Abstract
This work evaluated the efficiency of an ultrasound-assisted extraction method using diluted acids to determine micronutrients and toxic elements in samples of craft chocolates with different cocoa content. Ca, Fe, K, Mg, Na, P, and Zn were determined through inductively coupled plasma optical emission spectroscopy (ICP-OES) and As, Cd, and Pb through inductively coupled plasma mass spectrometry (ICP-MS). For method optimization, the Mixture Plan type simplex-centroid was used, investigating HNO3 (nitric acid), HCl (hydrochloric acid), and CH2O2 (formic acid) in the evaluation of the best extracting solution. The optimal condition indicated the usage of 10 mL of HNO3 (2.0 mol L−1) for extracting analytes from a mass of 200 mg at 50 ± 5 °C for 10 min of sonication. From the optimized method, limits of quantification vary from 0.030 to 15 mg kg−1 for As and P, respectively, and precision is expressed as relative standard deviation (%RSD) below 10%. Aiming to evaluate the influence of cocoa content and the chocolate production region, the method analyzed 17 samples of craft chocolate from two different suppliers. The results were assessed by principal component analysis and demonstrated a possible influence of cocoa planting location in the presence of micronutrients and product concentrations. The concentration range of analytes found in the samples was compared with the daily recommended ingestion (DRI) values, concluding that this follows the maximum recommended values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chocolate has had massive importance since the first records of civilizations in which it was present, and it is currently one of the most commercialized foods in the world. Its production is directly connected to cocoa cultivation, the fruit of the tree Theobroma cacao. In chocolate production, cocoa is selected, dried and roasted, peeled, and ground until it becomes a liquor, mixed with the other ingredients, such as powdered milk and sugar (Afoakwa et al. 2007). The final product is a semisolid suspension composed of solid particles of cocoa and sugar inserted into a continuous fat phase (Hartwig et al. 2016; Peixoto et al. 2012). However, variations in the quantities of raw materials will generate different types of chocolate, such as white, milk, and dark chocolate.
Nowadays, a specific type of chocolate has gained space in the world market—craft chocolate. Frequently, it is possible to face specialized establishments and gastronomic events exclusively dedicated to this variety of chocolate. Craft chocolate production is distinguished by adopting a characteristic and manual process compared to the large-scale production by the big industries. In this approach, craft chocolate manufacturers dedicate themselves to creating unique chocolates of high standard quality (Ferreira et al. 2014; Hartwig et al. 2016).
In chocolate, micronutrients from cocoa seeds are generally present, such as Ca, Fe, K, Mg, P, and Zn. The seed obtains these nutrients from the soil where the cacao is cultivated and plays significant roles during the plant’s life cycle. Besides these elements, some trace potentially toxic elements, such as As, Cd, and Pb, can be found in the product, originating from the soil and possibly from the production process. The presence of these can provoke damage to human organisms, such as anemia, abdominal pain, and hypertension, for example (Kruszewski et al. 2018; Vanderschueren et al. 2019). In this sense, some regulations indicate the maximum amount of these potentially toxic elements in chocolate. Among them, there is the European (EU) regulation N° 488/2014 that establishes the maximum content of Cd in different types of chocolate, being 0.10 mg kg−1 for chocolates with cocoa content less or equal to 30%, 0.30 mg kg−1 for chocolates with cocoa content superior or equal to 30% and less than 50%, and 0.80 mg kg−1 for chocolates with cocoa content higher or equal to 50%. As the European regulation, the Brazilian Resolution N° 42 from August 2013, established by the Brazilian Health Regulatory Agency (ANVISA), also describes the maximum amount of Cd, As, and Pb in chocolates: in chocolates with cocoa content less than 40%, the maximum limit is 0.20 mg kg−1, and for chocolates with cocoa content higher than 40%, it is 0.40 mg kg−1. Thus, determining chemical elements is essential to ensure chocolate quality and verify the adequate amount of micronutrients, such as the potentially toxic ones.
Among the most used techniques to determine the elementary composition of chocolate, inductively coupled plasma optical emission spectroscopy (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) stand out because they present adequate sensibility and multi-element character (Abt et al. 2018; Barreto et al. 2019; Costa et al. 2019; Giraldo et al. 2022; Hartwig et al. 2016; Karas et al. 2021; Manousi and Zachariadis 2021; Peixoto et al. 2012; Salama 2018; Vanderschueren et al. 2019). These techniques usually require the introduction of samples as an aerosol; therefore, the sample has to be in solution, and steps of sample preparation must be done.
An adequate method choice for sample preparation will help the efficacy of transportation from the aerosol to the plasma, reducing interferences. This preparation can be done through sample decomposition by wet via, which uses acids or alkaline reagents associated with heat (Ferreira et al. 2014).
Regarding chocolate, the usual method for sample preparation for multi-element determination is acid digestion in a closed system, assisted by microwave radiation, which could use highly concentrated acids and acid mixtures (Chekri et al. 2017; Hartwig et al. 2016; Junior et al. 2018; Lo Dicoa et al. 2017; Mrmolanin et al. 2018; Pedro et al. 2006; Peixoto et al. 2012). Although the use of a closed system causes the reduction of problems involved in contamination and losses of analytes, this method demands high amounts of acids that could cause interference and generate harmful waste. An alternative for sample preparation of chocolate is the use of ultrasound-assisted extraction, as reported by Jalbani et al. (2007), Ferreira et al. (2014), and Silva et al. (2021).
Despite the advantages of using ultrasound-assisted extraction, published papers that used the extractive technique for chocolate samples still have limitations, as the use of concentrated acids, long periods of sonication, and high temperatures of the ultrasound bath (Ferreira et al. 2014; Jalbani et al. 2007; Silva et al. 2021) what difficulties the application and repeatability of methods. Besides, a few analytes were studied, and applying for multi-analyte analysis was challenging.
Thereby, interest arises in developing and optimizing a fast method for sample preparation through ultrasound-assisted extraction, using a minimal amount of diluted reagents that efficiently extract chemical elements in chocolate samples with different amounts of cocoa. The amount of cocoa causes a direct impact on the sample matrix, which is needed to evaluate the behavior of the experimental system to reach the optimal condition to ensure efficient extraction of analytes, independently of cocoa content in the samples.
Herein was done a multivariate optimization using the Mixture Plan type simplex-centroid for preparing samples of craft chocolates through ultrasound-assisted extraction to find an excellent method to determine As, Ca, Cd, Fe, K, Mg, Mn, Na, P, Pb, and Zn in samples with different contents of cocoa.
Materials and Methods
i. Instrumentation
Micronutrient elements were determined on an ICP-OES (700 DV—Perkin Elmer, USA), while the trace elements were determined using ICP-MS (NexION 300D—Perkin Elmer, USA). Ar was used for plasma maintenance, sample aspiration, and auxiliary gas (99.999% pure—White Martins, Brazil). Operation conditions were as follows: auxiliary gas flow, 0.8 L min−1 (ICP-OES) and 1.2 mL min−1 (ICP-MS); plasma gas flow, 16.0 L min−1 (ICP-OES and ICP-MS); nebulizer gas flow, 0.8 L min−1 (ICP-OES) and 1.1 mL min−1 (ICP-MS); and nebulizer, Concentric Meinhard Type C (ICP OES) and Seaspray (ICP-MS). The ICP-OES was used in the determination of the following elements, with their respective wavelengths and plasma vision: Ca 317.933 nm (axial), Fe 238.204 nm (axial), K 766.490 nm (axial), Mg 285.213 nm (axial), Mn 257.610 nm (axial), Na 588.995 nm (radial), P 213.617 nm (axial), and Zn 206.200 nm (axial). In the ICP-MS, the measured isotopes were 75As, 114Cd, and 208Pb. A quartz torch was employed on both equipment, and the analysis was done in triplicate. An ultrasound bath (S40H—Elma, Germany) with a heating system and a centrifuge (Q222TM—QUIMIS, Brazil) was used for sample extraction.
ii. Reagents and Solutions
For sample preparation, HNO3 65% (m m−1) (Sigma-Aldrich, USA) and HCl 37% (m m−1) (Sigma-Aldrich, USA), previously purified by sub-boiling distillation Distillacid (BSB-939—Berghof, Germany), CH2O2 (Merck, Germany) and ultrapure water with resistivity of 18.2 MΩ cm−1 (PURELAB Ultra—ELGA, UK) were used. Analytical solutions were prepared with a multi-element standard of 1000 mg mL−1 (S171110020, SPC Science, Canada) for the analytes Ca, Fe, K, Mg, Mn, Na, P, and Zn. For As, Cd, and Pb, solutions were prepared using a 1000 µg mL−1 monoelemental standard (Absolut Standard INC, USA). All analytical solutions were diluted in 2% (v v−1) HNO3. Furthermore, all bottles were previously decontaminated in 15% (v v−1) HNO3 acid solution.
iii. Samples
Craft chocolate samples of the same brand but with different amounts of cocoa (46, 65, 70, and 85%) were used for method optimization.
For sample characterization by the established method, 17 chocolate bars from two different brands were used: A and B. Samples from brand A are artisanal chocolate produced in Linhares, Espírito Santo state, located in the Southeast region of Brazil. On the other hand, samples from brand B are artisanal chocolate produced in the Gramado, Rio Grande do Sul state, located in the South region of Brazil.
Both brand samples were divided into six classes according to the amount of cocoa: class 1, 35–40% cocoa (1a, 1b, 1c, and 1d); class 2, 45–65% cocoa (2a, 2b, and 2c); class 3, 70–80% cocoa (3a, 3b, 3c, and 3d); class 4, 80–90% cocoa (4a and 4b); class 5, 100% cocoa; and class 6, cocoa content not informed by the supplier (6a, 6b, and 6c) (Table 1). All samples were left in the freezer for 24 h before pre-processing and passed through a plastic grater. Crushed samples were stored in plastic bags in a refrigerator until sample preparation.
iv. Multivariate Optimization of the Extraction Procedure
Herein were used three acids: HNO3, HCl, and CH2O2. The last was used to evaluate the acids’ influence in analyte extraction for element determination. The multivariate optimization step of the extraction procedure by ultrasound with diluted acid was done employing the Mixture Plan type simplex-centroid. For so, four samples with different cocoa contents (46, 65, 70, and 85%) were used, resulting in four distinct plans, one for each cocoa.
For optimization, the following variables were fixed: temperature 50 ± 5 °C, time of sonication of 10 min, sample mass 0.2 g, solution volume of 10 mL, and extractive solution concentration of 2 mol L−1. The choice of the values of fixed variables and the extractive solution composition were made based on the literature (Ferreira et al. 2014; Jalbani et al. 2007; Junior et al. 2018; Lo Dicoa et al. 2017; Manousi & Zachariadis 2021; Vanderschueren et al. 2019). Nine experiments were done in triplicate for each sample, including the central point (Table 2). The controls were prepared without adding a sample, and the same conditions were used for each experiment. After sonication with different acid conditions, the raw data from ICP-OES and ICP-MS analysis were treated using multiple responses (MR) (Derringer and Suich 1980) to obtain mathematical models evaluated by ANOVA through regression significance and lack of fit.
All statistical parameters and math models were generated and evaluated by applying the software Statistica© 8.0 (StatSoft, Inc., Tulsa, USA). Routine calculations were done using the software Microsoft® Excel® 2016 (Microsoft, Inc., Redmond, USA).
v. Extraction Procedure
The optimal Plan condition resulted in adding 10.0 mL of HNO3 (2.0 mol L−1) in polypropylene tubes with around 200 mg of sample. The tubes were put on an ultrasound bath in the region of most intense cavitation, previously mapped according to the literature (Mason and Lorimer 2002). The sonication was done for 10 min at 50 ± 5 °C. The tubes were centrifuged for 10 min at 3800 rpm, and an aliquot of the supernatant (5 mL) was removed for posterior dilution to 25 mL. The solutions were stored under refrigeration at less than 5 °C until analysis. Before ICP-OES and ICP-MS analysis, the solutions were diluted 5 and 3 times, respectively.
vi. Multivariate and Nutritional Analysis
The optimized method was applied to 17 samples of craft chocolate from different brands and different cocoa contents bought from the local market. The results were evaluated using the Principal Component Analysis (PCA) software Statistica© 8.0 (StatSoft, Inc., Tulsa, USA). The nutritional evaluation of samples was realized based on Dietary Reference Intakes (DRIs) (Trumbo et al. 2001) that inform the recommended dietary amount related to the consumption of determined analytes.
Results and Discussion
i. Optimization of Procedure
Considering the study variables, each mixture plan was done nine experiments, including the triplicate on the central point (CP). To optimize the simultaneous extraction of higher amounts of elements, the results obtained at the four planned experiments were treated using multiple answers, according to Eq. 1. In this, the MRn represents the analytical signal for an element of a determined experiment; the MRmaxn is the maximum analytical signal in the set of experiments for that element n, being MR the sum of values obtained for each analyte (Derringer and Suich 1980).
The obtained results with the application of MR for each sample (cocoa content) are described in Table 2. A new MR was calculated from the MRs for each cocoa content to find the unique optimal condition for extracting all craft chocolates with different cocoa content. The values of global MR were used to calculate the mathematical models as being the vector y of the matrix of the experimental data plan.
From the data, linear and quadratic mathematical models were tested by variance analysis (ANOVA). Both showed a non-significative regression (QMreg/QMres < Ftabled). However, for the quadratic model, the Fregression value is near the tabulated value, and considering the number of analyzed variables in the mixture plan, the regression was enough to adequate the model. This can be confirmed by evaluating the non-significative lack of adjustment (QMfaj/QMep < Ftab), being considered satisfactorily, as an adequate determination coefficient (R2) that describes satisfactorily 84.75% of the explained variance. Thus, the equation represents the data set obtained using the quadratic model, with a confidence interval of 95%. From this, a contour plot was prepared (Fig. 1). It is possible to observe the behavior of MRs in different extracting solutions, where the red region represents the mixture’s proportionality for better results.
It is worth noting that the red area represents primarily a mixture of around 0.50 HNO3 and 0.40 HCl, in which HNO3 is variable with more significance, as expected, according to the general MRs shown in Table 1. This mixture has an intense oxidant action, and the reaction product will depend on the proportion of acids. In mixtures such as HNO3:HCl 1:3 (v v−1), it will form molecular chlorine and nitrosyl chloride (NOCl), which are highly oxidant and reactive. When HNO3 is in higher proportion, HNO3:HCl 3:1 (v v−1), chloride will act as an auxiliary to the oxidant action of nitric acid, acting as a ligand, stabilizing the analytes (Ferreira 2019).
Using an extractive solution of HNO3 2.0 mol L−1 (experiment 1) revealed advantages compared to the other solutions. This finding is supported by the results obtained by the mathematical model, which points to experiment 1 as the optimal condition due to the lower loss of information. Experiment 1 is the solution of HNO3 2.0 mol L−1, one of the most used extractive solutions for sample preparation of chocolate together with the mixture of HNO3 and HCl, done in experiment 4. According to the literature, both solutions stand out in relation to RMs; however, experiment 1 shows the advantage of not having HCl in the solution, which decreases the cost of sample preparation and contributes to green chemistry, reducing harmful products, besides avoiding interferences on determining As through ICP-MS (Abt et al. 2018; Chekri et al. 2017; Costa et al. 2019; Freitas et al. 2015; Jalbani et al. 2007; Lo Dicoa et al. 2017; Peixoto et al 2012; Salama 2018; Silva et al. 2021; Vanderschueren et al. 2019).
It can be observed in the contour plot that all regions corresponding to the variable CH2O2 did not significantly influence the results. Nevertheless, it was observed that in chocolates with cocoa content of 70 and 85%, the mean answer values (MRs) obtained on the central point, where there is the ternary mixture of HNO3, HCl, and CH2O2, were higher or close to the values obtained in experiments 1 and 4. Considering that these samples have higher amounts of carbohydrates than samples with 46 and 65% cocoa, formic acid may have contributed to the analysis efficiency of these samples, decreasing the control signal.
ii. Figures of Merit
Linearity, Limit of Detection (LD), and Limit of Quantification (LQ)
To validate the optimized method, the following figures of merit were verified: linearity, limits of detection and quantification, precision, and accuracy. The method linearity was determined based on the coefficient of determination of the analytical curve (R2) obtained from the external calibration in a solution of 2% HNO3 (v v−1). The analytical curves for each analyte were used to calculate the limit of detection (LD) and limit of quantification (LQ), calculated in relation to the background (BEC) (Thompsen 2012) for elements determined through ICP-OES and according to IUPAC (International Union of Pure and Applied Chemistry 1995) for elements determined through ICP-MS. The results obtained are shown in Table 3.
In the ultrasound extraction of chocolate samples done by Ferreira et al. (2014), the limits of quantification reached were 6 mg kg−1 for Fe and 400 mg kg−1 for Na and K, while Silva and coworkers (2021) reached limits of quantification of 0.294 mg kg−1 for Cd and 0.059 mg kg−1 for Pb. By comparing the LQ values from the method proposed here, it is noted that they are significantly close to the ones presented in the literature and below the maximum limits allowed by the European legislation N° 488/2014 for Cd, being this of 0.80 mg kg−1. The same was observed in Brazilian legislation N° 42 (ANVISA), which establishes maximum limits of 0.040 mg kg−1 for As, Cd, and Pb, demonstrating that the method can distinguish low concentrations of back signal caused by the sample’s matrix.
Precision
Seven independent samples were analyzed on the same day to obtain the precision of the developed method, obtaining the mean and standard deviation from the repetitions. The values for the coefficient of variation (COV) were calculated and compared with the maximum values of COV established by the Association of Official Analytical Chemists (AOAC) (2016) according to the concentration obtained for the studied analytes.
In a precision method, the individual measures should be close to the mean value, which indicates a reduction of random imprecisions. The calculated concentrations of the precision study were as follows: As, 0.20 ± 0.02 mg kg−1; Ca, 849.57 ± 29.21 mg kg−1; Cd, 0.98 ± 0.06 mg kg−1; Fe, 148.99 ± 7.69 mg kg−1; K, 9776.45 ± 307.29 mg kg−1; Mg, 2558.24 ± 102.93 mg kg−1; Mn, 132.18 ± 3.78 mg kg−1; Na, 238.27 ± 31.81 mg kg−1; P, 4574.81 ± 164.94 mg kg−1; and Pb and Zn, < LQ. The calculated values of %RSD (Table 3) were smaller than the maximum values allowed by AOAC (2016) as determined by the concentration in mg kg−1 of the sample analyte, being these 5.3% for Ca, Fe, Na, and Mn; 3.7% for K, Mg, and P; and 15% for As and Cd. These results demonstrate that this method is precise because the dispersion of the values is reduced, assuring the reliability and consistency of the analytical measures.
Accuracy
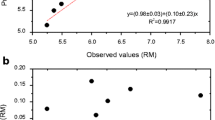
The method’s accuracy was evaluated by comparing the results obtained by the proposed method and two methods from the literature (Costa et al. 2019; Kruszewski et al. 2018) in analyzing one sample of craft chocolate with 70% cocoa. In both methods, the authors used acid digestion assisted by microwave. In Costa et al. (2019) method, the reagent used was diluted HNO3 (2.5 mol L−1), while the method developed by Kruszewski et al. (2018) used a solution of concentrated HNO3 and H2O2 (5:2). For the accuracy calculation, an analysis in triplicate was done for each method, obtaining the quadratic mean, standard deviation, and variance of measures for each analyte. From these values, an F-test was performed to evaluate the homogeneity between the variances of methods, obtaining a homogeneous variance between the methods of all studied analytes. From the result of the F-test, a t-test for homogeneous variance was used to evaluate if the means had a significant difference (Table 4). Both tests were done at 95% confidence; the T value was 2.78.
The t-test done between the method proposed by Kruszewski and coworkers (2018) and the optimized method demonstrated that for analytes As, Ca, Cd, Fe, K, Mg, Na, P, Pb, and Zn, there was no significative difference between the means. Regarding Costa and collaborators (2019), it was observed that excepting Mg, the obtained means for the optimized method and the author’s method did not present significant differences. In both cases, Mn showed a significant difference among the methods, indicating that the proposed extraction was insufficient for extracting this element from the sample matrix. However, observing the mean values in mg kg−1 and the deviations obtained for this analyte in both methods noted the proximity of the value of the optimized method.
Accuracy was also evaluated for recovery tests of the analyte, using the fortification of 1 mg L−1 for Ca, Fe, K, Mg, Mn, Na, P, and Zn and 2 µg L−1 of As, Cd, and Pb during the weight step. The recoveries were as follows: As, 107%; Ca, 93%; Cd, 107%; Fe, 88%; K, 127%; Mg, 96%; Mn, 89%; Na, 91%; P, 126%; Pb, 115%; and Zn, 92%. Compared with the established values by AOAC (2016), which describe the acceptable recovery ranges according to the analyte, the acceptable range of 95 to 105% was reached for Mg, Mn, and Zn. The same happened for Na (range 90 to 107%) and As and Cd (acceptable range 80 to 110%). A slight discrepancy was found for Ca, K, F, P, and Pb; however, this difference did not compromise the quantification of these analytes in the presence of a sample matrix, considering possible interferences and suppression effects or signal increase caused by the matrix.
iii. Application of the Analytical Procedure and Nutritional Information
Multivariate Analysis
After verifying the validated method, it was employed to analyze the 17 samples of craft chocolate from two brands (A and B) obtained on the local market (Table 1). Samples were separated into six classes according to cocoa content: class 1, 35–40% cocoa (1a, 1b, 1c, and 1d); class 2, 45–65% cocoa (2a, 2b, and 2c); class 3, 70–80% cocoa (3a, 3b, 3c, and 3d); class 4, 80–90% cocoa (4a and 4b); class 5, 100% cocoa; and class 6, cocoa content not informed by supplier (6a, 6b, and 6c).
The data obtained from sample analysis were treated using PCA to evaluate relationships between samples and variables and predict the influence of variables on these (Fig. 3). In the graph of PC1 versus PC2, in which PC1 represents satisfactorily 64.28% of data, it is possible to observe the presence of two big groups regarding the chocolates of each brand, and from the projection graph of variables, identify the primary influences suffered by them (Fig. 2). Samples of brand A are concentrated in the region of the second and third quadrants of the graph, indicating the significant presence of Mg, Cd, K, P, Fe, Zn, Mn, and Fe and small influence of Ca, Na, and Pb, contrary to what occurs with samples of brand B that are concentrated in the first and fourth quadrant.
Chocolates from brand A, manufactured in Linhares/ES, have cocoa cultivated in alluvial soil, formed by sediments transported by rivers, composed mainly of clay, sand, slime, and organic matter. Besides, the manufacturers use the harvest technique “cabruca” in which crops are put along “Rio Doce,” and the native trees are used to supply shadow to cacao. These aspects, summed with the fact that the municipality is in the Atlantic Forest region, contribute to the soil being rich in minerals such as K, P, and Mg, meaning that cocoa grown in this region also has these characteristics, which explains the influence of these elements on samples A (Ferreira et al. 2006).
Meanwhile, chocolates from brand B are manufactured in Rio Grande do Sul, where cocoa is harvested on Cambisol soil, characterized by its intermediary position in the development scale between incipient mineral soils and highly evolved soils. The technique of harvest used by the producers is the Forest System, an approach that incorporates planting diverse tree species together with cocoa plants. The chemical characteristics of this soil are influenced by particularities of the process of its local formation, but, in general, the cambisol in this region demonstrates a tendency to present elevated levels of Ca and Mg. The influence of these minerals on sample B is observed in Fig. 3, which is localized in a region that suffers the influence of Ca and Mg.
An example of a possible influence of the soil on samples’ composition from different brands can be noted by observing samples 3a(A), 3b(A), 3c(B), and 3d(B) in Fig. 3. All have 70% cocoa in their composition; however, the samples of brand B suffer more influence of Ca, while samples from brand A tend to be closer to regions with higher concentrations of K.
Besides the influence of soil in concentrations of analytes, by considering the classes of craft chocolate according to their cocoa content, it is noted that despite the existence of two groups, samples of different brands with the same range of cocoa content tend to be closer, demonstrating the possible influence of the concentration of elements on these, as occur with samples of class 1 and class 3 (Fig. 3).
Class 1 refers to samples with cocoa content between 35 and 40%. Between these samples, 1a(B) and 1b(B), which are close in Fig. 3, are craft milk chocolates with 36% cocoa in their formulation, while sample 1c(B) is craft chocolate with 39% cocoa without lactose and sample 1d(A) craft chocolate with 40% cocoa. The influence of milk in formulations is noted by the higher Ca and Na concentrations, which tend samples 1a(B) and 1b(B) to the fourth quadrant.
Samples of class 6 do not have information about cocoa content; however, they are craft milk chocolate samples with significant influence of Ca and Na. The localization of these concerning the others also indicates that they are samples with contents between 35 and 40% of cocoa. Regarding the determination of As, none of the evaluated samples showed the analyte on its composition. In contrast, 0.36 mg kg−1 of Pb was found in sample 6a(B); considering the possible content of cocoa, it is above the maximum limit (0.30 mg kg−1) suggested by the European regulation (N° 488/2014).
The results show the influence of the production place of the craft chocolates in the composition of micronutrients present in it, besides correlating the amount of cocoa with the concentration of the analyte. This demonstrates that the developed method is efficient in analyzing craft chocolate, allowing its application in samples with different amounts of cocoa without causing significant data losses. It is adequate for the precise determination of the analyzed components.
Nutritional Information of Samples of Craft Chocolate
Chocolate, particularly dark chocolate with a high cocoa content, may offer health benefits when consumed in moderation, as it helps in the organism’s antioxidant, cardioprotective, and anti-inflammatory capacity. However, when consumed in excess, chocolate can cause health risks, such as increased blood sugar levels, elevated cholesterol, and increased risk for heart disease (Efraim et al. 2011). The high content of micronutrients in the product can cause health damage. When consumed in excess, some minerals, like Fe and Zn, can interfere with absorbing other nutrients (Cozzolino 2007).
Considering the risks of excessive consumption of these elements, the results obtained were compared with the Dietary Reference Intakes (DRIs) (Trumbo et al. 2001), which are established values by health agencies, such as the Food and Nutrition Board (FNB) from the Institute of Medicine (IOM) from the USA to auxiliary in the evaluation and planning of adequate ingestion of nutrients.
Considering the maximum amount recommended by the Brazilian Ministry of Health for daily intake of chocolate (2008), the analyte concentrations were calculated concerning a total mass of 30 g and compared to maximum daily values of a nutrient that probably will not cause adverse effects to health described by DRIs. Table 5 presents the maximum, minimum, and mean values and standard deviation for each analyte obtained in the analysis of 17 samples. Assuming that one person, independent of age, eats 30 g of chocolate in one day, according to the results, it does not exceed the maximum daily limits described by DRIs, being 3000 mg/day for Ca, 45 mg/day for Fe, 350 mg/day for Mg, 11 mg/day for Mn, 2300 mg/day for Na, 4000 mg/day for P, and 40 mg/day for Zn.
Although the micronutrients identified in chocolate samples showed concentrations below the maximum limit for daily intake recommended by DRIs, relevant amounts of toxic metals such as Cd and Pb were noted in the studied samples. Among them, sample 6a(B) showed concentrations of 0.36 mg kg−1 (10.85 mg 30 g−1) of Pb above the maximum limit of 0.30 mg kg−1 described by the European regulation N° 488/2014. The relationship between the concentration of toxic elements in chocolate and the possible risks associated with the consumption of the food depends on several factors, for example, the rate of gastrointestinal absorption (Vanderschueren et al. 2023). However, obtaining an initial estimate of this information, considering the total concentration present in the food, is a first approximation of this information. So, the associated risks of taking these elements maybe high, mainly for infants and children. Pb can have a negative impact on neurological development, resulting in permanent damage to the brain, cognitive deficits, and behavior problems. At the same time, Cd can prejudice the central nervous system and kidney, besides compromising the immunological system (Magna et al. 2014).
Conclusion
Applying the Mixture Plan allowed the development of an analytical procedure for determining micronutrients (Ca, Fe, K, Mg, Mn, Na, P, and Zn) and contaminated elements (As, Cd, and Pb) in samples of craft chocolate with different contents of cocoa using ICP-OES and ICP-MS, from ultrasound-assisted extraction. The developed method has been demonstrated to be a fast and simple alternative to traditional acid digestion by microwaves, besides demanding a minor amount of reagents, which results in reduced costs and a small production of residues. Through the application of PCA on the obtained results from the analysis of samples of craft chocolate by the developed method, it was possible to observe the influence of soil composition in which cacao is cultivated on the presence and concentrations of the evaluated analytes, highlighting the uniqueness of the craft product.
References
Abt E, Sam J, Gray P, Robin L (2018) Cadmium and lead in cocoa powder and chocolate products in the US market. Food Addit Contam 11:92–102. https://doi.org/10.1080/19393210.2017.1420700
Afoakwa E, Paterson A, Fowler M (2007) Factors influencing rheological and textural qualities in chocolate–a review. Trends Food Sci Technol 18:290–298. https://doi.org/10.1016/j.tifs.2007.02.002
Barreto J, Assis R, Cassella R, Lemos V (2019) A novel strategy based on in-syringe dispersive liquid-liquid microextraction for the determination of nickel in chocolate samples. Talanta 193:23–28. https://doi.org/10.1016/j.talanta.2018.09.082
Chekri R, Bemrah N, Kandia D, Guérin T, Noel L, Jitaru P (2017) Essential and toxic trace elements in chocolate: occurrence data, assessment of nutritional merits and risk evaluation. 3rd IMEKOFOODS Conference: Metrology Promoting Harmonization and Standardization in Food and Nutrition 63:67.
Costa V, Pinheiro F, Amorim F, Silva E, Pereira-Filho E (2019) Multivariate optimization for the development of a sample preparation procedure and evaluation of calibration strategies for nutrient elements determination in handmade chocolate. Microchem J 150:104–116. https://doi.org/10.1016/j.microc.2019.104166
Cozzolino M (2007) Deficiências De Minerais Estudos Avançados 21:119–126. https://doi.org/10.1590/S0103-40142007000200009
Efraim P, Alves A, Jardim D (2011) Revisão: polifenóis em cacau e derivados. Braz J Food Technol 14:181–201. https://doi.org/10.4260/BJFT2011140300023
Ferreira B, Chaves E, Vialich J, Sauer E (2014) Extração assistida por ultrassom para determinação de Fe, K e Na em amostras de achocolatado em pó. Braz J Food Technol 17:236–242. https://doi.org/10.1590/1981-6723.1514
Ferreira P, Garcia G, Matos A, Ruiz H, Borges Junior J (2006) Transporte no solo de solutos presentes na água residuária de café conilon. Acta Sci Agron 28:1–8. https://doi.org/10.4025/actasciagron.v28i1.1295
Ferreira SB (2019) Avaliação de elementos traço em solos cultivados com cacaueiro na região sul da Bahia. Universidade Estadual de Santa Cruz, Dissertação
Freitas P, Carvalho R, Silva J, Prado T, Duarte E, Frescura V, Chaves E (2015) Ultrasound-assisted extraction for colorimetric determination of iron in soil: a comparison with inductively coupled plasma mass spectrometry. Quim Nova 38:570–574. https://doi.org/10.5935/0100-4042.20150021
Giraldo Y, Sanchez E, Torres L, Montenegro A, Pichimata M (2022) Development of validation methods to determine cadmium in cocoa almond from the beans by ICP-MS and ICP-OES. Talanta Open 5:100078. https://doi.org/10.1016/j.talo.2021.100078
Hartwig C, Pereira R, Rodrigo M, Rondan F, Cruz S, Duarte F, Flores E, Mesko M (2016) The synergic effect of microwave and ultraviolet radiation for chocolate digestion and further determination of As, Cd, Ni and Pb by ICP-MS. J Anal at Spectrom 31:523–530. https://doi.org/10.1039/C5JA00388A
International Union of Pure and Applied Chemistry, 1995International Union of Pure and Applied Chemistry (1995) Analytical chemistry division commission on analytical nomenclature + nomenclature in evaluation of analytical methods including detection and quantification capabilities. Pure & Applied Chemistry 67:1699–1723
Jalbani N, Kai T, Jamali MK, Afridi H, Sheerazi S, Ansari R (2007) Application of fractional factorial design and Doehlert matrix in the optimization of experimental variables associated with the ultrasonic-assisted acid digestion of chocolate samples for aluminum determination by atomic absorption spectrometry. J AOAC Int 90:1682–1688. https://doi.org/10.1093/jaoac/90.6.1682
Junior A, Maione C, Barbosa R, Gallimberti M, Paulelli A, Segura F, Souza V, Batista B, Barbosa F (2018) Elemental fingerprint profiling with multivariate data analysis to classify organic chocolate samples. J Chemom 32:30–36. https://doi.org/10.1002/cem.3036
Karas K, Zioia-Frankowska A, Bartoszewicz M, Krzysko G, Frankowski M (2021) Investigation of chocolate types on the content of selected metals and non-metals determined by ICP-OES analytical technique. Food Addit Contam 38:293–303. https://doi.org/10.1080/19440049.2020.1853821
Kruszewski B, Obiedzinski M, Kowalsda J (2018) Nickel, cadmium and lead levels in raw cocoa and processed chocolate mass materials from three different manufacturers. J Food Compos Anal 66:127–135. https://doi.org/10.1016/j.jfca.2017.12.012
Lo Dicoa G, Galvano F, Dugo G, D’ascenzi C, Mscaluso A, Vella A, Giangrosso G, Cammilleri G, Ferrantelli V (2017) Toxic metal levels in cocoa powder and chocolate by ICP-MS method after microwave-assisted digestion. Food Chem 245:1163–1168. https://doi.org/10.1016/j.foodchem.2017.11.052
Magna G, Machado S, Portella R, Carvalho M (2014) Avaliação da exposição ao Pb e Cd em crianças de 0 a 17 anos por consumo de alimentos vegetais cultivados em solos contaminados no município de Santo Amaro (BA). Engenharia Sanitaria e Ambiental 19:3–12. https://doi.org/10.1590/S1413-41522014019010000430
Manousi N, Zachariadis G (2021) A simple and rapid analytical method for the determination of nutrient and toxic elements in nut-based milk alternative beverages by ICP-OES. Food Anal Methods 14:1315–1321. https://doi.org/10.1007/s12161-021-01977-7
Mason TJ, Lorimer JP (2002) Applied sonochemistry: uses of power ultrasound in chemistry and processing. Wiley-VCH, Weinheim, p 314
Mrmolanin J, Pavlovic A, Krstic J, Mitic S, Tolic S, Stojkovic M, Micic R, Dordevic M (2018) Multielemental quantification in dark chocolate by ICP OES. J Food Compos Anal 67:163–171. https://doi.org/10.1016/j.jfca.2018.01.008
Pedro N, Oliveira E, Cadore S (2006) Study of the mineral content of chocolate flavoured beverages. Food Chem 95:94–100. https://doi.org/10.1016/j.foodchem.2004.12.021
Peixoto R, Oliveira A, Cadore S (2012) Multielemental determinations in chocolate drink powder using multivariate optimization and ICP OES. J Agric Food Chem 60:8117–8122. https://doi.org/10.1021/jf303022r
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219. https://doi.org/10.1080/00224065.1980.11980968
Salama A (2018) Health risk assessment of heavy metals content in cocoa and chocolate products sold in Saudi Arabia. Toxin Rev 38:318–327. https://doi.org/10.1080/15569543.2018.1471090
Silva L, Campos N, Lisboa T, Faria L, Matos M, Matos R, Sousa R (2021) Simultaneous determination of cadmium, lead and copper in chocolate samples by square wave anodic stripping voltammetry. Food Addit Contam 38:418–426. https://doi.org/10.1080/19440049.2020.1857443
Thompsen V (2012) Spectral background radiation and the “background equivalent concentration” in elemental spectrochemistry. Spectroscopy 27:28–36
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes. J Am Diet Assoc 101(3):294–294
Vanderschueren R, Montalvo D, Ketelaere B, Delcour J, Smolders E (2019) The elemental composition of chocolates is related to cacao content and origin: a multi-element fingerprinting analysis of single origin chocolates. J Food Compos Anal 83:103–110. https://doi.org/10.1016/j.jfca.2019.103277
Vanderschueren R, Doevenspeck J, Goethals L, Andjelkovic M, Wargeneers N, Smolders E (2023) The contribution of cacao consumption to the bioaccessible dietary cadmium exposure in the Belgian population. Food Chem Toxicol 172:113599. https://doi.org/10.1016/j.fct.2023.113599
Funding
This work was supported by FAPES (Fundação de Amparo à Pesquisa do Espírito Santo), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and LabPetro/UFES—Laboratório de Pesquisa e Desenvolvimento de Metodologias para Análise de Petróleo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies involving human participants or animals performed by any of the authors.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is an original research article that has neither been published previously nor considered presently for publication elsewhere. All authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Supplementary Information
ESM 1
(DOCX 28 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santana, G.F.R., Rodrigues, R.S., Brandão, G.P. et al. Multi-element Determination in Craft Chocolate with Different Cocoa Contents Using Ultrasound-Assisted Extraction and Dilute Acid as Sample Preparation. Food Anal. Methods 17, 475–485 (2024). https://doi.org/10.1007/s12161-024-02586-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-024-02586-w