Abstract
The aim of the study was to characterize the volatile profile of three different unifloral honeys (apple, cherry and saffron) from Kashmir Valley of India by using solid-phase microextraction and gas chromatography–mass spectrometry in order to discriminate between their floral origins. A total number of 42 volatiles, particularly aldehydes, esters, ketones, organic acids, hydrocarbons and alcohols, were detected, out of which 29 volatile components were detected for the first time as compared to data available from other countries. Only two compounds furfural and o-hydroxyanisole were found in all honey samples in the average range of 0.082–0.122 and 0.007–0.011 µg/g, respectively. Some volatile compounds present in each specific honey have also been detected in their respective floral origin, thus could be used as chemical markers in defining a given honey to its floral origin. Results showed unique floral markers to authenticate the botanical origin of apple honey, saffron honey and cherry honey allowing their marketing as monofloral rather than generic honey.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey is a very complex product, because its properties and composition depend not only on floral source visited by bee, but also on other factors such as bee species, geographic area, season and mode of storage [1, 2]. With the current globalization of the honey market, which involves nearly 150 countries, the identification of origin of honey as well as the proof of its authenticity has become an important task and has got importance for both industries as well as consumers [3]. Therefore, the search for reliable chemical markers which can indicate its floral or geographical origin has been the focus of many studies in last decade [4, 5]. It is difficult to find unanimous opinion about the floral markers of honey collected from different floral origins because the indicators that might distinguish one honey from another depend on many factors [3]. Therefore, unambiguous and unique indicators for honey collected from the same plant source have not been identified until now [6].
Aroma is one of the distinguishable characteristics of honey collected from different flowers formed by volatile compounds that may come from the nectar or honeydew [7]. The flavor and aroma of honey vary due to its floral source as light-colored honey is mild in flavor and darker honey has a more pronounced flavor [8, 9]. More than 600 organic compounds have been identified in honey samples originating from different floral sources which belong to seven major groups including alcohols, esters, hydrocarbons, acids, cyclic compounds, ketones and aldehydes [10–12]. Such compounds are specified as floral markers of the corresponding honey which have been used for the geographical discrimination of honey. Many studies have been carried out in order to specify volatile compounds as chemical markers for a fast and reliable identification of honey [12–16]. It has been reported that 2-aminoacetophenone has been found in chestnut honey (Castanea sativa), heptanal in lavender honey (Lavandula latifolia), linalool in Australian leatherwood honey (Eucryphia lucida), thymol in New Zealand thyme honey (Thymus vulgaris) and lime tree honey (Tilia sp.), and 2-methylbenzofuran and 2-hydroxyacetophenone in New Zealand manuka honey (Leptospermum scoparium), while sinensal has been found in Citrus sp. honey and lilac aldehydes-A, B, C, D in orange honey (Citrus sinensis) which are powerful odorants and used as major markers [17–21]. It has also been found that phenolic components and polyphenols can be used as a marker compounds as reported for ellagic acid in heather honey (Calluna vulgaris) and quercetin in sunflower honey (Helianthus annuus) [22]. Kus et al. [23] reported that the rare apple honey (Malus domestica Borkh.) from Poland and Spain is characterized by high percentage of shikimic acid-pathway derivatives, as well as terpenes, norisoprenoids and some other compounds such as coumaran and methyl 1H-indole-3-acetate.
Jammu and Kashmir is located in the northern part of the Indian subcontinent with varied climates and habitats which is broadly divided into subtropical zone, intermediate zone and temperate zone [24]. In Jammu and Kashmir, there are more than 1,600 honey-producing units and honey production is about 2,000 metric tons [25]. In Kashmir, the bee-keeping activity is instigated with the appearance of early blossoms in February to March from floral sources of poet’s daffodil (Narcissus poeticus), pear (Pyrus pyrifolia), cherry (Prunus avium), plum (Prunus domestica) and almond (Prunus amygdalus). During April–June, blossoms of fruits like apple (M. domestica), mustard (Brassica campestris), vegetable blossoms and other early flowering herb greatly help in encouraging brood-rearing activity and in building up some reserves of nectar and pollen. During August–September, colonies are migrated to higher altitudes where pollen and nectar sources such as zinnia (Zinnia elegans), thyme (T. vulgaris), cyanoglossum (Cynoglossum officinale) and balsam (Impatiens glandulifera) play an important role in gearing up the bee activity. Saffron (Crocus sativus) blossoms during late October to mid-November are of immense value to bees for brood rearing [26]. In India, a lot of information is available on physicochemical analysis, antioxidant capacity, mineral content and rheological study of honey obtained from berseem clover (Trifolium alexandrinum), mustard (B. campestris), sunflower (H. annuus) and eucalyptus (Eucalyptus lanceolatus) [27]. But no research work has been done or reported related to the presence of volatile components in honey and their application as honey marker in India so far. So the main objective of this work was to identify the chemical markers for the discrimination of honey collected from three unifloral sources by evaluating their volatile organic compound profiles through solid-phase microextraction and gas chromatography coupled to mass spectrometry (SPME–GC–MS).
Materials and methods
Honey samples
Twenty-one samples of honey from three different sources were collected between March 2012 and November 2013 from different regions (Pulwama, Srinagar, Baramullah, Budgam and Pampore) of Kashmir Valley of India as shown in Table 1. To confirm the floral origin of the samples, the bee keepers of state were contacted, who were keeping the hives in the areas where apple (M. domestica), cherry (P. avium) and saffron (C. sativus) were in major quantity. The origin of each honey sample was further confirmed by melissopalynological analysis. Samples of honey collected from bee keepers were classified according to their botanical origin using the method described by Von der Ohe et al. and Louveaux et al. [28, 29]. The following terms were used for frequency classes: predominant pollen (>45 % of pollen grains counted), secondary pollen (16–45 %), important minor pollen (3–15 %) and minor pollen (<3 %). The collected samples were stored between 0 and 4 °C.
Sample preparation
A 2- to 3-g honey sample was weighed into a 10-ml vial, and then 0.5 g of anhydrous sodium sulfate and 0.5 ml of internal standard solution were added. The internal standard was benzophenone (grade 99.9 %); the solution was prepared by dissolving 15 mg of benzophenone in 8 ml of acetone in a 25 ml volumetric flask and then diluting the whole solution to the mark with distilled water. The vial was then sealed with a Teflon-coated silicon septum (Alltech, Milano, Italy).
SPME analysis
The SPME procedure was performed using a manual SPME holder equipped with a 65-μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) coating because more volatile polar analytes are adsorbed more efficiently and released faster with a 65-μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) coating. This fiber coating has proved to be reliable for the extraction of volatile compounds from honey by improving performances in terms of number of detected compounds, total compound peak area and reproducibility with respect to other fibers [30–32]. Before the use of the fiber, it was conditioned at the GC injection port, at a temperature of 250 °C for 30 min. The investigated sample placed in a 10-ml closed vial was heated to 40 °C for 60 min, and the fiber was then exposed to honey headspace. After 30 min, the SPME fiber was withdrawn from the vial and introduced into the GC injector under the conditions reported below.
Gas chromatography–mass spectrometry (GC/MS) procedure
GC/MS analyses were performed using Agilent 7000C GC coupled to an Agilent triple-quad mass detector. The volatile compounds adsorbed on SPME fiber were desorbed in GC injector at 220 °C for 5 min in splitless mode, and chromatographic separation was carried out on a 30 m × 0.25 mm × 0.25 μm film thickness HP-5MS (5 % phenyl methyl siloxane) capillary column. The injector port was equipped with a narrow-bore (0.75 mm id) glass liner to minimize peak broadening. The GC oven temperature was programmed from 40 °C (held for 5 min) to 250 °C at a rate of 5 °C/min. Helium was used as a carrier gas at a constant flow of 0.9 ml/min. Mass spectra were recorded in EI mode at 70 eV, scanning the 20–550 m/z range. The interface and source temperatures were 150 and 230 °C, respectively.
Optimization of SPME–GC–MS method
The analyses of volatiles present in honey were performed by SPME–GC–MS technique. As honey is a complex mixture of sugars, different parameters (temperature, fiber polarity, honey matrix weight and SPME fiber exposure time) were considered in order to optimize the sensitivity of SPME technique. For optimization, quantitative analysis was performed using benzophenone as internal standard and response factor was assumed to be 1 for compounds whose commercial standards were not available (Table 2). Using identical experimental conditions of fiber, sampling time and temperature, three successive injections of the sample were made in order to address the optimized method reproducibility. The method proved to be very sensitive as reflected from the values of average recovery (84–93 %), limits of detection (LOD) (0.005–0.19 µg/g) and limits of quantification (LOQ) (0.08–0.23 µg/g).
Peak identification
The identification of the isolated volatile compounds was achieved by comparing obtained mass spectra of unknown peaks with those stored in the NIST.02 (US National Institute of Standards and Technology). In addition, a hydrocarbon mixture from n-C8 to n-C20 (Sigma-Aldrich, Milan, Italy) was used for relative retention index calculation. The relative concentration was calculated (μg/g of honey) by comparing peak surface area of the internal standard (benzophenone) to peak areas of the eluting compounds.
Results and discussion
The pollen spectra of honey samples studied have been briefly described in the following section, and the percentages are relative to pollen of nectar producing plants. Apple honey (M. domestica) contained 54–60 % pollen of M. domestica sp. Cherry honey (P. avium) contained 46–57 % pollen of P. avium, while saffron honey (C. sativus) contained 47–49 % pollen of C. sativus.
Identification of the volatile constituents in honey
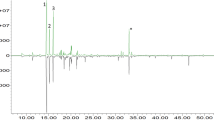
The identified volatile compounds of the apple honey (M. domestica), saffron honey (C. sativus) and cherry honey (P. avium) are presented in Tables 3, 4 and 5 and their exemplary volatile profile in Figs. 1, 2 and 3, respectively. In the analyzed honey samples, 42 volatile components were identified which belonged to different chemical classes of alcohols, ketones, organic acids, esters, aldehydes and aliphatic hydrocarbons.
Exemplary volatile profile of apple honey (Malus domestica). IS internal standard. Bold numbers refer to marker compounds listed in Table 3
Exemplary volatile profile of saffron honey (Crocus sativus). IS internal standard. Bold numbers refer to marker compounds listed in Table 4
Exemplary volatile profile of cherry honey (Prunus avium). IS internal standard. Bold numbers refer to marker compounds listed in Table 5
Honey markers for the characterization of different unifloral honeys
Our investigation showed that out of forty-two identified volatile compounds, only two compounds furfural and o-hydroxyanisole were found in all honey samples in the range of 0.082–0.122 and 0.007–0.011 µg/g, respectively, while 1,6-anhydro, β-d-glucopyranose and 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl were present in apple honey (M. domestica) and saffron honey (C. sativus). Alcohol is an important compound present in various honeys. Among the identified alcohols, oleyl alcohol and 1-heptacosanol were identified in apple honey (M. domestica), 1-heptanol and 4-methoxycyclohexanol in cherry honey (P. avium), while phenyl ethyl alcohol, maltol and 2-(4-methyl-1-piperazinyl) ethanol were identified in saffron honey (C. sativus). The alcohols identified in three analyzed samples have never been reported in earlier studies except maltol reported in sage honey (Salvia officinalis and Lavandula hybrida Reverchon II honey, phenyl ethyl alcohol reported in kanuka honey (Kunzea ericoides), lime honey (Tilia sp.), Palestinian honey and Brazilian cashew honey (Anacardium occidentale), while 1-heptanol has been reported in L. hybrida Reverchon II honey, Lavender honey (L. latifolia) and Lavandin honey (Lavandula angustifolia × latifolia) [20, 33–39]). Interestingly, 1-heptacosanol has been identified in apple skin wax, phenyl ethyl alcohol in saffron and 1-heptanol has been reported as an aromatic active volatile compound in cherry [18, 27]. Such volatile alcoholic compounds could be suggested as chemical markers in analyzed samples of apple honey (M. domestica), saffron honey (C. sativus) and cherry honey (P. avium).
Carboxylic acids are chemical compounds that can be distinguished by typical aromas, ranging from spicy to rancid. Acetic acid has spicy flavors and aromas, while butanoic acid and hexanoic acids are linked to rancid aroma. Of the organic acids identified, hexadecanoic acid was present in apple honey (M. domestica) (0.0162 µg/g) and in cherry honey (P. avium) (0.0762 µg/g), acetic acid (0.0519 µg/g) and stearic acid (0.0011 µg/g) only in cherry honey and apple honey, respectively, while there was no organic acid found in saffron honey (C. sativus). These results were in agreement with the studies conducted by Jerkovic et al. [11] and Lusic et al. [34] which found hexadecanoic acid in the unifloral honeys of black locust (Robinia pseudoacacia L.), chestnut (C. sativa L.) and fir honeydew honey (Abies alba), respectively. Hexadecanoic acid has been reported in apple blossom (M. domestica) as well as in Mahaleb cherry (Prunus mahaleb), thus revealing similarity of both volatile profiles [23, 40]. Stearic acid has been reported in Malaysian Tualang honeys (Koompassia excelsa)) by Syazana et al. [41]. Acetic acid is among those acids that occurs naturally in honey synthesized during bee metabolism [10]. Acetic acid has been found as major organic acid in cherry, while it has been identified in pure honey sources of lemon (Citrus limon), orange (C. sinensis), lavandin (L. angustifolia × latifolia), lavender (L. latifolia), rape (Brassica napus), acacia (R. pseudoacacia), lima (Tilia sp.), buckwheat (Fagopyrum esculentum), heather (C. vulgaris) and honeydew [39, 42–44]. The ketonic compound 3-hydroxy-2 butanone has been identified as a distinctive feature of eucalyptus honey (Eucalyptus sp.) and has been confirmed as a floral marker for this type of honey [16, 45]. It has been also identified in lime (Tilia sp.) and rosemary honey (Rosmarinus officinalis), while acetone has been reported as a relevant compound of acacia (R. pseudoacacia) and rosemary honey (R. officinalis) [14]. 2-Aminoacetophenone has been suggested as a major chemical marker of chestnut honey (C. sativa), [17, 18]. The ketones were only identified in saffron honey (C. sativus) which include tetrahydro[2]bifuranyl-5-one (0.5132 µg/g); 2,3-butanedione (0.0867); 2,3-dihydroxynapthalene-1,4-dione (0.0954 µg/g); and 2,6,6-trimethyl-2-cyclohexane-1,4-dione (0.0197 µg/g). Of the ketones identified in saffron honey, 2,6,6-trimethyl-2-cyclohexane-1,4-dione has also been detected in sage honey (S. officinalis), kanuka honey (K. ericoides) and heather honey (C. vulgaris) [20, 35, 46]. Three ketones 2,6,6-trimethyl-2-cyclohexane-1,4-dione; 2,3-butanedione; and 2,3-dihydroxynapthalene-1,4-dione have been reported as the unique volatile components in saffron [47, 48]. Thus, these three ketones analyzed in saffron honey could be characterized as unique markers.
Aldehydes have also been described as a characteristic aromatic compound of monofloral honeys. Benzaldehyde has been suggested as potential aromatic compound in chestnut honey (C. sativa) and heather honey (C. vulgaris), heptanal in lavender honey (L. latifolia), nonanal in rosemary honey (R. officinalis), while sinensal has been suggested as a major marker of citrus honey (Citrus sp.) [17, 21, 22, 49]. Only two aldehydes were identified in the analyzed samples in the form of furfural (0.0824–0.1224 µg/g) which was detected in all three honey types, while cis-9-hexadecenal was found in saffron honey (C. sativus) with a concentration of 0.0074 µg/g. Compounds produced during sugar degradation like furfural, furfuryl alcohol and 5-methyl-furfural derived from furan have been identified in fresh citrus honey (Citrus sp.). Furfural has also been identified as a relevant compound of lime honey (Tilia sp.), lavender honey (L. latifolia) and acacia honey (R. pseudoacacia), while 2-furanmethanol (furfuryl alcohol) has been reported in lavender honey (L. latifolia) [14]. The concentration of these compounds in the honey samples increased with storage, and this increase is greater when temperature is raised from 10 to 40 °C [50]. Compounds derived from furan are considered to be indicators of thermal processes and storage and thus are not considered appropriate floral markers as they lose freshness with long periods of storage or exposure to high temperatures [51]. The mild heating experienced by samples during SPME which is necessary in order to improve the extraction process and to reduce the equilibrium time could be partially responsible for some of these compounds [52].
The aliphatic hydrocarbons identified in the tested samples include triacontane in apple honey (M. domestica) (0.0134 µg/g) and cherry honey (P. avium) (0.0021 µg/g), while tricosane (0.0023 µg/g), hexacosane (0.0087 µg/g) and nonacosane (0.0026 µg/g) were present only in apple honey (M. domestica) (Tables 3, 5). 1-Methyl-1-(2-hydroxyethyl)-1-silacyclobutane (0.0234 µg/g) was found only in cherry honey and has never been reported in any type of honey before. This compound may be naturally present in cherry honey (P. avium), while no hydrocarbon was found in saffron honey (C. sativus). The hydrocarbons reported in our analyzed apple honey (M. domestica) have been reported by Syazana et al. [41] in Malaysian Tualang honeys (Koompassia excelsa). Omata et al. [53] have also identified same hydrocarbons in apple flowers and thus disclosing similarity of both profiles. The hydrocarbons are less related to honey origin as they are well constituents of beeswax. Esters like ethyl octanoate, ethyl decanoate and ethyl dodecanoate have been reported in Spanish chestnut honeys (C. sativa) [54]. Among the esters, pentanedioic acid bis (1-methylpropyl) ester (0.0231 µg/g), phosphonic acid bis (1-methylethyl) ester (0.0013 µg/g) and pentadecyl heptafluorobutyrate (0.0032 µg/g) were present in apple honey (M. domestica) and 1,2,3-propanetriol monoacetate (0.0054 µg/g) in saffron honey (C. sativus), while six esters viz. hexadecanoic acid octadecyl ester (0.0486 µg/g), succinic acid heptadecyl 2-methylallyl ester (0.0083 µg/g), fumaric acid 3-methylbut-3-enylundecyl ester (0.0093 µg/g), octanoic acid 3-en-2-yl ester (0.0523 µg/g) acetoxyacetic acid 5-tetradecyl ester (0.0027 µg/g) and ethyl hexadecanoate (0.0101 µg/g) were identified in cherry honey (P. avium). The esters identified in the analyzed samples have never been reported in any previous study. Ethyl hexadecanoate has been reported in sweet Chinese cherries that could be used as marker compound of cherry honey (P. avium) [44].
It is not necessary that all the volatile compounds (few may be present) found in a particular honey from a particular country or region will be same from the same honey variety from different region or country since volatile profile of a particular honey depends on nectar composition, floral origin, soil type, climate, growing area, detection and isolation techniques, which play a vital role in determining the results. The research conducted by Panseri et al. [55] on buckwheat honey (F. esculentum), from different geographical origins (Russia, Italy and Poland), shows that all volatile compounds were not present in three buckwheat honeys: phenylacetaldehyde was only present in Russian sample, eugenol only in Italian sample, while α-terpinene only in Poland sample and Russian sample.
Pyran derivative 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl was identified in apple honey (M. domestica) and saffron honey (C. sativus) in a concentration range between 0.8044 and 1.0245 µg/g. Pyran-derived compounds are produced by Maillard reaction and are considered as a loss of freshness, which could negatively influence the sensory properties of honey [45]. These results were consistent with the results reported by Manyi-Loh et al. [43].
The presence of a particular compound in a particular type of honey, and the absence in others, could be characteristic for a specified type of honey. In our study, 2,6,6-trimethyl-2-cyclohexane-1,4-dione; 2,3-butanedione; 2,3-dihydroxynapthalene-1,4-dione; phenyl ethyl alcohol; and cis-9-hexadecenal are the volatile compounds that could be used as marker compounds for saffron honey (C. sativus). These volatile compounds have also been reported in saffron as discussed above, and they are absent in other two types of honeys. However, the volatile compound 2,6,6-trimethyl-2-cyclohexane-1,4-dione has been reported in other honeys too [35, 46]. Cherry honey (P. avium) is characterized by the presence of aromatic alcohols and esters; 1-heptanol, acetic acid and ethyl hexadecanoate have been reported in cherries as discussed above thus can be used as unique markers for cherry honey (P. avium). In addition, octanoic acid oct-3-en-2-yl ester; 4-methoxycyclohexanol; and 2-imidazoline-4-carboxylic acid could also be used as markers for cherry honey (P. avium) as these are altogether absent in other apple honey and saffron honey. Apple honey (M. domestica) is characterized by the presence of 1-heptacosanol as reported in apple wax; tricosane, hexacosane and hexadecanoic acid are reported in apple flowers [23, 53], while oleyl alcohol, stearic acid and pentadecyl heptafluorobutyrate are also identified in apple honey (M. domestica). Such compounds could be characterized as marker compounds of apple honey (M. domestica) as these are altogether absent in other two types of honeys. The structure of some of the volatile compounds (as markers) detected in three honey samples is shown in Fig. 4.
Conclusion
In order to confirm any volatile compound as a “marker” of honey floral source, the chemical marker should be present only in honey samples coming from that floral source or at least the chemical marker should be present in a high and quite constant amount. Comparison of our honey samples with other monofloral honeys is not possible because the volatile compounds are derived from plant or nectar source; thus, all volatile compounds (few may be present) present in a particular unifloral honey from a particular region will not be same from same floral origin from different country. Most of the volatile compounds (70 %) identified in honeys from Kashmir Valley have been reported for the first time except few which have been reported in other monofloral honeys as discussed above. Interestingly, some of the volatile compounds that have been detected in flowers and fruits of apple, cherry and saffron have also been detected in our tested honey samples of apple, cherry and saffron too thus could be used as unique markers of the respective honeys. These results can be used to assess certain markers for the types of honey selected. Also, we concluded that the presence of a particular compound in a particular type of honey, and the absence in others, could be characteristic for a specified type of honey.
References
Bertoncelj J, Dobersek U, Jamnik M, Golob T (2007) Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem 105:822–828
Guler A, Bakan A, Nisbet C, Yavuz O (2007) Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem 105:1119–1125
Kaskoniene V, Venskutonis PR (2010) Floral markers in honey of various botanical and geographical origins: a review. Compr Rev Food Sci Food Saf 9:620–634
Al-Mamary M, Al-Meeri A, Al-Habori M (2002) Antioxidant activities and total phenolics of different types of honey. Nutr Res 22:1041–1047
Oddo LP, Piazza MG, Sabatini AG, Accorti M (1995) Characterization of unifloral honeys. Apidologie 26:453–465
Cabras P, Angioni A, Tuberoso C, Floris I, Reniero F, Guillou C, Ghelli S (1999) Homogentisic acid: a phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J Agric Food Chem 47:4064–4067
Careri M, Mangia A, Barbieri G, Bolzoni L, Virgili R, Parolari G (1994) Sensory property relationship to chemical data Italian-type dry-cured ham. J Food Sci 27:491–495
Conte L, Plasenzotto BS, Rouff K, Sanz J, Denemont J (2002) Determination of honey volatiles in relation to their botanical origin. In: Symposium II, Ruolo della ricerca in apicoltura, Bologna, pp 247–252
Schieberle P, Komarek D (2003) Changes in key aroma compounds during natural beer aging. Freshness Shelf Life Foods 836:70–79
Bastos C, Alves R (2003) Compostos volateis em meis florais. Quim Nova 26:90–96
Jerkovic I, Mastelic J, Marijanovic Z, Klein Z, Jelic M (2007) Comparison of hydrodistillation and ultrasonic solvent extraction for the isolation of volatile compounds from two unifloral honeys of Robinia pseudoacacia L. and Castanea sativa L. Ultrason Sonochem 14:750–756
Zhou Q, Winterstreen L, Cadwallader K (2002) Identification and quantification of aroma-active components that contribute to the distinct malty flavor of buckwheat honey. Food Chem 50:2016–2021
Serra-Bonvehi J, Ventura-Coll F (2003) Flavors index and aromas profiles of fresh and processed honey. J Sci Food Agric 83:275–282
Cuevas-Glory LF, Pino JA, Santiago LS, Sauri-Duch E (2007) A review of volatile analytical methods for determining the botanical origin of honey. Food Chem 103:1032–1043
De la Fuente E, Martinez-Castro I, Sanz J (2005) Characterization of Spanish unifloral honeys by solid phase microextraction and gas chromatography–mass spectrometry. J Sep Sci 28:1093–1100
Perez A, Brunete CS (2002) Analysis of volatiles from Spanish honeys by solid-phase microextraction and gas chromatography–mass spectrometry. Food Chem 50:2633–2637
Bouseta A, Scheirman V, Collin S (1996) Flavor and free amino acid composition of lavender and eucalyptus honeys. J Food Sci 61:683–687
Rowland CY, Blackman AJ, Darcy BR, Rintoul GB (1995) Comparison of organic extractives found in leatherwood (Eucriphia lucida) honey and leatherwood flowers and leaves. J Agric Food Chem 43:753–763
Revell LE, Morris B, Manley-Harris M (2014) Analysis of volatile compounds in New Zealand unifloral honeys by SPME-GC–MS and chemometric-based classification of floral source. Food Meas 8:81–91
Beitlich N, Koelling-Speer I, Oelschlaegel S, Speer K (2014) Differentiation of Manuka honey from Kanuka honey and from jelly bush honey using HS-SPME-GC/MS and UHPLC-PDA-MS/MS. J Agric Food Chem 62:6435–6444
Oelschlagel S (2011) Apimondia, congresses proceedings. http://www.apimondia.com. Accessed 17 Jan 2014
Serra-Bonvehi J, Ventura-Coll F (1995) Characterization of Citrus honey produced in Spain. J Agric Food Chem 43:2053–2057
Kus PM, Jerkovic I, Tuberoso CIG, Sarolic M (2013) The volatile profiles of a rare Apple (Malus domestica Borkh.) Honey: Shikimic acid-pathway derivatives, terpenes, and others. Chem Biodivers 10(1638):1652
Raina AN (2003) Radha Krishan Anand and Co. Pacca Danga, Jammu, pp 4–5
Press information bureau, Government of India (2013) http://pib.nic.in/newsite/erelease.aspx?relid=96239. Accessed 23 Dec 2013
Kashmir Valley Honey (2014) http://kashmirhoney.net/store/8-bee-keeping. Accessed 18 Feb 2014
Nanda V, Sarkara BC, Sharma HK, Bawa AS (2003) Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India. J Food Compos Anal 16:613–619
Von Der Ohe W, Oddo LP, Piana ML, Morlot M, Martin P (2004) Harmonized Methods of melissopalynology. Apidologie 35:18–25
Louveaux J, Maurizio A, Vorwohl G (1978) Methods of melissopalynology. Bee World 59:139–157
Alissandrakis E, Tarantalis PA, Harizanis PC, Polissiou M (2007) Aroma investigation of unifloral Greek citrus honey using solid-phase microextraction coupled to gas chromatography–mass spectrometric analysis. Food Chem 100:396–404
Ampuero S, Bodganov S, Bosset JO (2004) Classification of unifloral honeys with an MS-based electronic nose using different sampling modes SHS, SPME and INDEX. Eur Food Res Technol 218:194–207
Pontes M, Marques JC, Camara JS (2007) Screening of volatile composition from Portuguese multifloral honeys using headspace solid-phase microextraction–gas chromatography–quadrupole mass spectrometry. Talanta 74:91–103
Radovic BS, Careri M, Mangia A, Musci M, Gerboles M, Anklam E (2001) Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chem 72:511–520
Castro-Vazquez L, Diaz-Maroto MC, Gonzalez-Vinas MA, Perez-Coello MS (2009) Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem 112:1022–1030
Lusic D, Koprivnjak O, Curic D, Sabatini AG, Conte LS (2007) Volatile profile of Croatian lime tree (Tilia sp.), fir honeydew (Abies alba) and sage (Salvia officinalis) honey. Food Technol Biotech 45:156–165
Odeh I, Abu-Lafi S, Dwik H, Al-najar I, Imam A, Dembitsky VM, Hanus OL (2007) A variety of volatile compounds as markers in Palestinian honey from Thymus capitatus, Thymelaea hirsuta, and Tolpis virgate. Food Chem 101(4):1393–1397
Moreria RFA, Trugo LC, Pietroluonga M, De Maria CAB (2002) Flavor composition of cashew (Anacardium occidentale) and marmeleiro (Croton species) honeys. J Agric Food Chem 50:7616–7621
Jerkovic I, Marijanovic Z (2009) Screening of volatile composition of Lavandula hybrida REVERCHON II Honey using headspace solid-phase microextraction and ultrasonic solvent extraction. Chem Biod 6:421–430
Castro-Vazquez L, Leon-Ruiz V, Alanon ME, Perez-Coello MS, Gonzalez-Porto AV (2014) Floral origin markers for authenticating Lavandin honey (Lavandula angustifolia × latifolia). Discrimination from Lavender honey (Lavandula latifolia). Food Control 37:362–370
Jerkovic I, Marijanovic Z, Stover M (2011) Screening of natural organic volatiles from Prunus mahaleb L. honey: coumarin and vomifoliol as nonspecific biomarkers. Molecules 16(3):2507–2518
Syazana MS, Gan SH, Halim AS, Shah NSM, Gan SH, Sukari HA (2013) Analysis of volatile compounds of Malaysian Tualang (Koompassia excelsa) honey using gas chromatography mass spectrometry. Afr J Tradit Complement Altern Med 10(2):180–188
Plutowska B, Chmiel T, Dymerski T, Wardencki W (2011) A headspace solid phase microextraction method development and its application in determination of volatiles in honeys by gas chromatography. Food Chem 126:1288–1298
Manyi-Loh CE, Clarke AM, Ndip RN (2011) Identification of volatile compounds in solvent extracts of honeys produced in South Africa. Afr J Agric Res 6:4327–4334
Shu YS, Wen GJ, Yu PZ (2010) Characterization of the aroma-active compounds in five sweet cherry cultivars grown in Yantai (China). Flav Fragr J 25:206–213
Castro-Vazquez L, Diaz-Maroto MC, Perez-Coello MS (2006) Volatile composition and contribution to the aroma of Spanish honeydew honeys. Identification of a new chemical marker. J Agric Food Chem 54:4809–4813
D’Arcy BR, Rintoul GB, Rowland CY, Blackman AJ (1997) Composition of Australian honey extractives. Norisoprenoids, monoterpenes, and other natural volatiles from blue gum (Eucalyptus leucoxylon) and yellow box (Eucalyptus melliodora) honeys. J Agric Food Chem 45:1834–1843
Carmona M, Martınez J, Zalacain A, Rodrıguez-Mendez ML, Saja JA, Alonso GL (2005) Analysis of saffron volatile fraction by TD–GC–MS and e-nose. Eur Food Res Technol 223:96–101
Ling HJ, Yi L, Yu ZL (2011) Analysis of volatile components in saffron from Tibet and Henan by ultrasonic assisted solvent extraction and GCMS. J Chin Pharm Sci 4:404–406
Piasenzotto L, Gracco L, Conte L (2003) Solid phase microextraction (SPME) applied to honey quality control. J Sci Food Agric 83:1037–1044
Castro-Vazquez L, Diaz-Maroto MC, Perez-Coello MS (2007) Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem 103:601–606
Barra MPG, Ponce-Diaz MC, Venegas-Gallegos C (2010) Volatile compounds in honey produced in the central valley of Nuble province, Chile. Chil J Agric Res 70:75–84
Soria AC, Martinez-Castro I, Sanz J (2003) Analysis of volatile composition of honey by solid phase microextraction and gas chromatography–mass spectrometry. J Sep Sci 26:793–801
Omata A, Yomogida K, Nakamura S (1990) Volatile components of Apple Flower. Flavor Fragr J 5:19–22
Castro-Vazquez L, Diaz-Maroto MC, Torres C, Perez-Coello MS (2010) Effect of geographical origin on the chemical and sensory characteristics of chestnut honeys. Food Res Int 43:2335–2340
Panseri S, Manzo A, Chiesa LM, Giorgi A (2013) Melissopalynological and volatile compounds analysis of buckwheat honey from different geographical origins and their role in botanical determination. J Chem. doi:10.1155/2013/904202
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nayik, G.A., Nanda, V. Characterization of the volatile profile of unifloral honey from Kashmir Valley of India by using solid-phase microextraction and gas chromatography–mass spectrometry. Eur Food Res Technol 240, 1091–1100 (2015). https://doi.org/10.1007/s00217-015-2413-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2413-2