Abstract
Mango is a commercially important and popular tropical fruit. While there is a growing commercial interest to incorporate fruit pieces in juices and soft drinks, such implementation for mango pieces is impaired by the sensitivity of the pieces texture during beverage processing (i.e., thermal treatment) and shelf life. In this work, we have evaluated the evolution of texture, color and composition of mango pieces from two non-fully ripe ripening stages in a mango juice drink after pasteurization (77 °C for 15 min) and shelf life (8 weeks at 21 °C). Our results indicated that the firmness of the pieces from the earlier ripening stage was significantly improved by pasteurization and preserved during storage. On the other hand, no improvement in texture was observed for the riper pieces most likely due to a more degraded cell wall structure at the later ripening stage preventing the beneficial firmness increase occurring due to starch gelatinization. Those results, combined with the observed migration of sugars and acids between the pieces and the juice, suggest that the utilization of non-fully ripe mango pieces could present a promising opportunity for the addition of mango bits to fruit beverages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (Mangifera indica L.) is one of the most popular tropical fruits in the world. It softens quickly and extensively as the fruit approaches the fully ripened stage. The loss of fresh mango firmness during shipping and storage is presumably related to the depolymerization and solubilization of pectin substances in the middle lamella of the cell wall [1].
The usage of fresh-cut fruit pieces in a fruit beverage [addition of approximately 5 % (w/w) pieces] is an upcoming trend with consumers demanding high quality, natural fruit products with fresh appearance, texture and flavor (e.g., peach bits in peach juice drink products; apple juice, lemon juice and grape juice with aloe bits, orange juice with orange bits). In this context, mango juice with added mango pieces is of interest, but its production is challenging by the fact that fresh-cut ripe mango pieces are too soft to survive required processing steps (such as pasteurization) and storage. The softening of mango during ripening (from the core to outside of the flesh) is correlated with progressive changes in the composition of hemicellulose, pectin, cellulose and starch. However, information regarding textural changes during thermal processing and the following shelf life in a beverage is scarce, especially for mango pieces. In this manuscript, two critical process factors (pasteurization and storage) that affect the quality of mango pieces in beverages are studied.
In relation to quality evolution of fresh-cut mango pieces during storage, Beaulieu and Lea [2] indicated that firm fresh-cut mangoes were not sufficiently ripe to deliver an optimum product to consumers, while the soft ripe mango resulted in a product with a short shelf life with quality loss due to tissue damage, faster mushiness, etc. Allon et al. [3] suggested that to be used as fresh-cut products, mangoes must be at least partially ripened (almost ready to eat) to assure sufficient flavor quality [3]. Tovar et al. [4] also indicated that mango slices continue to ripen after cutting, but do not reach the same level of ripening as in the whole fruit. Other authors indicated that mangoes having a yellow color of the flesh or 12.5–14 % soluble solids are at the optimum stage in terms of maintaining appearance, texture and taste for utilization as fresh-cut fruits [3]. Sriwimon et al. [5] suggested that sensory properties of partially ripe mango pieces could be improved by impregnation of the pieces, under vacuum infusion, in mango juice. The benefit of such methodology is explained by the fact that the juice, as the optimal source of typical mango flavors, allows the pieces to desorb organic acids from the pieces that will negatively affect the sensorial properties while absorbing other typical flavor compounds resulting in improved sensorial properties compared with non-immersed pieces [5].
In the context of the upcoming trend of fruit juices containing fruit bits, the objective of the presented work was to study texture evolution, color and some compositional changes in mango pieces from two non-fully ripe stages during thermal processing and storage in a mango juice drink. Such objective was pursued with a goal to obtain mango pieces capable of providing necessary textural and sensorial properties (firm yet sweet), after thermal processing and during the expected shelf life, for the beverage industry.
Materials and methods
Materials
Individually quick frozen (IQF) Ngowe mango cubes (1 × 1 × 1 cm) at two mature yet non-fully ripen stages with a total soluble solids of 8.6 and 9.9 Brix° were obtained from Kenya. Those two ripening stages were selected in order to obtain pieces with a perspective to have on the one hand textural properties capable of withstanding thermal processing and storage and on the other hand not to be too acidic for consumption. The use of frozen cubes was opted for as this starting material is highly relevant for the food beverage industry. Prior to utilization, the frozen mango cubes were thawed in the refrigerator overnight. Mango juice concentrate (from the Totapuri variety) having 17° Brix was used as a starting material to prepare the model mango juice drink (10° Brix, 0.2 % acidity) by dilution with distilled water and adjustment of sucrose and citric acid. Each measurement was repeated three times.
Methods
Pasteurization and storage
Four percent (w/w) of thawed IQF mango cubes were immersed in a glass bottle containing the mango juice drink. The bottles were pasteurized at 77 °C for 15 min (holding time) using a laboratory-scale pasteurization unit (Miele, G7835 CD, Germany) and stored at 21 °C in an incubator protected from light for 0, 2, 4 and 8 weeks. Samples were taken immediately after pasteurization and after 2, 4 and 8 weeks of storage and frozen in liquid nitrogen until chemical analysis (i.e., dry matter, sugar and acid profile). However, color, texture and weight gain analysis were performed on non-frozen samples.
Texture analysis
The firmness of mango cubes was measured, similarly to previous works [6], by a compression test using a Texture Analyser (model TA-XT2i, 200 ma, UK) with a load cell of 25 kg and a cylindrical probe (25 mm diameter). The test speed was 1 mm/s, and the hardness of the mango cubes was defined as the maximum force recorded in the force–time curve (compression up to 70 % of its original thickness). For each treatment repetition, the mean value of compression forces of 15 mango pieces was considered as a single data point.
Total soluble solids (TSS)
Mango pieces were homogenized using a mixer (Retsch, GM 200, Germany) three times at 7500 rpm for 20 s to prepare a mango puree. TSS was determined by measuring the refractive index of the pureed mango pieces or of the mango juice drink using a refractometer (RE 50, Mettler Toledo, Japan).
Color analysis
The color of the pureed mango pieces was evaluated in terms of L* (lightness), a* (redness and greenness) and b* (yellowness and blueness) values by using a colorimeter (HunterLab, Inc., Vinton, Virginia, USA) following a previously reported procedure [7].
Weight gain analysis
Weight gain was analyzed as previously described [8]. Briefly, raw frozen mango pieces were thawed in the refrigerator overnight; they were left for 10 min on a tissue paper to remove dripping water. All mango pieces (for a single bottle) were then weighted before filling (Wi). After pasteurization and after each storage time, all mango pieces of each single bottle were weighted after removal of dripping liquid for 10 min on a tissue paper (Wt). The weight gain of samples (all mango pieces) was calculated as:
Dry matter
Dry matter (DM) was determined for both mango pieces and mango juice after drying using a vacuum oven (vacuum drying oven, 1445-2, 1400 W, Germany) at 70 °C under 0.2–0.8 bar pressure following a slightly modified previously published method [9]. Briefly, 3.0 g of wet samples (WS) was transferred to a porcelain dish, the samples were dried under 0.2, 0.4 and 0.6 bar for 1 h and 0.8 bar for 30 min, and then the weight of dry samples (DS) was determined. The dry matter content was calculated as:
Alcohol-insoluble residue extraction yield
Alcohol-insoluble residue (AIR) samples from mango pieces at different processing and storage stages were prepared following a previously described procedure [10]. Approximately 30.0 g of frozen mango cubes sample was weighed and homogenized using a mixer (Retsch, GM 200, Germany) followed by an additional homogenization in 192.0 mL of 95 % ethanol using a Buchi mixer (B-400, Flawil, Switzerland). The residue was vacuum filtered (Merck Eurolab filter no. 413, L 90 mm), re-homogenized in 96 mL of 95 % ethanol and filtered again. The residue was homogenized again in 96 mL of acetone at 4 °C before final vacuum filtration. The obtained AIR was dried in an incubator at 60 °C overnight followed by grinding using a mortar and pestle and storage in a desiccator until analysis.
Water-soluble pectin fractionation
Water-soluble pectin (WSP) was fractionated from the isolated AIR using a modified hot water extraction procedure according to the method described by Braga and co-workers [11]. In this method, exactly weighed (0.5 g) AIR samples were stirred in 90 mL of hot water (100 °C) for 5 min. The resulting solution was cooled in a sink, the pH was adjusted to 6.5, and the volume was adjusted to 100 mL. The mixture was filtered using a filter paper (Schleicher and Schuell, MN 615 φ 90 mm). The water-soluble pectin fraction (WSP) was freeze-dried (Christ alpha 2-4, type 102042, Osterode, Germany), and the dry powder was kept in a desiccator until molar mass distribution analysis.
Uronic acid content
Ten milligram of dried AIR was weighed into a beaker and placed in an ice bath. For hydrolysis, 8 mL of concentrated sulfuric acid (98 %) was added followed by a dropwise addition of 2 mL water during stirring. After 5 min, additional 2 mL water was added and the sample was hydrolyzed for 1 h [12]. The hydrolyzed sample was transferred into a volumetric flask, and deionized water was added to make up to 50 mL. The uronic acid content was determined quantitatively using a UV/Vis spectrophotometer (Ultrospec 2100 pro from GE Healthcare, Uppsala, Sweden) at 520 nm [13] using a calibration curve based on anhydrous galacturonic acid (GalA).
Determination of molar mass distribution
The molar mass distribution of the water-soluble fraction (WSF) containing mostly pectin polymers was characterized by size exclusion chromatography (SEC) using a series of three Waters columns (Waters, Milford, MA), namely Ultrahydrogel 250, 1000 and 2000 with exclusion limits of 8 × 104, 4 × 106 and 1 × 107 g/mol, respectively [14]. The Agilent HPLC system (Agilent technologies 1200 Series, Diegem, Belgium) consisted of a 1200 series HPLC pump, degasser, autosampler G1329A and variable wavelength detector G1316A. The eluent was monitored by a refractive index detector (Shodex RI-101, Showa Denko K.K., Kawasaki, Japan) coupled with a multi-angle laser light scattering (MALLS) detector (PN3621, Postnova Analytics, Germany). Prior to analysis, the mobile phase of 100 mM MES buffer together with 0.1 M NaCl was filtered through 0.1-μm filter and WSF was dialyzed (Spectra/por® dialysis tubing, 3.5 kDa MWCO) for 48 h against demineralized water. The resulting dialysates were freeze-dried and dissolved overnight in 100 mM MES buffer together with 0.1 M NaCl (0.3 % w/v). Eluents and samples were prepared in demineralized water (organic free, 18 MΩ cm resistance). Filtered (0.45 μm) polysaccharide-containing samples (100 μL) were eluted for 100 min at 35 °C at a flow rate of 0.5 mL/min. The dn/dc value used for the calculation of molar mass distribution was 0.146 mL/g based on the value reported in the literature for pectin in NaNO3 [15]. Molecular weight was calculated by the software (Nova Mals, version 1.0.0.18, Postnova Analytics, Germany). Debye plots with a fitted second-order regression were used for molar weight calculation.
Acid and sugar profiles
The levels of individual sugars (glucose, fructose and sucrose) and organic acids (malic and citric acids) were determined by a proprietary procedures (The Coca-Cola Company, Belgium) on an Acquity UHPLC (Waters, Milford, MA, USA). For the organic acid profiles, the separation was obtained on a HSS T2 column (Waters, Belgium) and the detection was performed by MS using a Quattro Premier Mass Spectrometer (Waters, Milford, MA, USA) [16], while for the sugar profiles the separation was obtained using a Xbridge amide column : 4.6 mm × 250 mm 3.5 µm (Waters, Belgium) and the detection was performed using ELS detector (2424 ELS Detector, Waters, Milford, MA, USA) [17].
Microscopy analysis
Mango cubes were dehydrated in an ethanol solution of 70 % to remove water and then cut into 120 µm thin layers using a Micro HM 355 (Microm Laborgerate GmbH, Walldorf, Germany) microtome. The layers were fixed on microscopy slides and stained with 0.1 % toluidine blue for 2 min followed by washing with water for cell wall analysis or with iodine solution (0.2 %) for starch analysis [18]. Fixed and stained layers were visualized at 40× magnification using an Olympus BX-41 microscope (Olympus, Optical Co. Ltd, Tokyo, Japan).
Statistical analysis
Data reported are the mean and standard error of the replicate samples. Where significance is reported, the data were subjected to one-way analysis of variance (ANOVA) followed by means comparison using Tukey’s multiple comparison test (Origin 8, OriginLab, MA, USA) and were considered significant at P < 0.05 [19].
Results and discussion
Impact of ripening stage, processing and storage on appearance of mango pieces
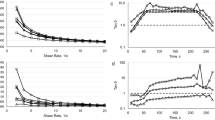
Visually, after pasteurization and during storage of up to 8 weeks in the mango juice drink, the pieces from both ripening stages remained intact. The color of pasteurized pieces was slightly darker, and some browning was clearly observed after 8 weeks of storage especially for the later ripening stage. The visually observed results were supported by optical color measurements (Fig. 1), showing that for both ripening stages the lightness (L* value) decreased due to the pasteurization and remained quite constant during storage, the redness (a*) was stable, while the yellowness (b*) decreased during storage in the mango juice. When comparing the two ripening stages, the appearance of mango pieces changed from light white-green to dark yellow-orange as the redness (a*) and yellowness (b*) increased for the later ripening stage, possibly due to degradation of chlorophyll (green) which masks the presence of carotenoids (yellow) [20]. Most likely the yellowness decreased during storage due to leaching of carotenoids from the pieces to the juice [5].
Impact of ripening stage, processing and storage on the texture of mango pieces
Effect of ripening stage and preprocessing (freezing/thawing) on texture of mango pieces
In Table 1, the firmness (measured as compression force) of raw unsoaked and soaked (in mango juice drink) mango pieces showed no significant difference between the two ripening stages studied after the freezing–thawing cycle. Similarly, light microscopy of mango starch (Fig. 2) and cell walls (Fig. 3) did not reveal clear difference between the raw mango samples at the two ripening stages. The visualization of starch (Fig. 2) showed at both ripening stages a similar starch granular size and shape and the cell walls were relatively intact, indicating that the mango materials were still relatively early in their maturity. Mango softening during ripening is believed to be related to depolymerization and solubilization of pectin substances in the middle lamella of the cell wall and to involve cell wall hydrolysis [21]. Starch is a major polysaccharide present in unripe mango, and its hydrolysis products are responsible for the sweetness development during ripening and also contribute to cell structure loosing [22]. Previous works presented a correlation between ripening, pectin solubilization, starch degradation and texture loss of mango [23]. Most likely, the lack of a significant difference in texture of raw pieces between the two ripening stages originates, at least partially, from the selection of relatively close ripening stages (8.6 ± 0.1 and 9.9 ± 0.2° Brix for the earlier and later ripening stage, respectively). Additionally, the freezing/thawing cycle is suggested to significantly diminish the difference in firmness between the ripening stages (in comparison with fresh pieces) due to cell wall damage, as will be further discussed. As expected, the soaking of the pieces in the juice did not result in any changes in firmness (Table 1, compared to unsoaked), yet the dry matter % of the soaked pieces increased significantly due to soaking. Additionally, the weight of the pieces slightly increased due to the soaking itself, without a significant effect of the ripening stage. The alcohol-insoluble residue (AIR) extraction yield of the earlier ripening stage was insignificantly higher (6.87 ± 0.45 % and 6.22 ± 0.55 % of fresh weight, respectively, not shown) possibly due to a slightly higher starch content and polysaccharides concentrations. During ripening, starch is known to convert to sugars resulting in lower concentration of AIR [24]. Our results showed that the decrease in the AIR extraction yield with increasing degree of ripening was correlated with increasing relative amounts of galacturonic acid (GalA) as an indicator of pectin content in the AIR [5.08 ± 0.10 % w/w for the earlier and 7.25 ± 0.25 % w/w for the later stage (P < 0.05)]. Our results are in line with a trend previously described in a study by Bhagyalakshmi et al. [22] that showed a decrease in the yield of AIR in ripe mango compared with unripe.
Light microscopy of mango starch granules before and after pasteurization and after pasteurization and 8 weeks of storage. a–c: Earlier ripening stage—a raw (after freezing and thawing), b after pasteurization, c after pasteurization and 8-week storage. d–f: Later ripening stage—d raw (after freezing and thawing), e after pasteurization, f after pasteurization and 8-week storage. Scale bar 100 µm
Light microscopy of cell wall material using toluidine blue staining before and after pasteurization and after 8 weeks of storage. a–c: Earlier ripening stage—a raw (after freezing and thawing), b after pasteurization, c after pasteurization and 8-week storage. d–f: Later ripening stage—d raw (after freezing and thawing), e after pasteurization, f after pasteurization and 8-week storage. Scale bar 100 µm
Molecular weight profile of the water-soluble fraction obtained from the alcohol-insoluble residue (containing mostly pectic material) at each ripening stage was determined (Fig. 4). The first (from the left) visible peak on the concentration elution chromatogram (low retention time) corresponds to the higher molecular weight pectin polymers (and possibly some residual starch), while the second peak (longer retention times) represents lower molecular weight polymers. With ripening, the peak related to the larger polymers mostly disappeared, while the peak related to the smaller polymers significantly increased, suggesting that the concentration of filterable (0.45 µM) high molecular weight pectin decreased, while the concentration of lower molecular weight pectin increased. As a result, the weight average molecular weight of the water-soluble fraction (WSF) was significantly lower for the later ripening stage (807 kDa and 255 kDa, for the earlier and later ripening stage, respectively) despite the relative closeness in the total soluble solids content that can approximately place both ripening stages in the unripe to half-ripe (semi-ripe) region [25, 26]. The decrease in MW is probably due to depolymerization of pectin and possibly the depolymerization of some residual starch molecules during ripening and can be correlated with the expected general trend of cell wall degradation during ripening. Interestingly, the observed decrease in molecular weight was not correlated with a measurable decrease in firmness (Table 1) for the riper pieces.
When discussing the texture of mango pieces, it is important also to take into account the preprocessing step of the freezing/thawing cycle. During freezing, the physical changes in the matrix occurring due to ice crystal formation result in mechanical damage to the pieces [27]. Although several authors indicated that there was no significant change in the water content after thawing of mango slices [27, 28], we visually observed that the shape of mango pieces after thawing was not as intact as of fresh-cut pieces, probably due to the destruction of cell walls resulting in texture loss. Unripe mango was reported to have a stronger cell structure with less ice crystals damage than the semi-ripe mango during freezing [29].
Effect of thermal processing and storage in mango juice on texture, sugar and acid profile of mango pieces
By visual observation, after pasteurization, the shape and integrity of the pieces (at both ripening stages) improved compared with the frozen-thawed unprocessed raw mango pieces that were deformed after thawing due to structural changes and water loss. The firmness (measured as the compression force) of mango pieces from the earlier ripening stage significantly improved after pasteurization followed by a slow decrease during storage (significantly only after 8 weeks compared with week 0) (Table 1). The improved texture of those mango pieces could be explained by starch gelatinization and swelling during the thermal treatment in the mango juice as shown in Fig. 2. Previous studies found that mango starch gelatinization takes place between 70 and 80 °C [18, 30], indicating that during the pasteurization process in our study (77 °C) starch gelatinization occurred. The decrease in texture during the 8 weeks of storage is likely to be due to starch hydrolysis and continuing cell wall degradation, and such hypothesis is strengthened by microscopy images (Figs. 2, 3) showing after 8 weeks of storage some degradation and solubilization of starch and cell walls. The thermal treatment in addition to gelatinization of starch can simultaneously induce starch hydrolysis [30] and amylose solubilization [18] that are possibly involved in the decrease in texture during storage. While pasteurization significantly increased the texture of the less ripened pieces, no comparable texture increase appeared for the pieces at the later ripening stage, despite the fact that starch gelatinization and swelling were demonstrated for those pieces as well (Fig. 2). It seems that starch gelatinization and swelling by itself cannot induce texture improvement of the riper mango pieces after pasteurization, most likely due to a more extensive partial destruction of cell walls during ripening, freezing and thawing and the following heat treatment. Some authors indicated that, in mango, starch was responsible for mango softening in the unripe stage, but in the ripened stage the cell wall had a larger effect [24, 30]. Several explanations can be suggested for such phenomena: Structural differences such as chain length and chain structure between mango starch in different ripening stages can perhaps lead to changes in texture. Additionally, the starch content is known to decrease with ripening [22, 30]; therefore, the lower expected concentration of starch could contribute to the lack of a comparable increase in texture for the more ripe pieces. Additionally, for the riper pieces no continuing significant decrease in the firmness of the pieces was observed during the 8-week storage, unlike what was observed for the less ripened pieces. It is possible that the reason for such phenomena originates from the initially lower firmness values, making a statistically significant conclusion regarding a decrease harder.
In addition to starch gelatinization and hydrolysis, migration of other components between mango pieces and the juice was observed which could have an important contribution for possible utilization of non-fully ripe pieces in beverages. Such migration can contribute to sensorial changes in the pieces during processing and storage and to the observed weight changes that can occur not only due to the presented (Fig. 2) starch swelling (with the absorbed water) but also caused by mass transfer of soluble solids such as degraded pectin or starch, sugar or other soluble components between the pieces and the juice, as further described. The weight gain of pieces (in comparison with unsoaked) immediately after pasteurization from the earlier ripening stage (Table 1) was ~12 %, and no significant changes during 8 weeks of storage were observed. While water absorption by starch granules is clearly responsible at least partially for the weight gain, Table 1 also shows an increase in the % dry matter of those pieces in comparison with unsoaked raw pieces, suggesting that solid components such as sugars were also absorbed by the pieces and possibly contributing to the weight gain. The higher concentration of starch in mangoes during earlier ripening steps [31, 32] will absorb more water during pasteurization and storage, hence resulting in a larger weight gain as was observed in this study.
In the present study, we followed the migration of both sugars and organic acids between the juice and the pieces. Sucrose is a main sugar in mango with 3.0 and 5.2 % w/w, for earlier and later ripening stages, respectively, in comparison with only 0.2 and 0.3 % w/w glucose content 1 and 1.1 % w/w of fructose (Table 2). After pasteurization and during storage, only the glucose concentration increased in comparison with the unprocessed mango pieces without significant changes in sucrose and fructose concentrations (Table 2). After pasteurization, the increase in glucose content was smaller for the more ripe pieces (from 0.30 to 0.50 %w/w) compared with the less ripe (from 0.20 to 0.80 %w/w), but after 8-week storage the glucose content of both was similar. The soaking in the juice itself caused an increase in Brix° of mango pieces (Table 1) at both ripening stages enhancing the conclusion that some soluble solids such as glucose are absorbed from the juice due to differences in concentration, explaining the increase in dry matter percentage of mango pieces. Treatments such as blanching are known to enhance solids uptake as permeability in the disrupted fruit tissue increases [33]; additionally, mango tissue is fairly porous, allowing solution uptake [34]. In contrast, immediately after pasteurization the dry matter percentage of both ripening stages decreases, remaining practically constant during the storage. Such results can be explained by the increase in water content, most likely due to starch swelling, and/or a decrease in solid content of the mango pieces. The fact that the dry matter percentage and the Brix° of the pieces increased due to the soaking while texture was not affected, points toward the conclusion that texture increase is not directly related to the influx of water-soluble compounds.
In addition to sugar migration, the migration of two main organic acids from the pieces to the juice was also observed after pasteurization (Table 2). Both malic acid and citric acid show a clear decrease due to both soaking and pasteurization in the juice, with the later ripening stage showing a higher decrease due to the soaking process possibly being related to the more damaged cell walls of the mango pieces at the later ripening stage. The pasteurization process most likely further enhanced the diffusion of acids from the pieces to the juice. A required and beneficial result of this migration of acids is an improved and less acidic taste profile of mango pieces that is a clear prerequisite for a successful utilization of not fully ripe mango pieces in beverages. A small increase in the concentration of citric and malic acids in the juice was observed after pasteurization, yet such increase is not expected to affect sensory properties of the juice given the low concentration of the pieces (4 % w/w) in the beverage. The organic acid concentrations in mango pieces and juice were stable during storage.
Conclusion
In this manuscript, the effect of heat treatment and storage in mango juice on the texture and composition of mango pieces from two ripening stages of non-fully ripe mango was studied. For the pieces from the earlier ripening stage, the measured firmness increased due to pasteurization, mostly due to starch gelatinization. We suggest that the lack of a similar effect for the later ripening stage is due to a more degraded cell wall structure and a lower starch concentration. In addition to texture, changes in the profile of sugars and organic acids in the pieces were observed. Increase in glucose concentration and decrease in organic acids after immersion and pasteurization of the pieces in the mango juice are likely to lead to improved sensorial properties. The fact that for a certain ripening stage we can obtain pieces with better firmness after processing that are able to remain firm during storage and that at the same time the soaking and pasteurization remove natural acidity of non-fully ripe fruits and enhance the sweetness, provide a feasible opportunity of utilization of such pieces in the beverage industry. An optimization and a clear defining of the optimal ripening stage for firmness enhancement without taste compromise will significantly increase such opportunities. Additionally, further research is needed both to increase our understanding on the effects of thermal treatment on the sensorial properties of fruit pieces and to fully develop the presented concept. Better understanding of the effects of the freezing thawing cycle can hopefully lead to better control of the negative aspects of this process on textural properties.
References
Brinson K, Dey PM, John MA, Pridham JB (1988) Post-harvest changes in Mangifera indica mesocarp cell walls and cytoplasmic polysaccharides. Phytochemistry 27:719–723. doi:10.1016/0031-9422(88)84082-2
Beaulieu JC, Lea JM (2003) Volatile and quality changes in fresh-cut mangos prepared from firm-ripe and soft-ripe fruit, stored in clamshell containers and passive MAP. Postharvest Biol Technol 30:15–28. doi:10.1016/S0925-5214(03)00081-4
Allong R, Wickham L, Mohammed M (2000) The effect of cultivar, fruit ripeness, storage temperature and duration on quality of fresh-cut mango. Acta Hortic 509:487–494
Tovar B, Garcı́a H, Mata M (2001) Physiology of pre-cut mango. I. ACC and ACC oxidase activity of slices subjected to osmotic dehydration. Food Res Int 34:207–215. doi:10.1016/S0963-9969(00)00154-X
Sriwimon W, Boonsupthip W (2011) Utilization of partially ripe mangoes for freezing preservation by impregnation of mango juice and sugars. LWT Food Sci Technol 44:375–383. doi:10.1016/j.lwt.2010.08.012
Christiaens S, Van Buggenhout S, Houben K et al (2011) Towards a better understanding of the pectin structure–function relationship in broccoli during processing: part I—macroscopic and molecular analyses. Food Res Int 44:1604–1612. doi:10.1016/j.foodres.2011.04.029
Yam KL, Papadakis SE (2004) A simple digital imaging method for measuring and analyzing color of food surfaces. J Food Eng 61:137–142. doi:10.1016/S0260-8774(03)00195-X
Van Buggenhout S, Messagie I, Maes V et al (2006) Minimizing texture loss of frozen strawberries: effect of infusion with pectinmethylesterase and calcium combined with different freezing conditions and effect of subsequent storage/thawing conditions. Eur Food Res Technol 223:395–404. doi:10.1007/s00217-005-0218-4
Park YW, Bell LN (2004) Determination of moisture and ash contents of foods. In: Handbook of food analysis, 2nd edn, vol 1, pp 55–82
McFeeters RF, Armstrong SA (1984) Measurement of pectin methylation in plant cell walls. Anal Biochem 139:212–217
Braga M, Pessoni R, Dietrich SM (1998) Cell wall polysaccharide composition of leaves of tropical Rubiaceae differing in phytoalexin response. Rev Bras Fisiol Veg 10:71–78
Ahmed AE, Labavitch JM (1980) Cell wall metabolism in ripening fruit: I. Cell wall changes in ripening ‘bartlett’ pears. Plant Physiol 65:1009–1013. doi:10.1104/pp.65.5.1009
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Shpigelman A, Kyomugasho C, Christiaens S et al (2014) Thermal and high pressure high temperature processes result in distinctly different pectin non-enzymatic conversions. Food Hydrocoll 39:251–263. doi:10.1016/j.foodhyd.2014.01.018
Fishman ML, Chau HK, Kolpak F, Brady J (2001) Solvent effects on the molecular properties of pectins. J Agric Food Chem 49:4494–4501
Feng S, Zeng W, Luo F et al (2010) Antibacterial activity of organic acids in aqueous extracts from pine needles (Pinus massoniana Lamb.). Food Sci Biotechnol 19:35–41. doi:10.1007/s10068-010-0005-2
Benvenuti ME, Burgess JA (2012) Monitoring sugar content of fruit juice using ACQUITY UPLC H-Class and BEH amide column chemistry with evaporative light scattering detection (ELSD). Waters company (Library Number: APNT134706653). http://www.waters.com/waters/library.htm?lid=134706653&locale=/
Bello-Pérez LA, Aparicio-Saguilán A, Méndez-Montealvo G et al (2005) Isolation and partial characterization of mango (Magnifera indica L.) starch: morphological, physicochemical and functional studies. Plant Foods Hum Nutr 60:7–12. doi:10.1007/s11130-005-2534-z
Vervoort L, Van der Plancken I, Grauwet T et al (2012) Thermal versus high pressure processing of carrots: a comparative pilot-scale study on equivalent basis. Innov Food Sci Emerg Technol 15:1–13
Minguez-Mosquera I, Gallardo-Guerrero L (1995) Disappearance of chlorophylls and carotenoids during the ripening of the olive. J Sci Food Agric 69:1–6
Huber DJ (1983) The role of cell wall hydrolases in fruit softening. Hortic. Rev (Am. Soc. Hortic. Sci). Wiley, New York, pp 169–219
Bhagyalakshmi N, Prabha T, Yashodha H et al (2002) Biochemical studies related to textural regulation during ripening of banana and mango fruit. Acta Hortic 575:717–724
Payasi A, Mishra N, Chaves A, Singh R (2009) Biochemistry of fruit softening: an overview. Physiol Mol Biol Plants 15:103–113. doi:10.1007/s12298-009-0012-z
Cárdenas-Coronel WG, Velez-de la Rocha R, Siller-Cepeda JH et al (2012) Changes in the composition of starch, pectin and hemicellulose during ripening of mango (Mangifera indica cv. Kent). Rev Chapingo Ser Hortic 18:5–19
Okoth EM, Sila DN, Onyango CA et al (2013) Evaluation of chemical and nutritional quality attributes of selected mango varieties at three stages of ripeness, grown in lower Eastern Province of Kenya—part 2. J Anim Plant Sci 17:2619–2630
Appiah F, Kumah P, Idun I (2011) Effect of ripening stage on composition, sensory qualities and acceptability of Keitt mango (Mangifera indica L.) chips. Afr J Food Agric Nutr Dev 11:5096–5109
Chassagne-Berces S, Fonseca F, Citeau M, Marin M (2010) Freezing protocol effect on quality properties of fruit tissue according to the fruit, the variety and the stage of maturity. LWT Food Sci Technol 43:1441–1449. doi:10.1016/j.lwt.2010.04.004
Marin M, Cano P, Fuster C (1992) Freezing preservation of four Spanish mango cultivars (Mangifera indica L.): chemical and biochemical aspects. Zeitschrift für Leb und Forsch 194:566–569. doi:10.1007/BF01185485
Prasanna V, Yashoda H, Prabha T, Tharanathan R (2003) Pectic polysaccharides during ripening of mango (Mangifera indica L.). J Sci Food Agric 83:1182–1186. doi:10.1002/jsfa.1522
Yashoda HM, Prabha TN, Tharanathan RN (2006) Mango ripening: changes in cell wall constituents in relation to textural softening. J Sci Food Agric 86:713–721. doi:10.1002/jsfa.2404
Simão RA, Silva APFB, Peroni FHG et al (2008) Mango starch degradation. I. A microscopic view of the granule during ripening. J Agric Food Chem 56:7410–7415. doi:10.1021/jf800467v
Morga NS, Lustre AO, Tunac MM et al (1979) Physico-chemical changes in Philippine Carabao mangoes during ripening. Food Chem 4:225–234. doi:10.1016/0308-8146(79)90007-4
Torreggiani D (1993) Osmotic dehydration in fruit and vegetable processing. Food Res Int 26:59–68. doi:10.1016/0963-9969(93)90106-S
Rincon A, Kerr WL (2010) Influence of osmotic dehydration, ripeness and frozen storage on physicochemical properties of mango. J Food Process Preserv 34:887–903. doi:10.1111/j.1745-4549.2009.00404.x
Acknowledgments
The research was financially supported by the Seventh Framework Programme (FP7) of the European Union under the Marie Curie Initial Training Network “HST FoodTrain” (Grant Agreement 264470).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animals subjects.
Rights and permissions
About this article
Cite this article
Nguyen, H.H., Shpigelman, A., Van Buggenhout, S. et al. The evolution of quality characteristics of mango piece after pasteurization and during shelf life in a mango juice drink. Eur Food Res Technol 242, 703–712 (2016). https://doi.org/10.1007/s00217-015-2578-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2578-8