Abstract
p35S promoter and tNOS terminator are the two primary targets for genetically modified organism (GMO) screening. An increasing number of genetic constructions do not contain p35S and tNOS elements; therefore, new screening assays are required. The use of a larger number of screening methods provides a better coverage of the EU-unapproved GMOs and is a cost-effective approach due to the decrease of tests required for identification. In the present study, new real-time PCR screening assays were developed targeting 10 promoter and terminator elements used in genetically modified constructs: pFMV, pNOS, pSSuAra, pTa29, pUbi, pRice actin, t35S, tE9, tOCS, and tg7. Specificity was verified against different plant species, and the limit of detection was determined on plasmid and genomic reference materials. Criteria of performance were successfully tested taking into account the recommendations of international guidelines. It means that these assays can be considered as ready for an inter-laboratory validation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The detection of a genetically modified organism (GMO) or a product derived from a GMO can be performed by the identification of a newly expressed protein or a fragment of the genetic construct integrated into the plant genome. Proteins subject to degradation during a product transformation process (e.g., cooking) are not ideal targets; however, techniques based on DNA are typically less dependent of alterations or damage. DNA is a relatively robust molecule, even if damaged by physical processes [1]. Segments to amplify must be of small size (<120 bp) in order to allow efficient detection in processed products.
Screening is typically the initial step to be carried out when searching for genetic modifications. Given a positive result, further testing can be done to subsequently identify and quantify the potential GM event(s) and for all these steps, DNA-based methods can fit. Screening must span the widest possible range of GM events that can be encountered on the market. To date, most screening tests are based on the detection of the cauliflower mosaic virus (CaMV) 35S promoter (p35S) or the Agrobacterium tumefaciens nopaline synthase terminator (tNOS). These targets have been widely applied and thus cover a large number of GM events. Controls must also be used to check that the signals are not due to the presence of the donor organisms [2, 3].
Tests for the detection of p35S and/or tNOS were successfully developed for classical PCR [4–7], real-time PCR [4, 8–14], isothermal PCR [15], microarrays [16], and biosensors [17]. In a first time, a majority of GM plants were transformed with constructs containing the p35S promoter and/or the tNOS terminator sometimes next to other promoters or terminators. Now, however, the p35S promoter and tNOS terminator are completely absent in some new transgenic constructs. PCR assays based on these new elements for screening are therefore essential. Alternatives to p35S/tNOS screening have been available for almost a decade but their interest in screening is more recent. For example, the literature reports classical PCR tests for the rice actin gene promoter [6], the CaMV terminator [6], a multiplex Microdroplet PCR test for the figwort mosaic virus promoter [18], a real-time PCR test for the maize Ubiquitin promoter [19], a duplex real-time PCR test for the CaMV terminator and the nopaline synthase promoter [20]. A commercial real-time PCR assay was also developed for the figwort mosaic virus promoter; however, the sequences used were not provided [21].
In this paper, we present new real-time PCR tests in TaqMan® format with similar thermal cycling conditions for the promoter of figwort mosaic virus (pFMV), A. tumefaciens Nopaline Synthase promoter (pNOS), Arabidopsis thaliana SSU promoter (pSSuAra), tobacco TA29 promoter (pTA29), maize Ubiquitin promoter (pUbi), rice actin promoter (pRice actin), CaMV terminator (t35S), pea E9 terminator (tE9), A. tumefaciens Octopine Synthase terminator (tOCS), and A. tumefaciens g7 terminator (tg7).
Materials and methods
Samples
We obtained certified transgenic reference material (CRM) as samples, sold by the Institute for Reference Materials and Measurements (IRMM, Geel, Belgium), the American Oil Chemists’ Society (AOCS, Urbana, Illinois, USA), and Bayer CropSciences (Diegem, Belgium). Coop de Pau (France) provided homozygous T25. The certified reference materials used are described in Table 2. Plants used for specificity testing were collected in gardens or fields. A. tumefaciens strains DNA was provided by the Department of Agroenvironmental Sciences and Technologies (DiSTA-Plant Pathology Unit) of the University of Bologna (Italy).
DNA extraction
Genomic DNA was extracted and purified from all samples following the CTAB-based method described in the Annex A.3.1 of the ISO 21571:2005 international standard [22]. The quality and quantity of DNA extracted from samples were estimated spectrophotometrically using a Nanodrop ND-1000 spectrophotometer at 260 nm (A260) and 280 nm (A280) absorbance. DNA purity was determined using A260/A280 ratio.
Primers and probes
Eurogentec (Seraing, Belgium) synthesized primers and probes. The probes were labeled with the reporter dye FAM™ at the 5′end, and the quencher dye TAMRA™ at the 3′ end. The primer and probe sequences are presented in Table 1.
Real-time PCR
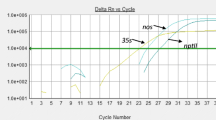
Real-time PCRs (total reaction volume of 25 μl) were performed on an ABI7000 and an ABI7500 fast (Applied Biosystems, Foster City, CA, USA) using Real-Time PCR Master Mix Diagenode (Universal Master Mix, GMO-UN-A600, Seraing, Belgium) or Applied Biosystems (TaqMan® Universal Master Mix, 4324020). The reaction mixture included 12.5 μl of Master Mix, 1.7 μl of each primer (5 μM), 1.5 μl of probe (9 μM), 2.6 μl of bidistilled water, and 5 μl of DNA. Reaction mixtures were distributed on 96-well reaction plates (Applied Biosystems) developed for the specific thermocyclers. Wells were covered with adhesive film and centrifuged (500 rpm, 10 s) to eliminate possible air bubbles in the well bottoms. PCR conditions were as follows: 2 min at 50 °C, 10 min to 95 °C, 50 cycles of 15 s at 95 °C, and 1 min at 60 °C. Baseline (normalized fluorescent signal before exponential PCR amplification occurs) was adjusted at three cycles earlier than the most abundant sample, and a threshold fluorescence level was fixed in the middle of the linear region of the amplification curve represented in a semilogarithmic graph (Y-axis with log fluorescence level in the function of PCR cycles).
Cloning into pCR2.1
The amplified fragments were ligated into the 3.9 kb pCR®2.1-TOPO plasmid vector (Invitrogen, Merelbeke, Belgium) following the TOPO® TA Cloning® kit instructions (Invitrogen, Merelbeke, Belgium). PCR results were visualized on 2.5 % agarose gels and the concentration estimated by visual comparison to a quantitative molecular weight marker (Smart Ladder, Eurogentec, Seraing, Belgium). Plasmid DNA was isolated from bacterial cultures using the High Pure Plasmid Isolation kit (Roche Diagnostics, Mannheim, Germany).
Subcloning into pUC18
pUC18 (10 μg) vector was cut with 100 units of HindIII and XbaI (Roche Diagnostics, Germany) to prepare the vector for the desired fragment insertion. The pCR®2.1 plasmid (10 μg), with integrated targets, was cut with 100 units of HindIII and XbaI to retrieve the fragment for insertion into pUC18.
Band purification and extraction on low melting point gels was conducted using the QIAquick Gel extraction kit (Qiagen, Hilden, Germany). DNA amounts were estimated on gels by visual comparisons relative to a quantitative molecular weight marker (Smart Ladder, Eurogentec, Liège, Belgium); and 10 ng of the fragments was used to ligate into the pUC18 vector in a 4:1 molar ratio for the insert toward the plasmid. Ligation was performed in a final volume of 60 μl with two units of T4 DNA Ligase (Roche Diagnostics, Germany) following the manufacturer’s recommendations (Cat no. 716359, Roche Diagnostics). Subsequent transformation to competent E. coli One shot® TOP10 cells (Invitrogen, Merelbeke, Belgium), and plasmid DNA extraction were completed as described above. All cloned inserts in pUC18 were verified by sequencing.
Limit of detection (LOD) determination
Target sensitivity was evaluated following the recommendations of the former (this standard no longer exists but the principles detailed are still applicable) AFNOR XP V03-020-2 standard [23], but adapted to plasmid targets. Based on the standard, the absolute limit of detection (LOD) was determined for the PCR assay (primers + probe + amplification program) on the dilutions of homozygous material (100 %) or reference material.
The subsequent dilutions must contain approximately 50, 20, 10, 5, 2, 1, and 0.1 copies of the target. Six PCRs must be achieved for each dilution. The method’s LOD is the smallest copy number for which the six PCRs are positive, and only if PCR on the final dilution containing the 0.1 copy generates a maximum of one positive signal on the six replications. If more than one positive signal is observed for the 0.1 copy, an evaluation of DNA quantities must be revised. The highest acceptable absolute LOD required for a test is 20 copies.
The zygosity of some reference material was not always indicated by the provider. For the calculation purposes of copy numbers of the target per haploid genome equivalent, such material was considered as homozygous for the transgenic trait. By doing so, the measured LOD remains valid because it is expressed as “below or equal to (≤)” a figure of copy numbers. If the certified reference material would have been heterozygous, then the measured LOD is overestimated but is indeed below the figure given. LOD was also checked on the cloned target. This has the advantage of better copy number control than on certified reference material.
Dilutions
Dilutions for LOD determination were conducted in water until reaching an estimate of 20,000 copies/5 μl. Further dilutions below that estimated copy number used a solution containing 5 ng/μl of salmon sperm DNA as background DNA. Low binding tubes were chosen to minimize DNA loss due to tube wall binding.
Estimated number of haploid genomes
The mean estimated DNA quantities necessary to obtain 20,000 target copies are based on the data of Arumuganathan and Earle [24] about sizes of haploid genome per plant species and were as follows: soybean: 23.00 ng, rapeseed: 24.60 ng, maize: 52.00 ng, sugar beet: 15.70 ng, potato: 35.85 ng, cotton: 46.55 ng, and rice: 8.00 ng. These quantities can be used to estimate transgenic target number. Here, it must be emphasized that the material used was 100 % GM, and the genetic modification was inserted once per haploid genome. Calculations were based only on figures of Arumuganathan and Earle [24] and not on that of other references [25–27] because the latter ones do not cover all species handled in this article.
Results and discussion
Targets based on promoters and terminators
We assessed the occurrence of the p35S and tNOS targets on 224 GM plants (stacked events excluded) listed in the GMOSeek matrix [28, 29]. Among the most representative genetically modified plant species, we note 16 soybean events, 27 rapeseed events, 41 maize events, 30 cotton events, 11 potato events, 7 sugar beet events, 18 tomato events, 8 wheat events, and 42 rice events. The GMOSeek Matrix sorting functionalities indicated that 87 events possessed a p35S, 78 have tNOS, 117 p35S or tNOS, and 48 p35S and tNOS.
With p35S and tNOS tests functional on all plants, only ~52 % of the GM plants (stacked events excluded) can be detected.
Therefore, the need for new screening tests is justified. On the same event list, the most frequent alternative promoters and terminators used were as follows (numbers between brackets provide the screening element occurrence in absolute numbers): pFMV35S (10), pNOS (16), pSSuAra (10), pTA29 (11), pUbi from Zea mays (20), pRice actin (19), t35S (27), tE9 (16), tOCS (11), and tg7 (7). This means that in view of a matrix-based screening approach, it is possible with the selection of a minimum set of tests to cover a maximum number of GMOs, thereby reducing the number of costly event-specific tests to be performed. Moreover, the availability of a larger set of screening methods allows a larger coverage of unauthorized GMOs [30].
Primers and probes (Table 1) were designed for PCR assays to specifically detect these 10 targets, with primary interest in the screening step. Size of the developed targets (Table 2) did not exceed 120 bp, which increases the potential to detect the targets in processed products.
The PCR assays developed in our study were selected based on the occurrence of the corresponding targets in GM plants. Testing all screening elements in addition to p35S and tNOS is not necessary, as some co-occur, for example, pSSuAra and pTA29 are in most cases introduced in the same GM event. However, it is valuable to possess a screening test that confirms a positive signal on co-occurring targets. In addition, increasing the number of screening elements leads to a reduction in the number of positive result candidates, and subsequently a decrease in the number of identification tests required to determine the presence of GM plants. Such strategy can be supported by a decision support system (DSS) to help the analyst for taking decision in front of the possible numerous results of the screening tests [31–33].
A wide screening strategy is also one of the ways to face the emerging challenge of detection of unknown GMOs: as very few information is available on the GM construction, a test with a large set of screening targets could give indications on the elements in presence and give a start point to apply sequencing strategies [34].
Target specificity
Target specificity was evaluated on 33 crop plants (see Table 3). The plant list includes the donor organisms of some developed targets (Arabidopsis: pSSuAra, tobacco: pTA29, maize: pUbi, rice: pRice actin, and pea: tE9). DNA extracts from the CaMV and A. tumefaciens were also included in these specificity checks. DNA from the figwort mosaic virus was not tested because we were unable to obtain the extracts of this material. At least an equivalent of 2,500 haploid genomes of the tested DNA was used for the specificity tests, as recommended in the GMOseek guideline D8/01 [35].
No aspecific signal was observed with the organisms tested, but signals were generated with donor organisms. Tobacco is absent in food products; consequently, there is no interference risk with the pTA29 target and its donor organism in food or feed. Conversely, the tE9 promoter will result in positive results whenever peas are present in the product, and it is not necessarily flagging a GM event presence. The primers and probes for tE9 were tested on a variety of plants close to pea from a taxonomic point of view namely Arachis hypogaea (peanut), Phaseolus vulgaris (bean), Glycine max (soybean), Faba vulgaris (broad bean), Phaseolus mungo (green mung bean), Lathyrus odoratus (sweet pea, flower), and Lens culinaris (lentil). All these plants gave negative results on the contrary of three types of pea that all gave positive results.
The problem is more of a concern for pRice actin, as nine Asian Rice events listed in the GMOSeek Matrix contain the pRice actin promoter in their GM construct. Similarly, pUbi is present in eight GM maize events listed in the GMOSeek Matrix. Indeed, pRice actin and pUbi generate clear positive signals with their respective donor organisms or taxonomically close relatives of them. A similar problem was already encountered with a classical PCR pUbi target developed by Babekova [19].
Maize and rice are primary ingredients in food and feed. Therefore, the application of pRice actin and pUbi is more limited in screening as they do not necessary flag a genetic modification. In this case, differential quantitative PCR [3, 36] with maize and rice reference genes could be applied to compare the number of copies of the screening tests and of alternative endogenous reference systems and this might extend the application range of the pRice actin and pUbi screening PCR assays.
However, in the case of raw material devoid of rice or maize, the targets maintain their interest as promoters are successfully used in other GM plants. Moreover, the pUbi promoter is frequently observed in Asian GM rice.
The screening targets were also successfully tested on the available GM reference material that contained the promoters and/or terminators we evaluated. One exception was t35S. Primer and probe establishment for t35S was rather difficult because some GM plants have a very short t35S of 70 bp (see Table 4). Moreover, the common sequence of 70 bp (CTTAGTATGTATTTGTATTTGTAAAATACTTCTATCAATAAAATTTCTAATTCCTAAAACCAAAATCCAG) is 78 % AT rich and does not permit the selection of primers and probe. Therefore, a 118-bp target was selected for t35S. This target was positively tested on T25, TC1507, and DAS59122 maize but failed to detect the Bt176 and event 3272 maize having a very short t35S.
Determination of LOD
The limit of detection (LOD) for the different promoter and terminator targets was determined following the former AFNOR XP V03-020-2 standard. The minimal requirement is to reach a 20-copy LOD. The LOD was determined on DNA dilutions of available reference material, but also on a cloned target in order to obtain better control of the copy number. The minimal performance criteria of 20 copies as highest acceptable absolute LOD was reached for all tested sample material (Table 2). For some events obtained as CRM, a LOD of one copy was reached, which suggested the target might be present more than once per haploid genome equivalent, or an underestimation of the DNA copies was introduced to the PCR tubes. For the cloned targets, the lowest estimated LOD reached was two copies.
Conclusion
Nine new PCR methods for GMO detection based on promoters (pFMV, pNOS, pSSuAra, pTA29, pUbi, pRice actin) and terminators (tE9, tOCS, tg7) were developed and successfully tested for qualitative purposes. A tenth method (t35S) was also developed, but its scope is more limited due to observed variation in sequences and length for different GM plant constructions.
In order to provide convincing evidence for a pre-validation report, we included LOD experiments, we checked specificity of the PCR assays, and we considered the recommended validation guidelines proposed in international documents as follows: AFNOR XP V03-020-2 standard [23], the Definition of minimum performance requirements for Analytical Methods for GMO testing [37], the Codex Alimentarius guidelines on performance criteria and validation [38], and the guidelines for qualitative methods described in the deliverable D8/1 of the GMOSeek project [35]. Next to specificity and sensitivity, another important performance criterion considered by Codex Alimentarius is robustness. This aspect was not thoroughly analyzed here, as there is no real consensus on how to practically address robustness. However, elements in favor of robustness [37, 38] were determined for pUbi and tE9 targets in the framework of the GMOSeek project, but not for all the targets.
Newly developed screening methods will permit greater coverage of the possible GM ingredient in food or feed products. The results of these tests can be compared with results obtained with other existing PCRs targeting the p35S and tNOS, and genes such as EPSPS and bar [39].
These methods will provide a better coverage of the potential GM events that could be present in a sample and constitute a step forward for the detection of the unauthorized/unknown GMOs. The reduction of subsequent identification tests is a cost-beneficial strategy. These methods were developed for merely qualitative purposes. However, differential quantitative PCR [3] might provide interesting information. Copy numbers in the same range for different screening targets might indicate the possibility of a single GM event (or a mix of several GM events showing the same screening elements), while important differences among copy numbers suggest the presence of several GM events in different concentrations.
This study provides an upgrade in useful screening elements targeting promoters and/or terminators. A second upgrade is required to target coding regions of genes often encountered in genetic constructs and taking into account the targets already developed for EPSPS [9, 40], bar [40], gox [41], pat [42], and the more complex cry family [43–47].
References
Debode F, Janssen E, Berben G (2007) Physical degradation of genomic DNA of soybean flours does not impair relative quantification of its transgenic content. Eur Food Res Technol 226:273–280
Chaouachi M, Fortabat M-N, Geldreich A, Yot P, Kerlan C, Kebdani N, Audeon C, Romaniuk M, Bertheau Y (2008) An accurate real-time PCR test for the detection and quantification of cauliflower mosaic virus (CaMV) applicable in GMO screening. Eur Food Res Technol 227:789–798
Cankar K, Chauvensy-Ancel V, Fortabat MN, Gruden K, Kobilinsky A, Zel J, Bertheau Y (2008) Detection of nonauthorized genetically modified organisms using differential quantitative polymerase chain reaction: application to 35S in maize. Anal Biochem 376:189–199
Mattarucchi E, Weighardt F, Barbati C, Querci M, Van den Eede G (2005) Development and applications of real-time PCR standards for GMO quantification based on tandem-marker plasmids. Eur Food Res Technol 221:511–519
Wolf C, Scherzinger M, Wurz A, Pauli U, Hübner P, Lüthy J (2000) Detection of cauliflower mosaic virus by the polymerase chain reaction : testing of food components for false-positive 35S-promoter screening results. Eur Food Res Technol 210:367–372
Matsuoka T, Kuribara H, Takubo K, Akiyama H, Miura H, Goda Y, Kusakabe Y, Isshiki K, Toyoda M, Hino A (2002) Detection of recombinant DNA segments introduced to genetically modified maize (Zea mays). J Agric Food Chem 50:2100–2109
Vollenhofer S, Burg K, Schmidt J, Kroath H (1999) Genetically modified organisms in food—screening and specific detection by polymerase chain reaction. J Agric Food Chem 47:5038–5043
Höhne M, Rosa Santisi C, Meyer R (2002) Real-time multiplex PCR: an accurate method for the detection and quantification of 35S-CaMV promoter in genetically modified maize-containing food. Eur Food Res Technol 215:59–64
Zeitler R, Pietsch K, Waiblinger HU (2002) Validation of real-time PCR methods for the quantification of transgenic contaminations in rape seed. Eur Food Res Technol 214:346–351
Alary R, Serin A, Maury D, Jouira HB, Sirven JP, Gautier MF, Joudira P (2002) Comparison of simplex and duplex real-time PCR for quantification of GMO in maize and soybean. Food Control 13:235–244
Kuribara H, Shindo Y, Matsuoka T, Takubo K, Futo S, Aoki N, Hirao T, Akiyama H, Goda Y, Toyoda M, Hino A (2002) Novel reference molecules for quantitation of genetically modified maize and soybean. J AOAC Int 85:1077–1089
Kimio M, Rie A, Naoki S, Masaki S, Hisatsugu I, Kazue S, Takashi T, Kunihiro K, Akihiro H, Kazuo S (2005) Detection of Genetically Modified Organisms in Foreign-made Processed Foods Containing Corn and Potato. J Food Hyg Soc Japan 46:79–85
Fernandez S, Charles-Delobel C, Geldreich A, Berthier G, Boyer F, Collonnier C, Coué-Philippe G, Diolez A, Duplan M-N, Kebdani N, Romaniuk M, Feinberg M, Bertheau Y (2005) Quantification of the 35S Promoter in DNA extracts from genetically modified organisms using real-time polymerase chain reaction and specificity assessment on various genetically modified organisms, part I: operating procedure. J AOAC Int 88:547–573
Reiting R, Broll H, Waiblinger HU, Grohmann L (2007) Collaborative study of a T-nos real-time PCR method for screening of genetically modified organisms in food products. J Verbr Lebensm 2:116–121
Fukuta S, Mizukami Y, Ishida A, Ueda J, Hasegawa M, Hayashi I, Hashimoto M, Kanbe M (2004) Real-time loop-mediated isothermal amplification for the CaMV-35S promoter as a screening method for genetically modified organisms. Eur Food Res Technol 218:496–500
Leimanis S, Hernandez M, Fernandez S, Boyer F, Burns M, Bruderer S, Glouden T, Harris N, Kaeppeli O, Philipp P, Pla M, Puigdomenech P, Vaitilingom M, Bertheau Y, Remacle J (2006) A microarray-based detection system for genetically modified (GM) food ingredients. Plant Mol Biol 61:123–139
Minunni M, Tombelli S, Mariotti E, Mascini M (2001) Biosensors as new analytical tool for detection of Genetically Modified organisms (GMOs). Fresenius Anal Chem 369:589–593
Guo J, Yang L, Chen L, Morisset D, Li X, Pan L, Zhang D (2011) MPIC: a high-throughput analytical method for multiple DNA targets. Anal Chem 83:1579–1586
Babekova R, Funk T, Pecoraro S, Engel K-H, Baikova D, Busch U (2008) Duplex polymerase chain reaction (PCR) for the simultaneous detection of cryIA(b) and the maize ubiquitin promoter in the transgenic rice line KMD1. Biotechnol Biotechnol EQ 22(2008/2):705–708
Pansiot J, Chaouachi M, Cavellini L, Romaniuk M, Ayadi M, Bertheau Y, Laval V (2011) Development of two screening duplex PCR assays for genetically modified organism quantification using multiplex real-time PCR master mixes. Eur Food Res Technol 232:327–334
Dörries H–H, Remus I, Grönewald A, Grönewald C, Harzman C, Berghof-Jäger K (2010) Development of a qualitative, multiplex real-time PCR kit for screening of genetically modified organisms (GMOs). Anal Bioanal Chem 396:2043–2054
ISO 21571:2005. (2005) Foodstuffs. Methods of analysis for the detection of genetically modified organisms and derived products. Nucleic acid extraction. International Organization for Standardization, Geneva
AFNOR XP V03-020-2 (2003) Produits alimentaires. Détection et quantification des organismes végétaux génétiquement modifies et produits dérivés. Partie 2: méthodes basées sur la réaction de polymérisation en chaîne. Norme expérimentale
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Nuclear Plant Mol Bio Rep 9:208–218
Bennett MD, Leitch IJ (2005) Nuclear DNA amounts in angiosperms: progress, problems and prospects. Ann Bot 95:45–90
Dolezel J, Greilhuber J (2010) Nuclear genome size: are we getting closer? Cytometry A 77:635–642
Praca-Fontes MM, Carvalho CR, Clarindo WR, Cruz CD (2011) Revisiting the DNA C-values of the genome size-standards used in plant flow cytometry to choose the “best primary standards”. Plant Cell Rep 30:1183–1191
GMOSeek project. http://www.nib.si/eng/index.php/aktualno/project/272-gmoseek-better-control-of-genetically-modified-organisms-gmos-for-an-improved-european-food-safety.html. Last consultation on the 5 of March 2012
Block A and Debode F, Grohmann L, Hulin J, Taverniers I, Kluga L, Barbau-Piednoir E, Broeders S, Huber I, Van den Bulcke M, Heinze P, Berben G, Busch U, Roosens N, Janssen E, Žel J, Gruden K, Morisset D (2013) The GMOseek matrix: a decision support tool for optimizing the detection of genetically modified plants (submitted for publication)
Holst-Jensen A, Bertheau Y, De Loose M, Grohmann L, Hamels S, Hougs L, Morisset D, Pecoraro S, Pla M, Van den Bulcke M, Wulff D (2012) Detecting un-authorized genetically modified organisms (GMOs) and derived materials. Biotechnol Adv 30:1318–1335
Querci M, Van den Bulcke M, Žel J, Van den Eede G, Broll H (2008) New approaches in GMO detection. Anal Bioanal Chem 396:1991–2002
Novak PK, Gruden K, Morisset D, Lavrac N, Stebih D, Rotter A, Žel J (2009) GMOtrack: generator of cost-effective GMO testing strategies. J AOAC Int 92:1739–1746
Gerdes L, Busch U, Pecoraro S (2012) GMOfinder—a GMO screening database. Food Anal Meth 5:1368–1376
Spalinskas R, Van den Bulcke M, Van den Eede G, Milcamps A (2012) LT-RADE: an efficient user-friendly genome walking method applied to the molecular characterization of the insertion site of genetically modified maize MON810 and rice LLRICE62. Food Anal Meth. doi:10.1007/s12161-012-9438-y
Morisset D, Broeders S, Block A, Berben G, Huber I, Debode F, Kluga L, Taverniers I, Grohmann L. (2011) Validation guidelines for qualitative methods. Deliverable D8/01. GMOseek project (FSA G03032, SAFEFOODERA)
Cankar K, Ravnikar M, Zel J, Gruden K, Toplak N (2005) Real-time polymerase chain reaction detection of cauliflower mosaic virus to complement the 35S screening assay for genetically modified organisms. J AOAC Int 88:814–822
European Network of GMO Laboratories (2008) Definition of minimum performance requirement for Analytical Methods for GMO testing. CRL GM Food and Feed. http://gmo-crl.jrc.ec.europa.eu/doc/Min_Perf_Requirements_Analytical_methods.pdf. Last consultation on the 5 of March 2012
Guidelines on performance criteria and validation of methods for detection, identification and quantification of specific DNA sequences and specific proteins in food (2010) ALINORM 10/33/23. Appendix III. http://www.codexalimentarius.net/download/report/738/al33_23e.pdf. Last consultation on the 5 of March 2012
Waiblinger H-U, Grohmann L, Mankertz J, Engelbert D, Pietsch K (2010) A practical approach to screen for authorised and unauthorised genetically modified plants. Anal Bioanal Chem 396:2065–2072
Grohmann L, Brunen-Nieweler C, Nemeth A, Waiblinger HU (2009) Collaborative trial validation studies of real-time PCR-Based GMO screening methods for detection of the bar gene and the ctp2-cp4epsps construct. J Agric Food Chem 57:8913–8920
Zhang Y, Zhang D, Li W, Chen J, Peng Y, Cao W (2003) A novel real-time quantitative PCR method using attached universal template probe. Nucleic Acids Res 31(20):e123. doi:10.1093/nar/gng123
Weighardt F, Barbati C, Paoletti C, Querci M, Kay S, De Beuckeleer M, Van den Eede G (2004) Real-time polymerase chain reaction-based approach for quantification of the pat gene in the T25 Zea mays event. J AOAC Int 87:1342–1355
Vaïtilingom M, Pijnenburg H, Gendre F, Brignon P (1999) Real-time quantitative PCR detection of genetically modified Maximizer maize and Roundup Ready soybean in some representative foods. J Agric Food Chem 47:5261–5266
Rho JK, Lee T, Jung SI, Kim TS, Park YH, Kim YM (2004) Qualitative and quantitative PCR methods for detection of three lines of genetically modified potatoes. J Agric Food Chem 52:3269–3274
Randhawa GJ, Chhabra R, Singh M (2010) Decaplex and real-time PCR Based Detection of MON531 and MON15985 Bt cotton events. J Agric Food Chem 58:9875–9881
Dinon AZ, Prins TW, VanDijk JP, Arisi ACM, Scholtens IMJ, Kok EJ (2011) Development and validation of realtime PCR screening methods for detection of cry1A.105 and cry2Ab2 genes in genetically modified organisms. Anal Bioanal Chemi 400:1433–1442
Guan Q, Wang X, Teng D, Yang Y, Tian F, Yin Q, Wang J (2011) Construction of a Standard Reference Plasmid for Detecting GM Cottonseed Meal. Appl Biochem Biotechnol 165:24–34
Acknowledgments
This research was conducted within a Belgian research project (Convention RT-06/6 GMODetec) financed by the Belgian Federal Public Service for Public Health, Food Chain Safety, and Environment in an endeavor involving three partners (ISP/WIV, ILVO, and CRA-W). Isabel Taverniers from ILVO (Belgium) kindly provided Arabidopsis DNA, and DNA from A. tumefaciens strains was kindly provided by Enrico Biondi from the University of Bologna (Italy). A part of this study (pUbi and tE9 targets) was financially supported by the UK Food Standard Agency (FSA, contract G03032), and the German Federal Office of Consumer Protection and Food Safety (BVL) through the project GMOseek, under the European ERA-NET consortium SAFEFOODERA. We are grateful to Marjorie Servais and Laurent Timmermans (HELHA Fleurus) for their technical help. We also thank Denis Roulez, Esther Arranz, Nicaise Kayoka-Mukendi, Cécile Ancion, and Gaëlle Antoine from our technical team (GMO team of CRA-W).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Debode, F., Janssen, E. & Berben, G. Development of 10 new screening PCR assays for GMO detection targeting promoters (pFMV, pNOS, pSSuAra, pTA29, pUbi, pRice actin) and terminators (t35S, tE9, tOCS, tg7). Eur Food Res Technol 236, 659–669 (2013). https://doi.org/10.1007/s00217-013-1921-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1921-1