figure 7 shows example proton density images for samples of fresh dough and frozen dough. The 2D proton density images indicated that the distribution of water in the dough sample was distinctly different before and after the freeze–thaw cycles, and the signal intensity for proton density of the CoAPPs dough decreased at a slower pace (Fig. 7). At first, the proton density was evenly distributed both internally and on the surface in the fresh dough. After freeze–thaw cycles, this homogeneous state was broken and the signal intensity for proton density was reduced. This phenomenon was due to the moisture content decrease in both kinds of dough after the freeze–thaw treatment. The vapor pressure of the freezing chamber was lower than that of the dough during freezing storage. This vapor pressure difference force drove the loss of moisture from the food surface to the air in the freezer, and the lost moisture was condensed and frozen on the inner surface of the package [28]. Temperature fluctuations led to the number of ice crystals reduced and the mean ice crystal size increased, damaging the dough’s gluten network. This reduced the water holding capacity of the gluten, and more water was lost when the dough was thawed again.
The signal intensity for proton density of the CoAPPs dough decreased at a slower pace when compared with the control groups (P < 0.05, Fig. 7), suggesting that the addition of CoAPPs inhibited the ice recrystallization and protected the gluten networks of the dough samples from the damage done by large ice crystals during the freeze–thaw treatment. This investigation is crucial for us to get fundamental understanding of water dynamics in frozen food quality and stability. The physicochemical mechanisms that water exerts such a strong influence on the properties of dough are now better understood.
Conclusions
The antifreeze peptides from pigskin collagen hydrolysates provided a significant cryoprotection on steamed bread dough during frozen storage. The CoAPPs weakened the influence of the freeze–thaw treatment on the water mobility. The physicochemical mechanism that water exerts strong influence on the properties of dough suggests that the addition of CoAPPs could inhibit the ice recrystallization and as a result protect the gluten networks. The study is of significance in suggesting that CoAPPs could potentially serve as a food-based cryoprotectant in the frozen dough industry.
Abbreviations
- AFPs:
-
Antifreeze proteins
- THA:
-
Thermal hysteresis ability
- RI:
-
Recrystallization inhibition
- APPs:
-
Antifreeze peptides
- CoAPPs:
-
Antifreeze peptides from pigskin collagen hydrolysates
- TPA:
-
Texture profile analysis
- CPMG:
-
Carr–Purcell–Meiboom–Gill
- MSE:
-
Multiple spin-echo
References
Wu JH, Rong YZ, Wang ZW, Zhou YF, Wang SY, Zhao B (2015) Isolation and characterisation of sericin antifreeze peptides and molecular dynamics modelling of their ice-binding interaction. Food Chem 174:621–629
Damodaran S (2007) Inhibition of ice crystal growth in ice cream mix by gelatin hydrolysate. J Agric Food Chem 55:10918–10923
Wang WL, Chen MS, Wu JH, Wang SY (2015) Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. LWT Food Sci Technol 63:878–885
Le-Bail A, Zia C, Giannou V (2012) Quality and safety of frozen bakery products. In: Sun DW (ed) Handbook of frozen food processing and packaging, seconded. CRC Press, Boca Raton, pp 501–528
Huen J, Weikusat C, Bayer-Giraldi M, Weikusat I, Ringer L, Lösche K (2014) Confocal raman microscopy of frozen bread dough. J Cereal Sci 60:555–560
Casey GP, Foy JJ (1995) Yeast performance in frozen doughs and strategies for improvement. In: Kulp K, Lorenz K, Brummer J (eds) Frozen and refrigerated doughs and batters. American Association of Cereal Chemists, Minnesota, USA, pp 19–51
Pablo D, Ribotta AE, León-María CA (2003) Effects of yeast freezing in frozen dough. Cereal Chem 80:454–458
Yadav DN, Patki PE, Sharma GK, Bawa AS (2009) Role of ingredients and processing variables on the quality retention in frozen bread doughs: a review. J Food Sci Technol 46:12–20
Loveday SM, Huang VT, Reid DS, Winger RJ (2012) Water dynamics in fresh and frozen yeasted dough. Crit Rev Food Sci Nutr 52:390–409
Li S, Dickinson LC, Chinachoti P (1998) Mobility of “unfreezable” and “freezable” water in waxy corn starch by 2H and 1H NMR. J Agric Food Chem 46:62–71
Zhang C, Zhang H, Wang L, Gao H, Guo XN, Yao HY (2007) Improvement of texture properties and flavor of frozen dough by carrot (Daucuscarota) antifreeze protein supplementation. J Agric Food Chem 55:9620–9626
Ding XL, Zhang H, Wang L, Qian H, Qi XG, Xiao JH (2015) Effect of barley antifreeze protein on thermal properties and water state of dough during freezing and freeze–thaw cycles. Food Hydrocoll 47:32–40
AACC International (2010) Approved method of analysis. 11th edn. AACC International, St. Paul, MN
Ktenioudaki A, Butler F, Gallagher E (2010) Rheological properties and baking quality of wheat varieties from various geographical regions. J Cereal Sci 51:402–408
Anja N, Birch-Frans WJ, Berg BG, Åse H (2013) Expansion profiles of wheat doughs fermented by seven commercial baker’s yeasts. J Cereal Sci 58:318–323
Fiszman SM, Salvador A, Varela P (2005) Methodological developments in bread staling assessment: application to enzyme-supplemented brown pan bread. Eur Food Res Technol 221:616–623
Li J, Hou GG, Chen Z, Chung AL, Gehring K (2014) Studying the effects of whole-wheat flour on the rheological properties and the quality attributes of whole-wheat saltine cracker using SRC, alveograph, rheometer, and NMR technique. LWT Food Sci Technol 55:43–50
Han M, Zhang Y, Fei Y, Xu X, Zhou G (2009) Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur Food Res Technol 228(4):665–670
Lu W, Grant L (1999) Effects of prolonged storage at freezing temperatures on starch and baking quality of frozen doughs. Cereal Chem 76:656–662
Jia CL, Huang WN, Wu C, Zhong J, Rayas-Duarte P, Guo CX (2012) Frozen bread dough properties modified by thermostable ice structuring proteins extract from Chinese Privet (Ligustrum vulgare) Leaves. Cereal Chem 89:162–167
Lin LY, Wang H, Lin SD, Liu HM, Mau JL (2013) Changes in buckwheat bread during storage. J Food Process Preserv 37:285–290
Li Z, Deng C, Li H, Liu C, Bian K (2015) Characteristics of remixed fermentation dough and its influence on the quality of steamed bread. Food Chem 179:257–262
Li J, Kang J, Wang L, Li Z, Wang R, Chen ZX, Hou GG (2012) Effect of water migration between arabinoxylans and gluten on baking quality of whole wheat bread detected by magnetic resonance imaging (MRI). J Agric Food Chem 60:6507–6514
Doona CJ, Baik MY (2007) Molecular mobility in model dough systems studied by time-domain nuclear magnetic resonance spectroscopy. J Cereal Sci 45:257–262
Ribotta PD, Leon AE, Anon MC (2001) Effect of freezing and frozen storage of doughs on bread quality. J Agric Food Chem 49:913–918
Vyavahare O, Ng D, Hsu SL (2014) Analysis of structural rearrangements of poly(lactic acid) in the presence of water. J Phys Chem B 118:4185–4193
Phimolsiripol Y, Siripatrawan U, Tulyathan V, Cleland D (2008) Effects of freezing and temperature fluctuations during frozen storage on frozen dough and bread quality. J Food Eng 84:48–56
Giannou V, Kessoglou V, Tzia C (2003) Quality and safety characteristics of bread made from frozen dough. Trends Food Sci Technol 14:99–108
Acknowledgements
This work was supported by Natural Science Foundation of China (No. 31571779, 31471623), the Special National Key Research and Development Plan (No. 2016YFD0400200) China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Xu Chen and Jin-hong Wu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, X., Wu, Jh., Li, L. et al. The cryoprotective effects of antifreeze peptides from pigskin collagen on texture properties and water mobility of frozen dough subjected to freeze–thaw cycles. Eur Food Res Technol 243, 1149–1156 (2017). https://doi.org/10.1007/s00217-016-2830-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2830-x