Abstract
Saffron spice is valuable for its colour, taste and aroma. All these properties depend on the different postharvesting treatments that Crocus sativus L. stigmas received to convert it into a spice. No previous scientific data relate the volatile content with its postharvesting process or origin. In this paper, two different analytical methods are used, CG-MS to determine volatile markers and an electronic nose based on metal oxides to determine the volatile profile of the samples from different geographic origins. It is demonstrated by means of CG-MS that 3,5,5-trimethyl-2-cyclohexenone, 2,6,6-trimethylcyclohexane-1,4-dione and acetic acid are capable to differentiate saffron from its origin. The content of acetic acid is very high in samples from Iran or Morocco, while low or undetectable content was observed in Greek and Spanish samples. In turn, using an e-nose (specifically optimised for this particular application), samples from different countries could be discriminated using Principal Component Analysis with a 90% of confidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saffron spice is one of the oldest spices and, due to its laborious harvesting procedures, is the most expensive spice in the world. The price of this spice greatly depends on the country of origin, not only because of the different labour costs but also because of its different capacity to transmit the three saffron attributes to foods: colour, aroma and taste. The compounds responsible for such attributes are crocins, a group of glycoside derivates from the carotenoid crocetin; terpenic aldehydes known as safranal and a glycoside terpenoid, picrocrocin, respectively. The content of these compounds depends on the dehydration postharvesting treatment necessary to convert Crocus sativus L. stigmas into saffron spice. The five main saffron-producing countries (Greece, India, Iran, Morocco and Spain) are known to use different dehydration techniques, whereas in India, Iran and Morocco, the dehydration process takes place at room temperature, directly under sunlight or in air-ventilated conditions [1–3]; in Greece stigmas are spread out on shallow layer trays with temperature set at 35–45 °C, during 12 h [4, 5]. In Spain, to produce the worldwide-recognized “Mancha” saffron, product with designation of origin (PDO), different heating sources may be used with average temperatures between 55 and 85 °C [6].
Although a large number of saffron volatile studies exist [7–15], the lack of relation between saffron dehydration and the compounds generated has not been taken into account. Together with the harsh extraction conditions employed in some methods, artefacts during the isolation previous to the analysis stage may be produced, also authors generally give poor attention to the origin of the samples since they acquire samples in local markets instead of buying certified saffron samples. In case of volatile analysis, this should be carried out without sample manipulation as proposed by Alonso [16, 17], in order that its original volatile content is reliable. After the isolation step, the recommended and the only explored possibility is to determine each compound content by GC-MS. The opposite focus, which is to consider the volatile profile as a whole and to study it by employing instruments based on sensor technology, the so-called electronic nose, has not yet been considered in saffron. The electronic noses based on Metal Oxide Sensors (MOS) have shown to be useful in the discrimination of the aromas of certain foods and beverages [18–23]. Nevertheless, it is clear now that the development of systems specific for a particular application may prove more successful than general-purpose instruments. In addition, one of the main problems when testing or training such an array of sensors is the need of having available well-characterised samples.
The aim of this study was to compare the volatile fraction of different saffron samples by means of two different analytical methods. The first method used was thermal desorption–gas chromatography–mass spectrometry (TD–GC-MS), able to separate and identify particular volatiles present in each variety of saffron. The second method was an electronic nose that analyses the volatile profile of saffron.
Materials and methods
Saffron samples
This study involved 13 well-defined samples of saffron from leading producers (3 from Spain, 7 from Iran, 2 from Greece, and 1 from Morocco) from the same harvesting year. The reduced number of samples was due that they were obtained directly from the producers with the guarantee of their origin and lack of adulteration.
Electronic nose device
MOS sensors and test system
Twenty-seven gas sensors based on metallic oxides were exposed to the saffron samples (Table 1). Sensors were placed inside a stank, stainless steel chamber, 80.5 mm long, 30.5 mm high, and 37.5 mm wide. The interior volume was 15 ml. The inside of the chamber was oval-shaped to eliminate possible dead volumes. Two 1/8 in. connector plugs were screwed in on both sides allowing gas to enter and exit the interior of the chamber. The hermetic seal was achieved with a 3 mm diameter toric joint. The chamber permitted the introduction of a maximum of 16 sensors, and a Pt100 probe for measuring internal temperature.
The sensors were exposed to the volatiles coming from the saffron samples using two different injection systems:
-
1.
In a static headspace autosampler unit Hewlett Packard HP 7694e (Palo Alto, CA), 1 g of saffron was placed into a 10 ml vial and kept at a constant temperature (40 °C) for 10 min. The vials were then pressurised for 8 s at 1.5 bar. The pressure gradient permitted to fill a 3 ml loop whose content was then injected to the sensor chamber. The carrier gas was high purity synthetic air and the flow rate was 100 ml/min. An injection was performed every 15 min.
-
2.
A dynamic headspace sampling method using a purge and trap TEKMAR 3100 sample concentrator was carried out. The saffron samples (1 g) were placed in a 30 ml tube and heated during 15 min at constant temperature of 50 °C. The volatile compounds were collected and concentrated on a tube filled with Tenax TA (100 mg, 60 mesh; Alltech, Lokeren, Belgium). After 5 min purging, the Tenax TA was subjected for a 5 min drying step to eliminate moisture that might have condensed inside, and subsequently it was thermally desorbed at 250 °C. Using nitrogen as carrier gas with a flow rate of 1 ml/min, the volatiles desorbed were injected into the sensor chamber.
Data acquisition and data analysis
As the inorganic gas sensors produce immediate responses, a fast data collection procedure was required. This step was performed through a PC-LPM-16 data acquisition card from National Instruments interfaced to a PC. The sensors were polarised using a constant voltage of 5 V provided by a Hameg 8142 programmable power supply. The resistance of each sensor was measured every 0.2 s and up to 16 sensors were measured simultaneously. Sensor selection was carried out by k-nearest neighbour (k-NN) methodology and with this new network of sensors the trials were repeated in triplicate (Table 1).
Data acquisition and processing software routines were written in Visual Basic version 5.0 from Microsoft™. The software allows representing in real time the relative or absolute change in the resistance of the sensors along the time. The data were stored in ASCII format for posterior data processing.
The effectiveness of the system to discriminate between saffron samples was studied using principal component analysis (PCA) taking into account the first 10 coefficients of the fast Fourier transform of the curves as the input variable, and performed through the software Matlab v5.0.
Identification and quantification of volatile compounds by thermal desorption–gas chromatography–mass spectrometry (TD–GC–MS)
A joined system made up of thermal desorption Perkin-Elmer ATD-400 equipment (Norwalk, CT, USA), a gas chromatograph HP-6890 and a mass spectrometer HP-5973 provided with a NIST library (Hewlett-Packard, Palo Alto, CA, USA) were used. A fused silica capillary column with stationary phase BP21 of 50 m length, 0.22 mm i.d. and 0.25 μm of film was used (SGE, Australia). The carrier gas was helium of chromatographic purity (220 kPa). Sample (20 mg) was introduced into the desorption tube and desorbed at 50 °C for 1 min. Other conditions for the thermal desorption equipment were oven temperature, 250 °C; cold trap temperature, −30 °C; transfer line temperature, 200 °C. Conditions for gas chromatography were 100 °C (5 min), 18 °C/min to 210 °C (15 min). In the mass spectrometer the electron impact mode (EI) was set up at 70 eV. The mass range varied from 35 to 500 uma and the detector temperature was 150 °C. Compounds identification was carried out using the NIST library and comparison with those reported in the literature. Identification of acetic acid, 3,7-dimethyl-1,6-octadien-3-ol (linalool), 2,6,6-trimethyl,1,3-cyclohexadiene-1-carboxaldehyde (safranal) and 3,5,5-trimethyl-2-cyclohexen-1-one (isophorone) has been carried out by comparing them with their respective commercial standards (Sigma–Aldrich, Madrid, Spain). Quantification was carried out in the single ion monitoring (SIM) mode and were determined if the peak heights were three times the baseline. The results were expressed as percentage of total area chromatograms. Data reported represent the average of three sample replicates.
Results and discussion
Assays carried out by e-nose
One of the main problems when testing or training the array of sensors is the need of having available well-characterised samples. This implies a degree of difficulty since samples have to be carefully selected and evaluated as it has been carried out for this study. Reason why the number of samples for the different countries is so reduced.
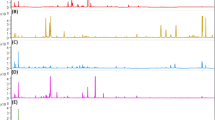
Two systems were tested for volatile compound extraction from the sample, a static headspace system (SHS) and a purge and trap system (DHS). In both cases, the exposure of the sensors to the aroma of saffron caused a fast change of the resistivity of the SnO2 films (expressed as the fractional change of resistance, (ΔR/R)×100) (Fig. 1). The changes were reversible and the baseline was completely recovered in all cases, although this process took a few minutes. In addition, the different sensing units showed different sensitivity towards the saffron headspace. It is worth noting that for the sensors suitable for the detection of VOCs and alcohols, increases in the resistances were observed, whereas the sensors devoted to the detection of reducing gases, the opposite tendency was observed.
When using purge and trap, the sensors showed a degree of selectivity towards the different classes of samples. As a first example, the polar graphs of the responses of the sensors S4 and S10 to the 13 saffron samples are shown in Fig. 2. These graphs were constructed using the changes of relative resistance observed in Fig. 1, and representing the peak height of each sensor as a radial vector. The response of the sensors was highly reproducible. The standard relative deviation for repeated measurements (n=5) ranged from 1.7 to 3.5%.
Saffron samples were repeatedly measured and the PCA analysis was conducted. The results obtained using the headspace and the purge and trap injection systems were compared. The following were tested as characteristic parameters of the curves obtained: the area under the curve, the height of the maximum, the width at the semi-height and the first terms of the fast Fourier transform. This last option is what offered the best results in the statistical analysis of the principal components, probably because it gathered more information about the form of the curve.
The headspace sampler allowed obtaining a primary classification between samples from different countries, but the discrimination was not complete. The clusters corresponding to saffron coming from Iran and Greece were clearly separated; nevertheless, the cluster corresponding to the Spanish saffron was too wide to assume a good discrimination from the Morocco saffron.
Results from the analysis of the principal components using purge and trap are shown in Fig. 3. In the histogram it is observed that the sensors were capable of perfectly differentiating the countries where samples originated. In good agreement with previously published results [24], the purge and trap system was chosen for its greater sensor signal intensity. The increase was due as much to trap concentration effect, when purging the sample for a long time, as to the drying stage to eliminate water. As well, the system would permit the determination of the origin of any sample if a sufficiently large collection of saffron was available of known origin.
Assays carried out by TD–GC–MS
The same samples analysed by the e-nose were analysed by TD–GC–MS, as the thermal desorption units work as a purge and trap device. Comparison of the different chromatograms revealed that all samples contained the same compounds (Table 2), although in different proportions, with one exception, compound 1, acetic acid. Quantification of 2,6,6-trimethyl,1,4-cyclohexadiene-1-carboxaldehyde, compound 4, was not possible because of its low concentration.
Because of the few available samples, before considering a statistical analysis, a previous descriptive analysis was done that consisted of comparing the significance of each compound in its country's volatile with respect to its importance in remaining countries' aromas. First of all, the average area of each component from each country was calculated, thus establishing a confidence interval (mean − 2DE < x < mean + 2DE) to assure that the average value was representative. Secondly, the importance of each compound in the volatile from each production area was evaluated according to the relationship between its average area value and the sum area of the remaining components. Lastly, the relative importance of each component was compared among countries starting from the quotient between the importance of each component in Greece, Iran and Morocco, in regard to its importance in Spanish saffron. The same conclusions were obtained independently of the compound considered most significant. To simplify matters, only the results obtained when safranal was considered the most important are shown in Table 3. The highest percentages within each country indicated compounds with the greatest importance, in regard to the importance of that same component in Spanish saffron. Except for compound 1 in the case of Greece, the results for all three countries were the same when compared to Spain. Acetic acid, isophorone (compound 5) and 2,6,6-trimethyl-1,4-cyclohexanedione (compound 9) were found at a much higher proportion. Returning to the raw data, it is easy to understand that these compounds may allow saffron origin to be differentiated. The proportion that they implied in regard to safranal was very different depending on the studied country (Table 4). These results are coherent with those obtained by Semiond [25] for compounds 5 and 9 in Spanish saffron, when compared with saffron from other origins. In relation to acetic acid, the fact that the same behaviour is observed in countries where temperature is raised during dehydration suggests that the presence or absence of this compound could be related to this process. It is possible that rapid elimination of water hinders the fermentation which generates acetic acid. In regard to 2-hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one (compound 8), the small variation on its content does not allow it to be used for differentiating between countries. On the other hand, this does not reduce importance of the role that both Cadwallader [7] and Knapp [26] attribute to it.
Once the result of the previous descriptive analysis was known, a discriminate analysis was performed in search of more information. Starting as much with the absolute as the relative quantification values for each component, various results were obtained (Fig. 4). The results confirmed the conclusions reached through the descriptive analysis. When absolute values were used, canonical discriminate functions depended on compounds 1 and 9. In spite of a great part of the variance being explained by both functions, 65.4% and 34.6% respectively, the desired separation was not obtained between Greece and Iran. Probably, this was because in these two countries the most important compounds at the moment of differentiation were 7 and 8. When component values were introduced as total volatile percentages, the program carried out separation of the countries according to components 1 and 5. In this case, the first canonical discriminate function explained 89.6% of the variance, and the most poorly separated countries were Greece and Spain. The proportion of compound 5 for these two countries was not very different, and since neither of them contained acetic acid, they seem to overlap. A justification of why different results were obtained through discriminate analysis depending on the way data were introduced could be that compounds 5 and 9 might be related. It should be pointed out that compounds 5 and 9, although they were at very different proportion, maintained a relationship between them from 7.2 to 8.0 in all countries, including Spain. To verify whether a relationship existed or not between compound 5 and 9, a Pearson correlation test was carried out by countries: Spain (0.94), Greece (0.98), Iran (0.61) and Morocco (1.00). The degree of correlation was high and statistically significant in every case (0.01). It remains to be demonstrated how the dehydration process used in different production areas had an influence on their larger or smaller proportion.
Conclusions
Thermal desorption–gas chromatography–mass spectrometry (TD–GC–MS) and e-nose have shown to be useful techniques to discriminate saffron samples of different origins. Besides this important utility, enormous possibilities for application open up in saffron by relating results from e-nose to those obtained from classical techniques as they could be of great utility to watch over and optimise dehydration, storage and packaging conditions. It would permit acquisition of saffron batches according to their volatile content but also depending on their future evolution.
References
Ait-Oubahou A, El-Otmani M (1999) Saffron Crocus sativus L. Medicinal and aromatic plants. Industrial profiles. In: Negbi M (ed). Harwood Academic Publishers, Amsterdam, Holland, pp 87–102
Hassnain FM (1998) Saffron cultivation in Kashmit. In: Sethi SS (ed). Rima Publishing House, Nueva Delhi, India
Nauriyal JP, Gupta R, George CK (1997) Arecaunt Spices Bull 8:59–72
Goliaris AH (1999) Saffron Crocus sativus L. Medicinal and aromatic plants. Industrial profiles. In: Negbi M (ed). Harwood Academic Publishers, Amsterdam, Holland, pp 73–87
Ordoudi S, Tsimidou M (2004) In: Dris R, Jain SM (eds). Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 209–260
Carmona M, Zalacain A, Pardo JE, López E, Alvarruiz A, Alonso GL (2005) J Agric Food Chem 53:3974–3979
Cadwallader KR, Baek HH, Cai M (1997) In: Riach SJ, Ho CT (eds). American Chemical Society Symposium Series 660, Washington, DC, pp 66–79
Cadwallader KR (2002) In: Winterhalter P, Rouseff R (eds). American Chemical Society Symposium Series 802, Washington, DC, pp 220–239
D'Auria M, Mauriello G, Rana GL (2004) Flavour Fragrance J 19:7–23
Kanakis CD, Daferera J, Tarantilis PA, Polissiou MG (2004) J Agric Food Chem 52:4515–4521
Rödel W, Petrizka M (1991) J High Res Chromatogr 14:771–774
Tarantilis PA, Polissiou MG (1997) J Agric Food Chem 45:459–462
Zareena AV, Variyar PS, Gholap AS, Bongirwar DR, Wani AM (2001) J Agric Food Chem 49:687–691
Zarghami NS, Heinz DE (1971) Lebensm Wissen Techn 4:43–45
Zarghami NS, Heinz DE (1971) Phytochemistry 10:2755–2761
Alonso GL, Salinas MR, Garijo J (1998) J Food Prot 61:1525–1528
Alonso GL, Salinas MR, Sánchez MA, Garijo J (2001) Food Sci Tech Int 7:225–229
Bartlett PN, Blair N, Gardner JW (1997) Food Technol 51:44–48
Gardner JW, Bartlett PN (1999) Electronic noses. Oxford University Press, Oxford
Di Natale C, Davide FAM, D'Amico A, Nelli P, Groppelli S, Sberveglieri G (1996) Sens Actuators B 33:83–88
Pearce TC, Schiffman SS, Nagle HT, Gardner JW (eds) (2002) Handbook of machine olfaction: Electronic nose technology, Chap 7, Wiley-VCH, Weinheim, Germany
Di Natale C, Macagnano A, Davide F, D'Amico A, Paolesse R, Boschi T, Faccio M, Ferri G (1997) Sens Actuators B 44:521–526
Orts J, Llobet E, Vilanova X, Brezmes J, Correig X (1999) Sens Actuators B 60:106–117
Schaller E, Zenhäusern S, Zesiger T, Bosset JO, Escher F (2000) Analysis 28:743–749
Semiond D, Dautraix S, Desage M, Majdalani R, Casabianca H, Brazier JL (1996) Anal Lett 6(29):1027–1039
Knapp H, Straubinger M, Witte A, Winterhalter P (2000) In: Schieberle P, Engel KH (eds) Proceedings of the 9th Weurman flavour research symposium, Garching, Germany, pp 440–444
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carmona, M., Martínez, J., Zalacain, A. et al. Analysis of saffron volatile fraction by TD–GC–MS and e-nose. Eur Food Res Technol 223, 96–101 (2006). https://doi.org/10.1007/s00217-005-0144-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0144-5