Abstract
Lactobacillus plantarum LB1 and Lactobacillus rossiae LB5, isolated from wheat germ and selected based on the kinetics of acidification, were used as starters for the manufacture of sourdough fermented wheat germ. A bread containing sourdough fermented wheat germ as an ingredient (SFWGB) was compared to breads made with (raw wheat germ bread, RWGB) or without (wheat flour bread, WFB) raw wheat germ. The higher concentration of free amino acids mainly differentiated SFWGB from WFB and RWGB. The in vitro protein digestibility of WFB was the highest, even if sourdough fermentation of wheat germ attenuated the difference. Phytase and antioxidant activities of SFWGB were highest. The specific volume and cell-total areas were also the highest for SFWGB. As determined by texture profile analysis, the values of hardness, resilience and fracturability of breads containing wheat germ were lower than those found in WFB. The crust lightness showed a decrease from WFB to SFWGB. As determined by sensory analysis, SFWGB had mainly acid taste and flavour and resulted more salty. Sourdough fermented wheat germ is an ingredient able to enhance nutritional, texture and sensory properties of bread.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat germ, corresponding to 2–3% of the total weight of wheat kernel, is almost systematically removed during milling since it adversely affects the keeping and processing quality of the flour [1, 2]. Due to the high concentration of α-tocopherol, vitamins of group B, dietary fibre, polyunsaturated fats, minerals and phytochemicals, wheat germ is one of the most attractive and promising source of vegetable functional compounds [1, 2]. Wheat germ is also considered to be the major alternative source of plant proteins [3]. Most of the essential amino acids contained in the proteins of wheat germ are present at a level higher than that found in the reference egg proteins [3, 4]. The poor stability of wheat germ during shelf-life of wheat flour and related baked goods is the main concern for the limited use in bakery industries. The high lipase and lipoxygenase activities cause sensitivity to oxidation which leads to the release of free fatty acids and, consequently, to the appearance of rancidity in baked goods [5]. Lipases of wheat germ are thermo-stable and maintain more than 20% of the residual activity at 60–90 °C for 1 h [5]. Lipases have an optimum of pH of ca. 8.0, but their activity markedly decreases under acid conditions [5]. Increasing efforts are being made to stabilize wheat germ towards oxidation [5]. Treatments by heat, microwave and extrusion cooking [6] or the addition of antioxidants [7] were considered. Despite their effectiveness, the above technological treatments are in some cases expensive, not completely resolving and they negatively affect the nutritional value of wheat germ. Besides, synthetic antioxidants are increasingly looked at with suspicious because of their potential risks for consumer health [7].
Recently, some studies have considered the potential of wheat germ in medical and cosmetic applications, and also the effect of processed wheat germ on the nutritional and sensory properties of cereal based foods [2]. Previously, two lactic acid bacteria, Lactobacillus plantarum LB1 and Lactobacillus rossiae LB5, were isolated from wheat germ, selected based on the kinetics of acidification and used as starters for the manufacture of sourdough fermented wheat germ [2]. Sourdough fermentation stabilized and enhanced some nutritional properties of the wheat germ. Due to lactic acidification, the lipase activity of the sourdough fermented wheat germ was markedly lower than that found in the raw wheat germ. As shown by SPME/GC/MS analysis, a very low level of volatile compounds deriving from lipid oxidation was found in the freeze-dried sourdough fermented wheat germ during 40 days of storage [2].
This paper aimed at using the sourdough fermented wheat germ as an ingredient for the manufacture of white bread. The nutritional, texture and sensory characteristics of this bread were compared to those of white breads made with or without the use of raw (non fermented) wheat germ.
Materials and methods
Wheat germ and chemical characterization
Six samples of wheat germ were supplied by the industry Tandoi Pellegrino (Corato, BA, Italy). Before use, samples were pooled as usually done at industry plant. The germ was separated from refined flour during milling of Triticum aestivum cv. Appulo. A degerminator and a set of rollermills were used (Bühler AG, Uzwil, Switzerland). Moisture, ash, proteins and fat were determined according to the approved methods of the American Association of Cereal Chemists [8]. Total titratable acidity (TTA) was determined on 10 g of wheat germ homogenized with 90 ml of distilled water and expressed as the amount (ml) of 0.1 M NaOH to get pH of 8.3. The values of pH were determined by a Foodtrode electrode (Hamilton, Bonaduz, Switzerland).
Sourdough fermentation of wheat germ
Lactobacillus plantarum LB1 and Lactobacillus rossiae LB5 were previously isolated from raw wheat germ and selected based on the kinetics of acidification [2]. Lactobacilli were cultivated in modified MRS (mMRS, maltose and fresh yeast extract were added to MRS at 1 and 5%, respectively, and the final pH was 5.6) until the late exponential phase of growth was reached (ca. 10 h), washed twice in phosphate buffer, pH 7.0, 50 mM and re-suspended in tap water. Two hundred grams of pooled wheat germ, 115 ml of tap water and 5 ml of the cell suspension, containing both lactic acid bacteria (final cell density in the dough ca. 108 cfu/g), were used to produce 320 g dough (dough yield of 160) with a continuous high-speed mixer (60×g, dough mixing time, 5 min). Sourdough fermentation was carried out at 30 °C for 24 h. After fermentation, sourdough wheat germ was freeze-dried and used for bread making. Fermentations were carried out in triplicate.
Serial dilution of freeze-dried sourdough fermented wheat germ were made and plated onto MRS (Oxoid LTD, Basingstoke, Hampshire, UK). Enumeration of lactic acid bacteria was carried out after incubation at 30 °C for 48 h.
Lipase activity
As previously shown [9], wheat germ lipase has good solubility in water/salt-buffers. Water/salt-soluble extracts from raw wheat germ and sourdough fermented wheat germ were prepared according to the method originally described by Osborne and modified by Weiss et al. [10]. The concentration of proteins in the water/salt-soluble extracts was determined by the Bradford method [11]. Tributyrin as the substrate and the agar diffusion assay [12] were used to determine the lipase activity of the water/salt-soluble extracts. Agar plates contained 1% (wt/vol) of triglyceride, 0.02% (wt/vol) sodium azide and 50 mM phosphate buffer, pH 8.0. As reported by Kapranchikov et al. [5], this value of pH was the optimum for wheat germ endogenous lipase activity. Lipase activity was expressed as the minimum dilution of the enzyme preparation that failed to give a detectable zone of hydrolysis after 24 h of incubation at 30 °C.
Bread making
The characteristics of the wheat flour (T. aestivum, cv Appulo) used for bread making were as follows: moisture, 14.2%; protein (N × 5.70), 11.5%, of dry matter (d.m.); fat, 1.6% of d.m.; ash, 0.6% of d.m.; and total soluble carbohydrates, 1.5% of d.m. According to typical Italian bread making, three breads having dough yield of 160 were manufactured at the pilot plant of the Department of Plant Protection and Applied Microbiology. The formulas were as follows: (1) wheat flour bread (WFB) made with 250 g flour, 150 g tap water and 2% (wt/wt) of baker’s yeast; (2) raw wheat germ bread (RWGB) made with 240 g flour, 10 g raw wheat germ (RWG) (4%, wt/wt of wheat flour), 150 g tap water and 2% of baker’s yeast (wt/wt); and (3) sourdough fermented wheat germ bread (SFWGB) made with 240 g flour, 10 g of freeze dried SFWG (4%, wt/wt of wheat flour), 150 g tap water and 2% (wt/wt) of baker’s yeast. A continuous high-speed mixer (60×g, dough mixing time 5 min) was used to prepare the doughs. Fermentation of doughs was allowed at 30 °C for 2.5 h. Before baking, doughs were characterized for pH, titratable acidity, organic acids, free amino acids, total phenols, and phytase as well as antioxidant activities.
The rheology properties of the doughs were determined by a Brabender Farinograph (mixer type S300H Brabender, Duisburg, Germany), according to the AACC method [8]. From the farinograph normal curve, three main parameters such as water absorption capacity, development time and level of dough softening were determined.
All breads were baked at 220 °C for 40 min (Combo 3, Zucchelli, Verona, Italy). Fermentations were carried out in triplicate and each bread was analysed twice. Breads were packed in polyethylene bags to maintain constant the moisture and stored at room temperature for 8 days.
Determination of organic acids and free amino acids
Water/salt-soluble extracts from fermented doughs were prepared as reported elsewhere. Organic acids were determined by high performance liquid chromatography (HPLC) using an ÄKTA Purifier system (GE Healthcare) equipped with an Aminex HPX-87H column (ion exclusion, Biorad) and a UV detector operating at 210 nm. Elution was at 60 °C, with a flow rate of 0.6 ml/min, using H2SO4 10 mM as mobile phase [5]. Total and individual free amino acids were determined by a Biochrom 30 series Amino Acid Analyzer (Biochrom Ltd., Cambridge Science Park, England) with a Na-cation-exchange column (20 by 0.46 cm inner diameter) as described by Rizzello et al. [5].
Phytase activity
Phytase activity from water/salt-soluble extracts was measured in terms of inorganic ortophosphate released from the phytic acid by phytase [13]. The reaction mixture, containing 150 μl of water extract and 600 μl of substrate (3 mM Na-phytate in 0.2 M Na-acetate, pH 4.0), was incubated at 45 °C. The reaction was stopped by adding 750 μl of 5% trichloroacetic acid. The released inorganic phosphate was measured by adding 750 μl of colour reagent, prepared daily by mixing four volumes of 1.5% (wt/vol) ammonium molybdate in 5.5% (vol/vol) sulphuric acid solution and one volume of a 2.7% (wt/vol) ferrous sulphate solution. The absorbance was measured at 700 nm. One unit (U) of phytase activity was defined as the amount of enzyme required to liberate 1 nmol of phosphate per min under the assay conditions.
Total phenols and antioxidant activity
Extracts from dough were prepared by weighing 5 g of sample and mixing with 50 ml of 80% methanol. The mixture was purged with nitrogen stream, mixed for 30 min and centrifuged at 6,000×g for 20 min. Extracts were transferred into culture tubes, purged with nitrogen stream and stored at ca. 4 °C before analysis. Analysis of total phenols was done according to the method of Slinkard and Singleton [14]. Gallic acid was the standard. The reaction mixture contained 20 μl of extract, 100 μl of Folin-Ciocalteu reagent (Sigma Chemical Co.) and 1.58 ml of distilled water. After few min, 300 μl of saturated sodium carbonate solution was added to the reaction mixture. Incubation was allowed at 20 °C for 2 h and the absorbance at 765 nm was determined. The concentration of total phenols was calculated as gallic acid equivalent.
The radical cation (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) (ABTS·+) scavenging capacity was measured using the Antioxidant Assay Kit CS0790 (Sigma Chemical Co.), following the manufacturer’s instruction. Trolox (6-hydroxy 2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as the antioxidant standard. The scavenging activity was expressed as Trolox equivalent.
In vitro protein digestibility
The in vitro protein digestibility of breads was determined according to the methods of Dahlin and Lorenz [15], with some modifications. Fifty millilitres of bread suspensions, containing 6.25 mg of crude protein/ml, was allowed to rehydrate at 5 °C for 60 min. After rehydration, the suspension was placed in a water bath at 37 °C and the pH was set to 8.0, using 0.1 N NaOH and/or 0.1 N HCl. Lyophilized, crystallized trypsin (Sigma Chemical Co., Milan, Italy), at the concentration of 1.6 mg/ml, was maintained in an ice bath and the pH was adjusted to 8.00 with 0.1 N NaOH and/or 0.1 N HCl. Five milliliters of the enzyme solution was added to the protein suspension under stirring at 37 °C. The activity of trypsin was 13,000 BAEE units/mg protein. A rapid decline in pH occurred immediately. The pH drop was recorded 15 s after enzyme addition and at 1-min intervals for 10 min. The enzyme solution was freshly prepared before each test. The percent protein digestibility (Y) was calculated according to the following equation [15]: Y = 210.4−18.1x, where x is the change in pH after 10 min.
Texture and image analyses
Instrumental texture profile analysis (TPA) was performed with a TA.XT2i Texture Analyzer, using a 35-mm flat-end aluminium compression disc (probe P/35). The selected settings were as follows: test speed 1 mm/s, 30% deformation of the sample and one compression cycle. TPA was carried out [16] using Texture Export Exceed software. Specific volume, height, width, depth and area of loaves were measured by the TA.XT2i system. The following textural parameters were obtained by the texturometer software: hardness (maximum peak force); fracturability (the first significant peak force during the probe compression of the bread); and resilience (area during the withdrawal of the penetration, divided by the area of the first penetration). Triplicate measurements for breads from each storage time were made.
The crumb grain of breads was evaluated after 24 h of storage using the image analysis technology. Images of the sliced breads were scanned full-scale using an Image Scanner (Amersham Pharmacia Biotech, Uppsala, Sweden), at 300 dots per inch and analysed in grey scale (0–255). Image analysis was performed using the UTHSCSA ImageTool program (Version 2.0, University of Texas Health Science Centre, San Antonio, Texas, available by anonymous FTP from ftp://maxrad6.uthscsa.edu). A threshold method was used for differentiating gas cells and non-cells [17]. Analysis was carried out on two sub-images of 500 × 500 pixels (field of view) selected from within the bread slice. Two slices were analysed per treatment. The crumb cell features recovered were number, area, perimeter, elongation, roundness and gas cell to total area ratio.
Colour measurement
Colour was measured at three different positions of the bread surface using a Minolta CR-10 camera [18]. The L*a*b* colour space analysis method was used, where L* represents lightness (white–black) and a* and b* the chromaticity co-ordinates (red–green and yellow–blue, respectively). Result was reported in the form of a colour difference, dE *ab , as follows:
where dL, da, and db are the differences for L, a, and b values between sample and reference (a white ceramic plate having L = 93.4, a = −1.8, and b = 4.4).
Sensory analysis
Sensory analysis of breads was carried out by ten non-trained panellists according to the method described by Haglund et al. [19]. Elasticity, colour, acid taste, acid flavour, sweetness, dryness and taste were considered as sensory attributes using a scale from 0 to 10, with 10 the highest score. Salty taste, previously described as another wheat sourdough bread attribute, was also included [20].
Statistical Analysis Data were subjected to one-way ANOVA; pair-comparison of treatment means was achieved by Tukey’s procedure at P < 0.05, using the statistical software, Statistica 7.0 for Windows.
Results
Raw and sourdough fermented wheat germ
The averaged values of the six samples of raw wheat germ were the following: moisture 11.11 ± 0.37%, protein (N × 5.70) 28.56 ± 0.88% of dry matter (d.m.); fat 7.99 ± 0.04% of d.m.; and ash 3.77 ± 0.007% of d.m. The pH was 6.34 ± 0.08 and TTA was 18.1 ± 0.23 ml of 0.1 M NaOH/10 g. After freeze-drying, sourdough fermented wheat germ had values of moisture of 11.09 ± 0.41%, pH 4.15 ± 0.05 and TTA 25.5 ± 0.11 ml of 0.1 M NaOH/10 g. It contained ca. 5.9 × 109 cfu/g of viable lactic acid bacteria. The water/salt-soluble extracts of raw wheat germ and sourdough fermented wheat germ were used to determine the lipase activity. The minimum concentration of the crude enzyme extract that failed to give a detectable zone of hydrolysis was, respectively, 52.7 ± 3.4 and 152.8 ± 1.9 μg/ml.
Chemical and nutritional characterization
The values of pH were 5.45 ± 0.08, 5.76 ± 0.05 and 4.86 ± 0.04 for WFB, RWGB and SFWGB, respectively (Table 1). TTA was 3.5 ± 0.11, 4.8 ± 0.09 and 6.4 ± 0.16 of 0.1 M NaOH/10 g for WFB, RWGB and SFWGB, respectively. Lactic and acetic acids were not detectable in WFB and RWGB. Due to the preliminary sourdough fermentation of wheat germ, SFWGB contained 5.28 ± 0.07 and 2.07 ± 0.05 mM of lactic and acetic acids, respectively. Consequently, SFWGB bread had the quotient of fermentation (QF, molar ratio between lactic and acetic acids) of 2.55. This value approached that usually found in sourdough baked goods.
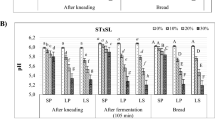
The concentration of total free amino acids of WFB, made without wheat germ, was 751 ± 48 mg/kg (Table 1). It increased to 1,356 ± 56 mg/kg for RWGB which used non-fermented wheat germ as an ingredient. The concentration of total free amino acids of SFWGB (1,686 ± 56 mg/kg) was significantly (P < 0.05) higher than that found in WFB and RWGB. Almost all free amino acids were found at higher concentration in RWGB and SFWGB breads with respect to WFB (Fig. 1). In particular, the concentration of Arg, Ser, Glu, Gly, Pro and Lys differentiated RWGB from WFB. By comparison RWGB, SFWGB had lower levels of Glu, Ser and Arg, but higher concentrations of Asp, Ala, Val, Ile, Leu, GABA and Lys.
Concentration of free amino acids and amino acid derivatives (mg/kg) of wheat flour (WFB), raw wheat germ (RWGB) and sourdough fermented wheat germ (SFWGB) breads. Details of the formulas for bread making are indicated in “Materials and methods”. Data are the means of three independent experiments twice analyzed. Bars of standard deviations are also represented
The phytase activity contained in the water/salt-soluble extract of SFWGB (2.27 ± 0.10 U) was significantly (P < 0.05) higher than that found in WFB and RWGB (0.78 ± 0.02 and 0.81 ± 0.08 U, respectively) (Table 1).
Under the conditions of this study, 80% methanol-extracts were used to determine the concentration of total phenols and antioxidant activities [21]. The concentration of total phenols significantly (P < 0.05) increased from WFB (0.18 ± 0.02 mM), RWGB (0.29 ± 0.01 mM) to SFWGB (0.36 ± 0.02 mM) (Table 1). The result of antioxidant activity, determined based on the scavenging activity towards ABTS radical [21], was as follows: 3.98 ± 0.12, 6.83 ± 0.24, and 8.50 ± 0.15 μmol/g for WFB, RWGB and SFWGB, respectively (Table 1).
The in vitro protein digestibility (IVPD) of WFB made without wheat germ was 68.37 ± 0.1% (Table 1). The use of raw wheat germ as the ingredient caused the decrease of the value of IVPD to 62.92 ± 0.2% (RWGB). Nevertheless, the use of sourdough fermented wheat germ (SFWGB) favoured a lower decrease (65.11 ± 0.2%) of IVPD with respect to RWGB.
Rheology properties and texture characterization
As determined by the Brabender farinograph, the water absorption capacity (62.5 ± 2.2%) of the WFB, RWGB and SFWGB doughs did not significantly (P > 0.05) differ. The development time was 4.8 ± 0.2 min for WFB dough and it slightly increased for RWGB and SFWGB doughs (5.3 ± 0.1 and 5.5 ± 0.2 min, respectively). WFB had the lowest value of dough softening (35 ± 3 Brabender Units, BU). It did not significantly (P > 0.05) vary between RWGB and SFWGB doughs (42 ± 3 and 38 ± 2 BU).
The volume of WFB, RWGB and SFWGB significantly (P < 0.05) differed. It was 666 ± 2, 706 ± 2 and 748 ± 2 cm3, respectively (Table 2). The same differences were found for the specific volume and, in an opposite order, for the density. The height but not the width of the loaves differentiated SFWGB from the other breads. Apart from fermentation, the use of wheat germ had a positive influence on the area of the bread (150 vs. 143 cm2 for RWGB and SFWGB vs. WFB). During 8 days of storage, the specific volume and moisture of packaged breads did not significantly (P > 0.05) vary. After baking, the value of hardness, corresponding to the peak force of the first compression of the product, was the highest for WFB (2,792 ± 12 g) and did not significantly (P > 0.05) differ between RWGB and SFWGB. The peak force required to puncture a 35-mm hole in breads was used as the index of the total textural attributes (toughness and crumbliness) [22]. After 4 days of storage, the peak force required for WFB and RWGB increased to 5,586 ± 24 and to 4,230 ± 22 g, respectively. At the end of storage, it was 6,466 ± 31 and 6,315 ± 29 g. On the contrary, the hardness of SFWGB increased slowly and reached the final value of 5,621 ± 28 g. Resilience indicate how well the bread recovers its original position. During storage, the lowest values of resilience were found for SFWGB (0.35 ± 0.03, 0.54 ± 0.02 and 0.26 ± 0.01 after baking, and 4 and 8 days of storage, respectively). WFB had the highest values of resilience. The fracturability point (the fracture of the bread) corresponds to the first significant peak force during compression of the bread. After baking, the values of fracturability only slightly differed between breads and ranged from 35,715 ± 24 to 39,897 ± 28 J. Fracturability markedly increased during storage highlighting the differences. It was the highest for WFB (114,706 ± 42 J) and the lowest for SFWGB (84,315 ± 37 J).
Image analysis and sensory characterization
The crumb grain of the breads was determined by image analysis technology. Digital images were pre-processed to detect crumb cell-total area by a binary conversion (black/white pixels) (Table 3). The cell-total areas (corresponding to the black pixel total area) were 37.1 ± 0.5, 43.2 ± 0.8, 48.4 ± 0.6% for WFB, RWGB and SFWGB, respectively (Table 3). Crumb cell detection of bread slice portions showed some differences in the number of gas cells. Cells detected in the sections of WFB were 482 ± 12 with the mean area of 2.68 ± 0.02 mm2 and the mean perimeter of 1.44 ± 0.03 mm. The number of cells of RWGB significantly (P < 0.05) differed (361 ± 10). No significant (P > 0.05) differences were found between SFWGB and WFB (487 ± 16 vs. 482 ± 12). While the mean area and mean perimeter of cells of RWGB markedly differed with respect to WFB, those of SFWGB had intermediate values. Elongation and roundness of WFB and SFWGB did not differ significantly (P > 0.05).
The visual inspection of the crust of breads made with wheat germ (RWGB and SFWGB) revealed a more intense colouring with respect to WFB. The crust lightness (L) showed a decrease from WFB (59.63 ± 0.11), RWGB (56.32 ± 0.09) to SFWGB (54.32 ± 0.05) (Table 2). The colour difference (dE), calculated based on the chromaticity co-ordinates (L, a and b), was 41.3 (WFB), 45.6 (RWGB) and 47.5 (SFWGB).
After baking (few hours), sensory analysis was carried out. The bread made without wheat germ (WFB) was characterized by the lowest value of elasticity and the highest score for dryness (6.5 ± 0.2 and 6.8 ± 0.2, respectively) (Table 4). As expected, the perception of the colour resulted in lower WFB with respect to RWGB and SFWGB. SFWGB made with fermented wheat germ received the highest scores for acid taste and flavour. The salty attribute also characterized the sensory profile of this bread, which also had the highest overall perception of taste (6.8 ± 0.1). Due to the use of non fermented wheat germ, sweetness was significantly (P < 0.05) more appreciated in RWGB bread. At the end of storage, dryness markedly increased in WFB, overall off-flavours were appreciated by panellists in RWGB and sensory attributes did not significantly (P > 0.05) vary in SFWGB (data not shown).
Discussion
Because of the high nutritional value, some studies considered the use of wheat germ for the manufacture of novel cereal based foods, especially healthy foods [3]. Replacement of wheat flour with defatted wheat germ at levels of 0–25% had positive repercussion on the functional and nutritional properties of cookies [23]. Pasta manufactured with semolina blended with 15% of raw and microwaved wheat germ showed an increase up to 17% of high nutritional value proteins [24]. Compared to breads made with refined flour, the concentration of minerals, proteins, fat and dietary fibres was higher in breads supplemented with wheat germ (7.5%) and bran [22]. Despite these findings, the use of wheat germ is still challenging due to the poor stability and the presence of anti-nutritional factors such as (1) raffinose which is not digested by pancreatic enzymes but metabolized by gas-producing bacteria of the large intestine, thus causing disorders such as flatulence [2]; (2) phytic acid which markedly decreases the mineral bioavailability [25]; and (3) wheat germ agglutinin (WGA) which is responsible for the hyperplastic and hypertrophic growth of the small bowel and pancreas [6]. To the best of our knowledge, no studies previously considered the stabilization of wheat germ by sourdough fermentation and the use of freeze dried sourdough fermented wheat germ for the manufacture of white bread. The sourdough fermentation of wheat germ was not only effective to partially inhibit the endogenous lipase activity and to increase the shelf-life of wheat germ, but also favoured the increase of the phytase activity and the decrease of the concentration of raffinose [2]. Overall, baking and even milder heat treatments almost completely eliminated the WGA activity from wheat germ [6].
This study used the freeze-dried sourdough fermented wheat germ as an ingredient (4%, wt/wt of wheat flour) for the manufacture of white bread. This concentration was chosen to mimic the percentage of wheat germ in the kernel. Higher concentrations of wheat germ (e.g., 6–8%) favoured an excessive sweet taste which seemed to be rather far from the main sensory attributes of the typical Italian breads.
The suitability for processing of the flours containing wheat germ was determined by Brabender farinograph analysis. It represents a simple test to assess the possibility of using functional ingredients in dough preparation [26]. The replacement of 4% of the wheat flour with RWG or SFWG did not modify the water absorption capacity and improved the softening of the doughs.
Bread leavening was carried out using baker’s yeast and the characteristics of this bread (SFWGB) were compared to those of white breads made with (RWGB) or without (WFB) the use of raw (non fermented) wheat germ. The use of freeze-dried sourdough fermented wheat germ had a positive effect on the nutritional properties of the white bread. The concentration of total free amino acids of RWGB and, especially, SFWGB were the highest. In particular, Lys, the major limiting amino acid of wheat flour, was found in SFWGB at the concentration of ca. 80 mg/kg. This concentration was ca. six-times higher than that found in WFB. GABA, a non-protein amino acid, which possesses well-known physiological functions such as neurotransmission, induction of hypotension, diuretic and tranquilizer effects [2], also increased from ca. 90 (WFB) to ca. 223 mg/kg (SFWGB). A recent report from the Federation of American Societies for Experimental Biology identified people sensitive to high daily doses of l-Glu [27]. Sourdough fermentation decreased the wheat germ endogenous concentration of Glu, probably due to its conversion into GABA through the activities of microbial and wheat germ glutamate decarboxylases. The concentration of Glu in SFWGB was ca. 23% of that found in RWGB. As previously reported [28], wheat germ has a value of in vitro protein digestibility (IPVD) lower than wheat flour. Sourdough fermentation increased the value of IPVD in raw wheat germ. The enhanced protein digestibility may be attributed to proteolysis by sourdough lactic acid bacteria and, probably, to the inactivation of some anti-nutritional factors such as trypsin inhibitor [29]. Overall, the sourdough fermentation increased the concentration of the wheat flour phenolic compounds [30]. This increase was also found during sourdough fermentation of wheat germ [2], and, as expected, the concentration of total phenols and the radical cation ABTS+ scavenging activities of SFWGB were higher than those found in the other two breads. Phytic acid is an excellent chelator of minerals such as Ca++, Mg++, Fe++, and Zn++, and complexes the basic amino acid group of proteins, thus decreasing the dietary bioavailability of these nutrients [25]. Phytic acid is largely distributed in the pericarp and aleurone layers of the kernel [25]. Compared to RWGB, the phytase activity of SFWGB was ca. 2.9-times higher. Sourdough lactic acid bacteria possess phytase activity. On the other hand, lactic acidification favoured more suitable condition of pH for the activity of flour and germ endogenous phytases [2].
The use of freeze-dried sourdough fermented wheat germ had positive effect also on the texture and sensory properties of the white bread. Under the experimental conditions of this study, highest specific volume and cell-total area of the crumb slices were found in SFWGB. Bread staling is a very complex physic-chemical phenomenon not fully understood. Firmness is one of the parameters used to evaluate staling, and TPA is one of the largely used method which gives different structure information [31]. After baking, breads were packaged in polyethylene films and stored for 8 days at room temperature. The values of hardness, resilience and fracturability of breads containing wheat germ (RWGB and SFWGB) were lower than those found in WFB. All these characteristics defined RWGB and, especially, SFWGB as the softest breads. Breads with wheat germ had a higher concentration of fat, which in part determined the increase in softness. Sensory analysis confirmed the elasticity of the breads containing wheat germ. Bread colour and aroma develop during baking, simultaneously with the crust formation, and derive from chemical reactions such as Maillard reaction and sugar caramelization [18]. The use of wheat germ leaded to an intense and appreciated colour of the crust, especially in SFWGB. Most of the sensory attributes differentiated RWGB and SFWGB, and both of them from WFB. RWGB was mainly defined by the sweetness note. SFWGB had mainly an acidic taste and flavour, due to either sourdough fermentation or consumption of wheat germ carbohydrates [2]. SFWGB also proved to be saltier than WFB and RWGB, probably due to the effect of acidification and proteolysis by selected lactic acid bacteria. This sensory attribute may assume particular importance for the manufacture of baked goods according to the new EC directive which aimed at decreasing the concentration of salt in bread [32].
Sourdough fermentation in part overlapped problems for stabilization and enhanced the nutritional properties of wheat germ, making it suitable for food processing [2]. Despite the health advantages, good sensory and texture properties still remain an essential requisite for baked goods. The use of freeze-dried sourdough fermented wheat germ enhanced the nutritional, texture and sensory characteristics of the white bread.
References
Amadò R, Arrigoni E (1992) Nutritive and functional properties of wheat germ. Int Food Ingred 4:30–34
Rizzello CG, Nionelli L, Coda R, De Angelis M, Gobbetti M (2009) Effect of sourdough fermentation on stabilization, and chemical and nutritional characteristics of wheat germ. Food Chem. doi: 10.1016/j.foodchem.2009.08.016
Ge Y, Sun A, Ni Y, Cai T (2001) Study and development of a defatted wheat germ nutritive noodle. Eur Food Res Technol 212:344–348
FAO/WHO/UNU (1995) Energy and protein requirements, report of joint FAO/WHO/UNU expert consultation, WHO Tech. Rep. Ser. No. 724. WHO, Geneva
Kapranchikov VS, Zherebtsov NA, Popova TN (2004) Purification and characterization of lipase from wheat (Triticum aestivum L.) germ. Appl Biochem Microbiol 40:84–88
Matucci A, Veneri G, Dalla Pellegrina C, Zoccatelli G, Vincenzi S, Chignola R, Peruffo ADB, Rizzi C (2003) Temperature-dependent decay of wheat germ agglutinin activity and its implications for food processing and analysis. Food Chem 48:3522–3527
Paradiso VM, Summo C, Trani A, Caponio F (2008) An effort to improve the shelf life of breakfast cereals using natural mixed tocopherols. J Cereal Sci 47:322–330
CC AA (2003) Approved methods of the American Association of Cereal Chemistry, 10th edn. AACC, Minnesota
Korneeva OS, Popova TN, Kapranchikov VS, Motina EA (2008) Identification of catalytically active groups of wheat (Triticum aestivum) germ lipase. Appl Biochem Microbiol 45:349–355
Weiss W, Vogelmeier C, Gorg A (1993) Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’ asthma. Electrophoresis 14:805–816
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Lawrence RC, Fryer TF, Reiter B (1967) Rapid method for the quantitative estimation of microbial lipase. Nature 213:1264
Shimizu M (1992) Purification and characterization of phytase from Bacillus subtilis (natto) N-77. Biosci Biotech Biochem 56:1266–1269
Slinkard K, Singleton VL (1997) Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic 28:49–55
Dahlin K, Lorenz K (1993) Protein digestibility of extruded cereal grains. Food Chem 48:13–18
Gámbaro A, Feszman S, Giménez A, Varela P, Salvador A (2004) Consumer acceptability compared with sensory and instrumental measures of white pan bread: sensory shelf-life estimation by survival analysis. J Food Sci 69:401–405
Crowley P, Grau H, Arendt EK (2000) Influence of additives and mixing time on crumb grain characteristics of wheat bread. Cereal Chem 77:370–375
Ahrné L, Andersson CG, Floberg P, Rosen J, Lingnert H (2007) Effect of crust temperature and water content on acrylamide formation during baking of white bread: steam and falling temperature baking. LWT 40:1708–1715
Haglund Å, Johansson L, Dahlstedt L (1998) Sensory evaluation of wholemeal bread from ecologically and conventionally grown wheat. J Cereal Sci 27:199–207
Lotong V, Chambers E IV, Chambers DH (2000) Determination of the sensory attributes of wheat sourdough bread. J Sens Stud 15:309–326
Ragaee S, Abdel-Aal ESM, Noaman M (2006) Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem 98:32–38
Sidhu JS, Al-Hooti SN, Al-Saqer JM (1999) Effect of adding wheat bran and germ fractions on the chemical composition of high-fiber toast bread. Food Chem 67:365–371
Arshad MU, Anjum FM, Zahoor T (2007) Nutritional assessment of cookies supplemented with defatted wheat germ. Food Chem 102:123–128
Pınarli İ, İbanoğlu Ş, Öner MD (2004) Effect of storage on the selected properties of macaroni enriched with wheat germ. J Food Eng 64:249–256
Febles CI, Arias A, Hardisson A, Rodríguez-Alvarez C, Sierra A (2002) Phytic acid level in wheat flours. J Cereal Sci 36:19–23
Jancurová M, Minarovičová L, Dandár A (2009) Rheological properties of doughs with buckwheat and quinoa additives. Chem Pap 63:738–741
Populin T, Moret S, Truant S, Conte L (2007) A survey on the presence of free glutamic acid in foodstuffs, with and without added monosodium glutamate. Food Chem 104:1712–1717
Bilgiçli N, Elgün A, Herken EN, Türker S, Ertaş N, İbanoğlu Ş (2005) Effect of wheat germ/bran addition on the chemical, nutritional and sensory quality of tarhana, a fermented wheat flour-yoghurt product. J Food Eng 77:680–686
Adams MR (1990) Topical aspects of fermented foods. Trends Food Sci Technol 1:140–144
Katina K, Arendt E, Liukkonen KH, Autio K, Flander L, Poutanen K (2005) Potential of sourdough for healthier cereal products. Trends Food Sci Technol 16:104–112
Meullenet JF, Lyon BG, Carpenter JA, Lyon CE (1998) Relationship between sensory and instrumental texture profile attributes. J Sens Stud 13:77–93
WHO, World Health Organization (2007) Reducing salt intake in populations: report of a WHO forum and technical meeting Paris, France, 5–7 October 2006
Acknowledgments
The authors thank Dott. Davide Minervini (Molini Tandoi-Pellegrino, Corato, BA-IT) for TPA analysis. This work was supported by the Italian Ministry of University and Research, project no. 12819, D.D. 1801 (31 December 2004) “PANTI”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizzello, C.G., Nionelli, L., Coda, R. et al. Use of sourdough fermented wheat germ for enhancing the nutritional, texture and sensory characteristics of the white bread. Eur Food Res Technol 230, 645–654 (2010). https://doi.org/10.1007/s00217-009-1204-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1204-z