Abstract
In this study, an efficient, sensitive, and rapid method based on microwave-assisted extraction coupled with dispersive liquid–liquid microextraction (DLLME) followed by gas chromatography–mass spectrometry for determination and quantification of seven nitrosamines (NAs) in heated meat products (sausage and salami) was developed. At extraction stage, nitrosamines were extracted from sausage and salami samples with 10 mL of a hydrolyzing solvent using microwave at 500 MHz for 1.5 min. Effective parameters on DLLME, such as volumes of extraction and disperser solvents, pH, and salt addition, were optimized using response surface methodology based on central composite design. This technique provided acceptable repeatability in the range of 3.5–5.4 % for spiked samples. The recoveries of NAs were in the range 83.9–109.4 %. Limits of detection and limits of quantification for NAs in the real samples were within the ranges of 0.11–0.48 and 0.41–1.45 ng g−1, respectively. Good linear ranges were obtained for seven NAs in the range of 0.1–200 ng mL−1, with the coefficient (R 2) higher than 0.99. The merit figures, compared with other methods, showed that new proposed method is an accurate, precise, and reliable sample pretreatment method that substantially reduces sample matrix interference and gives very good enrichment factors (126–152).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
N-nitrosamines (NAs) are unintentional by-products during food processing, which are formed by their action between secondary amines and nitrosating agents (such as nitrites and oxides of nitrogen) [1]. In addition, they may be formed through microbial reactions. Nitrites and nitrates are commonly used in the manufacturing of meat products to generate desirable color, texture, and flavor, as well as to increase the shelf life and to prevent from rancidity during storage [2–4]. The formation of NAs depends on the cooking method, temperature, time, concentration of nitrite, content of catalysts, and inhibitors [2, 4, 5]. The NAs promote the cell growth, thereby inducing the tumors in vivo, especially in liver, lung, esophagus, bladder, and pancreas [6]. A number of NAs, which increase the cancer risk in humans, have been classified by International Agency for Research on Cancer [7]. In 1987, the IARC have thought over N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) are probably carcinogenic to human, and N-nitrosodibutylamine (NDBA), N-nitrosopiperidine (NPIP), N-nitrosopyrrolidine (NPYR), N-nitrosomorpholine (NMOR), N-nitrosodi-n-propylamine (NDPA), and N-nitrosodiphenylamine (NDPheA) are possibly a carcinogen for human [8]. Hence, monitoring programs have been carried out in many countries to track the levels of volatile NAs in food. For example, a maximum level of 10 µg kg−1 body weight (bw) per day for NPYR in retail food products has been set in the USA [9].
Nitrosamines are classified to volatile and nonvolatile. Volatile nitrosamine compounds that are nonpolar and have low molecular weight are separated via distillation from foods. In meat products (e.g., sausage and salami), the NAs are linked to proteins and other materials, which makes the extraction of NAs a difficult work. Furthermore, because of the low contents of NAs in meat products, several preconcentration steps are required in their analytical procedures [10]. Mostly, primary extraction is necessary for isolation of analytes from solid samples. Alkaline extraction with thermal treatment can be utilized, as an established technique, to release the analytes from solid samples. However, this approach is a time-consuming step. Microwave-assisted extraction (MAE) has been exploited for the extraction of compounds from complex matrices; this method shows clear advantages such as reducing the extraction time [11]. MAE is a process that employs microwave energy to heat polar solvents in the vicinity of a sample, in order to release the analytes from the sample matrix into the solvent. Vacuum mineral oil distillation procedure [12] and solid-phase extraction [13, 14] are general methods of NAs analysis in meat products. Combination of techniques, for instance, alkaline extraction and distillation followed by liquid–liquid extraction (LLE) [5], and two-step solid-phase extraction (SPE) with Extrelut and Florisil as sorbents [10] have been also reported for this aim. LLE consumes large amounts of toxic and expensive organic solvents and is a time-consuming method [15, 16]. Usually, SPE uses less solvent than LLE, but it needs specific columns and there is the risk of contamination, loss of analytes, and decrease of analytical precision.
In recent years, the efficient separation of analytes from matrices, using small amounts of solvents, has been the main purpose of every sample preparation method. Therefore, analytical researchers have been looking for approaches with less consumption of toxic organic solvents [17–19]. Consequently, microextraction techniques were considered. Solid-phase microextraction (SPME) [20], headspace solid-phase microextraction (HS-SPME) [21], microwave-assisted extraction with dispersive liquid–liquid microextraction (MAE–DLLME) [22], and dispersive microsolid-phase extraction (D-µ-SPE) [23] were developed to determine the NAs in meat products and other complex matrices [24–28].
Dispersive liquid–liquid microextraction (DLLME), introduced in 2006 by Assadi et al. was exploited for determination of organic and inorganic analytes [29] and other compounds, due to simplicity, low organic solvent consumption, low cost, and facility [30–32]. This technique was performed with various solvents, even with ionic liquids as extractor solvents [33, 34]. DLLME is based on a ternary component solvent system for the isolation and preconcentration of analytes from the aqueous matrices. Extraction and disperser solvents are rapidly injected into the aqueous sample to form a cloudy solution, and equilibrium is quickly reached. In spite of the benefits of DLLME technique, like rapidity and ease of use, its application to solid samples is a challenging work. According to our previous research and other reports, the combination of MAE and DLLME is an attractive and economical tool for extraction of analytes from complicated samples [35]. MAE–DLLME could be the best way to extract NAs from solid food, since it offers favorable repeatability, high recovery, and large enrichment factor within a shorter time, and also it is simple and inexpensive.
The objective of this study was to apply and optimize a new method based on microwave-assisted extraction coupled with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry (MAE–DLLME–GC–MS) for determination of nitrosamines in sausage and salami. The volumes of extraction and disperser solvents, pH, and salt amount were optimized using response surface methodology (RSM) based on a central composite design (CCD). The proposed method was successfully applied to the analysis of trace amounts of 7 NAs in sausage and salami samples.
Materials and methods
Reagents and standards
A standard mixture stock solution (2,000 µg mL−1) of NDMA, NDEA, NDBA, NMEA, NPIP, NDPheA, and NPYR was purchased from Supelco (Harrison Road/Bellefonte, USA). A standard solution (100 µg mL−1) of 7 NAs was prepared in methanol. Stock and working solutions were kept in refrigerator at 4 °C. Ethyl alcohol (99.6 %) was supplied by Bidestan Company (Qazvin, Iran), and chloroform, hydrochloric acid, methanol, potassium hydroxide, and sodium chloride were acquired from Merck (Darmstadt, Germany). Potassium ferrocyanide and zinc acetate were obtained from Panreac (Belzhike). To prepare carrez solution I, 10.6 g of ferrocyanide was dissolved in 100 mL with distilled water. Carrez solution II was prepared by mixing 21.9 g of zinc acetate with 3 mL of acetic acid, and then, the volume of the solution was adjusted to 100 mL via distilled water. Biphenyl (Merck) was employed as internal standard and prepared in methanol at a concentration of 100 µg mL−1.
Instrumentation
In order to determine the nitrosamines, a gas chromatography–mass spectrometer (GC–MS), equipped with a 7890A gas chromatography (GC) system from Agilent Technologies (Palo Alto, CA, USA), a split/splitless injection port, and a 5975C inert mass selective detector (MSD) network, was utilized. A HP-5MS capillary column (30 m × 0.25 mm, ID; 0.25 µg, film thickness) was to separate the compounds. The temperature program started at 70 °C for 15 min was raised to 76 °C with a ramp of 3 °C min−1, held for 1 min, increased to 280 at 30 °C min−1, and held for 8 min under the helium flow rate of 0.8 mL min−1 in the split mode (1:50 ratio). About 3 µL of the sample was injected into in the split mode to GC–MS. The auxiliary and injector temperatures were fixed at 280 °C. The compounds were quantified in the selected ion monitoring (SIM) mode, and one qualifier ion was chosen for each compound. A Centrifuge (HeltichRotorfix 32A) with the speed of 4,000 rpm was used to separate the mixture components. A microwave oven (Delonghi type MW 602) was applied to accelerating the alkaline hydrolysis and primary extraction.
Experimental design
The RSM was employed to determine the optimal values for experimental variables. The experimental response (Y) in RSM is assumed to be a function of independent variables. In this study, the most effective independent factors on the DLLME technique, including disperser solvent volume (A), extraction solvent volume (B), pH (C), and amount of NaCl (D), were chosen according to the literature and the preliminary experiments. The CCD (full, and with alpha = 2) was to reach values of these factors as well as the best response. The above parameters (A, B, C, and D) were tested at five levels. Table 1 illustrates the factors and their levels.
The response surface design is constructed from several superimposed designs and composed of a factorial design (2f), which is augmented with 2ƒ star points and central points, where f is the number of factors that should be optimized. The total experimental points required (N) are determined by means of the equation N = 2f + 2ƒ + n [36, 37]. Our design was consisted of six replicates of the point and included 30 randomly performed experiments. For each experiment, three replicate extractions and quantitative measurements were conducted. A quadratic polynomial model (Eq. 1) was obtained to predict the response of the dependent variable for extraction of the NAs from the samples:
wherein Y is the dependent variable, x i is the independent variable, b 0 is the constant coefficient, b i is the coefficient of linear effect, and b ij is the coefficient of interaction effect. To achieve the most desirable extraction efficiency, the sum of the peak area ratios of NDMA, NDEA, NPYR, NPIP, NDBA, and NDPheA to the internal standard was exploited as the GC response. The sum of the GC responses for all of the extracted compounds was considered as the total GC response, to evaluate the extraction efficiency under different conditions. The software package Design-Expert 8.0.5 (Minneapolis, USA) was used to analyze the data, and the suggested quadratic model was employed to find the most important influences and interactions.
Sample preparation
A dry cured 70 % sausage was purchased from a supermarket and ground in a blender. A total of 150 g of this sample was mixed with 100 mL water and 100 µL of the mixed working standard solution (100 µg mL−1). The mixture was stirred with a shaker and put in the refrigerator (4 °C) for 48 h to dry. After that, it was extracted in various conditions according to the experimental design. Under the optimized conditions, 1.5 g of the homogenized sample was moved into a glass container, 10 mL of a hydrolyzing solution, composed of 2 mol L−1 potassium hydroxide and methanol (80:20), was added to it, and the glass was closed tightly afterward. The sample was placed in a microwave at 500 MHz for 1.5 min to undergo hydrolysis and saponification. Then, it was cooled, transferred into the centrifuge tube, and centrifuged at 4,000 rpm for 10 min. After separating the two phases, the aqueous phase was transported to another vessel and its pH was adjusted to 7.5 by the addition of hydrochloric acid (10, 5, 1, and 0.1 N). At the end, 1 mL of carrez solution I and 1 mL of carrez solution II were added into the vessel, and the vessel was centrifuged at 4,000 rpm for 10 min to precipitate the proteins.
Following the preliminary extraction and centrifugation, the clear phase was separated, 1.1 g sodium chloride was added into the vessel, and the vessel was shaken completely. The DLLME solution, containing 110 µL of chloroform as the extracting solvent, 500 µL of ethanol as the disperser solvent, and 1 µL of biphenyl (100 µg mL−1) as the internal standard, was injected to the vessel. The mixture was stirred gently by means of a shaker and then centrifuged at 4,000 rpm for 10 min. The dispersed particles of the extractor phase were settled at the bottom of the vessel, and approximately 3 µL of the sediment was injected directly into the GC–MS through a microsyringe.
Results and discussion
The ability to heat the solvents and samples quickly is the intrinsic characteristic and the principal advantage of the MAE technique. Usually, the amount of solvent consumption in this method is 10 times less than conventional techniques, and a typical extraction procedure (using MAE) takes 15–30 min. To reach an optimal extraction process, choosing an appropriate solvent is essential. Special attention must be given to the microwave absorbing properties of a solvent, the interaction of the solvent with sample, and the solubility of analytes in the solvent. The solvent should have high selectivity toward the target analytes and hinder the release of unwanted matrix elements. Generally, alkaline digestion is for the hydrolysis of food matrices [5]. For MAE process, we applied various combinations of KOH (2 mol L−1) and MeOH. The most favorable result was obtained when the ratio of KOH to MeOH in the hydrolyzing solution was 80:20. After early digestion, carrez solutions were added. The carrez I and II solutions have been exploited as effective agents to precipitate proteins in complex matrices [38].
Optimization of DLLME process
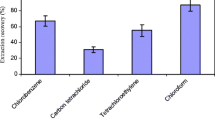
In DLLME method, some factors must be considered for the selection of extracting solvent. The most fundamental factors are high/low density (typically compared to water), low polarity, good chromatographic behavior, high extraction capability, high purity, and large partition coefficient for NAs [22, 39]. For this purpose, three organic solvents, comprising ethylene tetrachloride, carbon tetrachloride, and chloroform, were examined to choose the best solvent for the extraction of the NAs from the samples. When carbon tetrachloride was applied, some peaks (e.g., the peaks of NDMA and NMEA) were not detectable. On the other hand, when chloroform was employed as the extracting solvent, the most desirable results were attained. These results were presumably related to the higher polarity of chloroform compared with other solvents [40]. Thus, chloroform was selected as extracting solvent. Moreover, acetonitrile, ethanol, and acetone were explored to choose the disperser solvent, with chloroform as the extracting solvent. Ethanol afforded the most favorable results for all compounds.
The CCD was exploited to optimize the influential parameters in DLLME procedure, including volume of disperser solvent (A), volume of extraction solvent (B), pH (C), and amount of NaCl (D). The equation of RSM model is represented below, in terms of the coded factors:
where Y is the sum of the significant peak areas for all the nitrosamine compounds (total relative peak area). The CCD optimization technique could explain the interactions, as well as the quadratic effects of variables and linear effects of factors on the response. To estimate the interactions and the significance of each factor, the analysis of variance (ANOVA) was utilized (Table 2). The insignificant lack of fit relative to the pure error for all the investigated variables demonstrated that the quadratic polynomial model was admissible for accurate prediction of the valid response. The quality of the equation, which has been written as model, was confirmed by the cooperative of determination (R 2, adjusted R 2, and predicted R 2). The R 2 of 0.98, adjusted R 2 of 0.96, and predicted R 2 of 0.91 represented a good connection between the trial data and the fitted model and proved that the model could response. With regard to the ANOVA summary, it could be realized that the model was meaningful with a p < 0.0001 and an F value of 50.97. The disperser solvent (A), extracting solvent (B), pH (C), and amount of NaCl (D) possessed relevant influences on the model (p < 0.05). Significant interactions occurred between the extracting solvent and NaCl, disperser solvent and pH, and between pH and NaCl. The other interactions (AB, AD, and BC) were not important (p > 0.05).
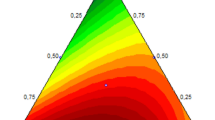
Figure 1 shows the predicted values versus the actual responses. Most of the points were dispersed monotonously around the line, which correlated between the predicted and the actual responses, and, in turn, the adequate fitness of the proposed quadratic model. As observed in Fig. 2, the residual plot is scattered randomly, demonstrating that the variance of the experimental measurements is constant for all the values of Y. The optimum amount of each parameter was subsequently figured out to gain the maximum response. To this aim, three graphs were used (Fig. 3).
Figure 3a depicts the response surface obtained by plotting the pH versus the volume of disperser solvent. The extraction efficiency improved with the increase of pH and the decrease of disperser solvent volume. The maximum response was achieved at pH 7.5 with 500 µL of the disperser solvent. Using high volumes of ethanol (700–1,000 µL) probably enhanced the solubility of the NAs in the aqueous solution, leading to the reduction of the extraction efficiency. Once the pH amount was decreased from 13.8 to 7.5, while the volume of the disperser solvent was constant at 500 µL, the extraction efficiency improved. Diminishing the pH to 7.5 presumably caused the nitrosamines to acquire a nonionic structure. Thus, mass transfer of the NAs into the extracting solvent could be done more easily. Nevertheless, the efficiency of extraction procedure slightly declined, when the pH value was decreased to less than 7.5. It might be due to the fact that the NAs found an ionic structure. The best result was obtained in the neutral pH. Figure 3b illustrates the interaction between the extracting solvent and salt. To enhance the ionic strength of the samples, sodium chloride (0–1.5 g) was added to the aqueous phase before blending the DLLME solution. By raising the amount of salt up to 1.1 g, the peak areas increased for all compounds. Diminishing the solubility of NAs was possibly owing to the salting-out effect. This phenomenon often has a positive impact on the extraction efficiency [22, 41]. The most favorable efficiency was gained with 110 µL of the extractor solvent, and at larger volumes, the efficiency of extraction almost remained constant. Another significant interaction was the interaction between pH and salt amount, which is presented in Fig. 3c. The influences of these variables have been described previously in this section. While the pH was lowered with increasing of the salt amount (a rise in the ionic strength), the extraction recovery did not change appreciably. This result verified that the structures of the NAs at pH less than 7.5 were unsuitable for their extraction via the organic solvent.
Quantitative analysis
Under the optimum conditions, the analytical performance of the MAE–DLLME–GC–MS technique was scrutinized. Table 3 shows the linearity, repeatability, R 2 value, enrichment factor (EF), recovery, limit of detection (LOD), and limit of quantification (LOQ); LOD and LOQ are defined as the lowest concentrations of the analyte in the sample that provide chromatographic signals three and ten times greater than the background noise, respectively. The figure of merit was compared with some of the other similar methods [21–23, 42–44]. For the nitrosamine compounds, the standard solution calibration curves were linear within the range of 0.1–200 ng mL−1. The R 2 value was more than 0.98. To estimate the repeatability, the comparative peak area from seven calibration levels with three replicates extractions from the sample was calculated and employed, and its value was displayed as relative standard deviation (RSD %). For all the NAs (n = 7), the RSD % was smaller than 5.4 %. The ratio of the definitive concentration in the essential solution was imparted as EF. The EF of the suggested technique was greater than 126. The recovery of each compound in the DLLME process was assigned by comparing the volumes of analytes, added to the meat product, with the concentrations found after the process. The recovery values were in the range 83.9–109.4 %. The LODs for the NAs were within the range of 0.11–0.48 ng g−1 in the real samples, using the optimized conditions and the GC–MS in the SIM mode, and the LOQs were in the range 0.41–1.45 ng g−1.
Application of the method to real samples
To evaluate the suitability of the proposed technique for detecting the NAs in the real samples, seven samples of heated meat products, comprising sausage and salami, were analyzed under the optimal conditions. The samples were purchased from a local supermarket (Tehran, Iran) and stored at 4 °C. The concentrations of some NAs in these samples were very low, and their analytical results were verified with the aid of the standard addition method (Table 4). These results proved that the MAE–DLLME followed by GC–MS (in the SIM mode) is a powerful technique for monitoring the NAs at very low concentrations in samples. Figure 4 depicts the chromatograms of the nonspiked (a) and spiked (b) (at 40 ng g−1 with seven NAs) sausage sample obtained by means of MAE–DLLME–GC–MS in the SIM mode. Good chromatograms were achieved, which were free of sample matrix interference.
Chromatogram obtained by MAE–DLLME–GC–MS for a sausage sample under optimum conditions, a nonspiked and b spiked with 40 ng g−1 of nitrosamines. a N-nitrosodimethylamine (NDMA) b Nnitrosomethylethylamine (NMEA), c N-nitrosodiethylamine (NDEA), d N-nitrosopyrrolidine (NPYR), e Nnitrosopiperidine (NPIP), f N-nitrosodibutylamine (NDBA), biphenyl (IS), g N-nitrosodiphenylamine (NDPheA)
Conclusion
In this research, the feasibility of the use of MAE–DLLME–GC–MS as a simple, fast, and reliable methodology to the analysis of nitrosamines in sausage and salami was examined. The factors affecting the MAE and DLLME efficiencies were explored in detail, and the optimal conditions were established. The microwave irradiation was beneficial to the transportation of the analytes from the solid sample matrix into the aqueous solution, as well as the acceleration of the extraction procedure. The results also confirmed that the application of carrez solution to sediment the proteins could substantially reduce the interference of the real sample matrix. The volumes of extracting and disperser solvent, pH, and salt amount possessed considerable influences on the DLLME procedure. Low solvent consumption, simple experimental setup, large EF, and excellent precision were the obvious advantages of this method. Besides, the method could offer an adequate repeatability and a favorable recovery within a short analysis time. This method was easier and faster relative to the other traditional extraction methods. The suggested method can be successfully exploited for the routine monitoring of nitrosamines in various types of real sausage and salami from different producers.
References
Masuda M, Mower HF, Pignatelli B, Celan I, Friesen MD, Nishino H, Ohshima H (2000) Formation of N-nitrosamines and N-nitramines by the reaction of secondary amines with peroxynitrite and other reactive nitrogen species: comparison with nitrotyrosine formation. Chem Res Toxicol 13:301–308
Honikel K-O (2008) The use and control of nitrate and nitrite for the processing of meat products. Meat Sci 78:68–76
Noel P, Briand E, Dumont J (1990) Role of nitrite in flavour development in uncooked cured meat products: sensory assessment. Meat Sci 28:1–8
Thomas C, Mercier F, Tournayre P, Martin J-L, Berdagué J-L (2013) Effect of nitrite on the odourant volatile fraction of cooked ham. Food Chem 139:432–438
Drabik-Markiewicz G, Dejaegher B, De Mey E, Impens S, Kowalska T, Paelinck H, Vander Heyden Y (2010) Evaluation of the influence of proline, hydroxyproline or pyrrolidine in the presence of sodium nitrite on N-nitrosamine formation when heating cured meat. Anal Chim Acta 657:123–130
Radomski J, Greenwald D, Hearn W, Block N, Woods F (1978) Nitrosamine formation in bladder infections and its role in the etiology of bladder cancer. J Urol 120:48–50
IARC (1978) Comprehensive analytical procedures for the determination of volatile and non-volatile, polar and non-polar N-nitroso compounds. IARC scientific publications 3
IARC (1987) The relevance of N-nitroso compounds to human cancer: exposures and mechanisms, IARC, Lyon, pp 503–506
USDA (2004) US code of federal regulations, food safety and inspection service, USDA 2004, Certain other permitted uses, 9 CFR Ch. III (1-1-03 ed)
Yurchenko S, Mölder U (2007) The occurrence of volatile N-nitrosamines in Estonian meat products. Food Chem 100:1713–1721
Sun L, Lee HK (2002) Microwave-assisted extraction behavior of non-polar and polar pollutants in soil with analysis by high-performance liquid chromatography. J Sep Sci 25:67–76
Association of Official Analytical Chemists, N-Nitrosamines (Volatile) in Fried Bacon. Mineral Oil Vacuum Distillation-Thermal Energy Analyzer Method. 1990. No. 982.22. 15th edition
Pensabene JW, Fiddler W, Gates RA (1995) Nitrosamine formation and penetration in hams processed in elastic rubber nettings: N-nitrosodibutylamine and N-nitrosodibenzylamine. J Agric Food Chem 43:1919–1922
Raoul S, Gremaud E, Biaudet H, Turesky RJ (1997) Rapid solid-phase extraction method for the detection of volatile nitrosamines in food. J Agric Food Chem 45:4706–4713
Fine DH, Rounbehler DP, Huffman F, Garrison AW, Wolfe NL, Epstein SS (1975) Analysis of volatile N-nitroso compounds in drinking water at the part per trillion level. Bull Environ Contam Toxicol 14:404–408
Goff E, Fine D (1979) Analysis of volatile N-nitrosamines in alcoholic beverages. Food Cosmet Toxicol 17:569–573
Chan ST, Yao MW, Wong Y, Wong T, Mok C, Sin DW (2006) Evaluation of chemical indicators for monitoring freshness of food and determination of volatile amines in fish by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur Food Res Technol 224:67–74
Prat C, Bañeras L, Anticó E (2008) Screening of musty-earthy compounds from tainted cork using water-based soaks followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur Food Res Technol 227:1085–1090
Wang Y-L, Zeng Z-R, Liu M-M, Yang M, Dong C-Z (2008) Determination of organophosphorus pesticides in pakchoi samples by headspace solid-phase microextraction coupled with gas chromatography using home-made fiber. Eur Food Res Technol 226:1091–1098
Forsyth D, Dusseault L (1997) Determination of methylcyclopentadienyl manganese tricarbonyl in beverages by solid-phase microextraction. Food Addit Contam 14:301–307
Andrade R, Reyes FG, Rath S (2005) A method for the determination of volatile N-nitrosamines in food by HS-SPME-GC-TEA. Food Chem 91:173–179
Campillo N, Viñas P, Martínez-Castillo N, Hernández-Córdoba M (2011) Determination of volatile nitrosamines in meat products by microwave-assisted extraction and dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. J Chromatogr A 1218:1815–1821
Huang M-C, Chen H-C, Fu S-C, Ding W-H (2013) Determination of volatile N-nitrosamines in meat products by microwave-assisted extraction coupled with dispersive micro solid-phase extraction and gas chromatography-Chemical ionisation mass spectrometry. Food Chem 138:227–233
Geng Y, Zhang M, Yuan W, Xiang B (2011) Modified dispersive liquid–liquid microextraction followed by high-performance liquid chromatography for the determination of clenbuterol in swine urine. Food Addit Contam 28:1006–1012
Hu R (1999) Solid phase microextraction of pesticide residues from strawberries. Food Addit Contam 16:111–117
Kamankesh M, Mohammadi A, Modarres Tehrani Z, Ferdowsi R, Hosseini H (2013) Dispersive liquid-liquid microextraction followed by high-performance liquid chromatography for determination of benzoate and sorbate in yogurt drinks and method optimization by central composite design. Talanta 109:46–51
Mohammadi A, Tavakoli R, Kamankesh M, Rashedi H, Attaran A, Delavar M (2013) Enzyme-assisted extraction and ionic liquid-based dispersive liquid–liquid microextraction followed by high-performance liquid chromatography for determination of patulin in apple juice and method optimization using central composite design. Anal Chim Acta 804:104–110
Víctor-Ortega MD, Lara FJ, García-Campaña AM, Olmo-Iruela Md (2012) Evaluation of dispersive liquid-liquid microextraction for the determination of patulin in apple juices using micellar electrokinetic capillary chromatography. Food Control 31:353–358
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatog A 1116:1–9
Pirsaheb M, Fattahi N, Shamsipur M (2013) Determination of organophosphorous pesticides in summer crops using ultrasound-assisted solvent extraction followed by dispersive liquid-liquid microextraction based on the solidification of floating organic drop. Food Control 34:378–385
Rezaee M, Yamini Y, Faraji M (2010) Evolution of dispersive liquid-liquid microextraction method. J Chromatog A 1217:2342–2357
Kujawski MW, Pinteaux E, Namieśnik J (2012) Application of dispersive liquid–liquid microextraction for the determination of selected organochlorine pesticides in honey by gas chromatography-mass spectrometry. Eur Food Res Technol 234:223–230
Han D, Tang B, Ri Lee Y, Ho Row K (2012) Application of ionic liquid in liquid phase microextraction technology. J Sep Sci 35:2949–2961
Ho Y-M, Tsoi Y-K, Leung KS-Y (2013) Ionic-liquid-based dispersive liquid-liquid microextraction for high-throughput multiple food contaminant screening. J Sep Sci 00:1–8
Ghasemzadeh-Mohammadi V, Mohammadi A, Hashemi M, Khaksar R, Haratian P (2012) Microwave-assisted extraction and dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry for isolation and determination of polycyclic aromatic hydrocarbons in smoked fish. J Chromatogr A 1237:30–36
Agudelo Mesa LB, Padró JM, Reta M (2013) Analysis of non-polar heterocyclic aromatic amines in beef burgers by using microwave-assisted extraction and dispersive liquid-ionic liquid microextraction. Food Chem 141:1694–1701
Ceylan H, Kubilay S, Aktas N, Sahiner N (2008) An approach for prediction of optimum reaction conditions for laccase-catalyzed bio-transformation of 1-naphthol by response surface methodology (RSM). Biores Technol 99:2025–2031
Drabik-Markiewicz G, Dejaegher B, De Mey E, Kowalska T, Paelinck H, Vander Heyden Y (2011) Influence of putrescine, cadaverine, spermidine or spermine on the formation of nitrosamine in heated cured pork meat. Food Chem 126:1539–1545
Delatour T, Périsset A, Goldmann T, Riediker S, Stadler RH (2004) Improved sample preparation to determine acrylamide in difficult matrixes such as chocolate powder, cocoa, and coffee by liquid chromatography tandem mass spectroscopy. J Agric Food Chem 52:4625–4631
Ripollés C, Pitarch E, Sancho JV, López FJ, Hernández F (2011) Determination of eight nitrosamines in water at the ng L−1 levels by liquid chromatography coupled to atmospheric pressure chemical ionization tandem mass spectrometry. Anal Chim Acta 702:62–71
Glória MBA, Barbour JF, Scanlan RA (1997) Volatile nitrosamines in fried bacon. J Agric Food Chem 45:1816–1818
Ozel MZ, Gogus F, Yagci S, Hamilton JF, Lewis AC (2010) Determination of volatile nitrosamines in various meat products using comprehensive gas chromatography-nitrogen chemiluminescence detection. Food Chem Toxicol 48:3268–3273
Sanches Filho PJ, Rios A, Valcárcel M, Zanin KD, Bastos Caramão E (2003) Determination of nitrosamines in preserved sausages by solid-phase extraction-micellar electrokinetic chromatography. J Chromatogr A 985:503–512
Yurchenko S, Mölder U (2006) Volatile Nitrosamines in various fish products. Food Chem 96:325–333
Acknowledgments
This study has been supported by the National Nutrition & Food Technology Research Institute of Iran. We gratefully acknowledge their assistance.
Conflict of interest
Houra Ramezani, Hedayat Hosseini, Marzieh Kamankesh and Vahid Ghasemzadeh-Mohammadi declares that they have no conflict of interest. Abdorreza Mohammadi has received research grants from National Nutrition and Food Technology Research Institute.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramezani, H., Hosseini, H., Kamankesh, M. et al. Rapid determination of nitrosamines in sausage and salami using microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Eur Food Res Technol 240, 441–450 (2015). https://doi.org/10.1007/s00217-014-2343-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2343-4