Abstract
A milk-clotting enzyme named YS-1 was purified from a Bacillus subtilis (B. subtilis) YB-3, which we have isolated from Tibetan Plateau, Gansu, China. The enzyme YS-1 was identified as a metalloproteinase. SDS-PAGE and MALDI-TOF-MS analysis of the purified enzyme gave a molecular weight of 42 kDa. YS-1 was stable over a wide range of temperature from 20 to 60 °C. Purified YS-1 was also active over a wide range of pH from 5.0 to 9.0. It can be activated by Ca2+ and Al3+ but inhibited by Zn2+, Fe2+ and Cu2+. The milk-clotting enzyme YS-1 exhibited high specificity to substrate β-casein and yak milk casein and led to a 75% more rapid coagulation of yak milk than cow milk, due to high β-casein content in yak milk. Together, our findings confirmed that the enzyme YS-1 has a potential to be used in yak cheese industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chymosin (milk-clotting enzyme; EC 3.4.23.4) is a neonatal gastric proteinase that is of commercial importance in cheese-making industry [1]. Milk-clotting enzyme (MCE) is widely distributed in many organisms and tissues with different physiological properties and functions [2–6]. While making cheeses from cow, goat and yak milk, cheese factory usually uses different types of MCEs to control rheological and organoleptic properties [5, 7, 8].
As one of the most remarkable domesticated animals inhabiting altitudes ranging from 2,500 to 5,500 m, yak has survived in the tough environments [9]. Tibetans have made yak milk into dairy products, in which yak cheese is the main and the most popular one. Yak milk is different from cow milk in its high content of total proteins, total caseins and the proportion of individual caseins, particularly high amount of β-casein [10, 11]. In addition, the processing of yak cheese requires neutral pH, from 6.0 to 8.0, and a high temperature, over 65 °C, to reduce microbial contamination. As such, the traditional chymosin, which only shows high specificity for κ-casein and is stable only up to 50 °C and pH 6.0 [7, 12, 13], is of limited use in processing and production of yak cheese. In light of the high nutritional value of yak cheese and its unique position in Tibetan dietary, it is urgent to develop new sources of MCE with strong yak milk coagulation activity at 50 °C and neutral pH.
In an effort to searching new enzyme sources for yak milk clotting, we have isolated a B. subtilis strain, named as YB-3, from aged yak cheese that could secret extracellular MCEs. Importantly, the MCE from B. subtilis YB-3 is specific for yak milk casein, and it could lead to a 25% more rapid coagulation of yak milk [14]. Here, we describe the isolation and characterization of the MCE YS-1 from this strain.
Materials and methods
Microbial growth and enzyme production
A B. subtilis strain, named as YB-3 (CCTCC M209075), was isolated from aged cheese samples taken from Gannan Prefecture (it is situated at the east edge of Qinghai-Tibet Plateau, the average air temperature is −1.7 °C, the average altitude is over 3000 meters, the oxygen content is roughly 40% lower than that in the plains). The MCE production was carried out in a 100-ml flask, containing 30 ml of fermentation medium. On the basis of previous research [14], the fermentation medium had the following basic composition (g/l): casein, 10; beef extract, 3; NaCl, 5; Na2HPO4·12H2O, 5; initial pH 7.0. The medium was inoculated with 1 ml of vegetative inoculum, and then incubated at 37 °C under shaking conditions (150 rev/min). After the fermentation, the culture was centrifuged at 3,020×g for 5 min at room temperature to remove the cells. The supernatant was referred as crude enzyme extracts. MCE produced by this stain is named as YS-1.
Production of the milk-clotting enzyme in a pilot plant fermentation unit
Pilot plant fermentation was carried out in an Eastbio GUJS-20 L fermenter (East Biotech Equipment and Technology Co., Ltd, Zhenjiang, China), using 14 l of the fermentation medium. The medium was sterilized in situ at 121 °C for 30 min. The inoculum consisted of 300 ml of a shake-flask culture in such a manner that an initial cellular density of 108 cells/ml was achieved. Fermentation was started with a constant air flow rate of 7 l/min (equivalent to 0.5 VVM) and a stirring speed of 200 rev/min under pH control (pH 7.30; the pH was controlled automatically with 1 M citric acid) at 37 °C. Addition of the anti-foam product was controlled automatically.
Purification of an extracellular MCE and zymography
At first, 30 ml of crude enzyme extracts, with protein content of 4.5 mg/ml, was precipitated by ethanol between 30 and 60%. The precipitate obtained after centrifugation at 12,000×g for 30 min was dissolved in 3 ml of 50 mM phosphate buffer (KH2PO4–Na2HPO4, pH 6.0) as partially purified enzyme. 1 ml partially purified enzyme, with protein content of 1.5 mg/ml, was put on a Waters protein pak i-125 molecular exclusion chromatography column (7.5 × 300 mm) (Milligen Corp., MA, USA), where the column was previously equilibrated with 50 mM phosphate buffer (pH 6.0) containing 0.15 M Na2SO4. The partially purified enzyme was eluted with the same buffer, and 1.0-ml fractions were collected at a flow rate of 1.0 ml/min. The absorbance of the fractions was monitored at 280 nm. The fractions containing clotting activity were pooled and concentrated by Amicon Ultra (Milipore Corp., MA, USA). The concentrates were subsequently analyzed by SDS-PAGE gel electrophoresis and MS–MS.
For zymogram analysis, MCE YS-1 was separated in a 12% SDS–polyacrylamide gel containing 0.1% casein as a substrate, which copolymerized with separating gel as described [15].
Determination of molecular mass
The molecular mass of MCE YS-1 was determined by SDS-PAGE (separating gel: T = 12%, pH 8.8; stacking gel: T = 5%, pH 6.8) according to the method of Laemmli [16] in mini VE vertical electrophoresis system (Amersham Biosciences, CA, USA), following by quantization of the SDS-PAGE bands using a ImageQuant TL 7.0 software (GE Healthcare). The subsequent MALDI-TOF-MS analysis was also introduced to determine the molecular mass of MCE YS-1. The MCE YS-1 purified by gel filtration chromatography was electrophoresed, and the stained MCE band was used for MS (Thermo Fisher Scientific Inc., MA, USA) analysis.
Protein sequence analyses and protein identification
An Agilent 1100 HPLC system was connected to an LTQ Orbitrap Mass Spectrometer (Thermo Fisher Scientific Inc., MA, USA). The instrument was equipped with a 20 mm × 100 μm i.d. Aqua™ C18 RP column (Phenomenex, CA, USA) and a 200 mm × 50 μm i.d. Reprosil C18 RP analytical column. The tryptic peptides were separated by using a 90-min 0.2 ml/min linear gradient from 0 to 60% solvent B [0.1 M acetic acid in 80% acetonitrile (v/v)], in which solvent A was 0.1 M acetic acid. The MS was operated in positive ion mode, and parent ions were isolated for fragmentation in data-dependent mode. The mass peak list obtained was submitted to Mascot (http://www.matrixscience.com) database for protein identification [15].
The determination of the homology between the metalloproteinase obtained from Bacillus pumilus and YS-1 from B. subtilis YB-3 was carried out. The metalloproteinase gene (1,563 bp) was amplified by PCR in 25 μl volumes containing 10 × PCR buffer (TaKaRa, Dalian, China), 0.25 mM of each dNTP, 0.5 μM of the upstream primer p1 (5′-ATGGGTTTAGGTAAGAAATTGTCT-3′), 0.5 μM of the downstream primer p2 (5′-TTACAATCCAACAGCATTCCAGGC-3′)], 1 U of DNA Taqase (TaKaRa, Dalian, China) and 50 ng of DNA template. For sequencing, amplified PCR products were purified using the PCR purification kit (Invitrogen, CA, USA). Sequence comparison with the databases was performed using BLAST through the NCBI server.
Enzyme assay
Milk-clotting activity (MCA) was measured by the method of Arima et al. [17], using 10% reconstituted skim milk containing 0.01 M CaCl2 as a substrate. The enzyme fraction (1 mg/ml protein) was added to 10 ml of substrate solution, and the reaction was carried out at 37 °C. The end point was recorded when curd particles were observed. Under the above assay conditions, one Soxhlet unit (SU) of the enzyme activity was defined as the amount of enzyme that clotted 10 ml of reconstituted skim milk in 40 min. The clotting activity was calculated according to the following formula: SU = 2,400 × 10/(1 × t), where t was the clotting time (s).
Effects of metal ions and protease inhibitors on MCA of purified YS-1
The effects of various 5 mM and 10 mM metal ions on MCA of purified MCE YS-1 were determined by pre-incubating the enzyme with the individual metal ions (Na+, K+, Li+, Ca2+, Mn2+, Zn2+, Cu2+, Fe2+, Mg2+, Co2+ or Al3+) in 50 mM phosphate buffer (pH 6.0) at 25 °C for 30 min, then the MCA was measured.
The effects of protease inhibitors, including phenylmethylsulfonyl fluoride (PMSF) at 1 mM and 2 mM levels, ethylenediaminetetraacetic acid (EDTA) at 10 mM level, β-mercaptoethanol (ME) at 10 mM level and pepstatin at 0.05 mM level on MCA of purified MCE YS-1 were also investigated. The purified enzyme was pre-incubated with each inhibitor for 30 min at 25 °C, and then the MCA was determined.
The effects of temperature and pH
The optimum temperature of the purified YS-1 was determined by assaying MCA at temperatures between 20 and 80 °C. The thermostability of purified YS-1 was examined by pre-incubated YS-1 at temperatures between 0 and 60 °C for 60 min and then brought to room temperature. Aliquots (0.5 ml) were removed at 10-min intervals and the MCA was measured.
The optimum pH of the purified YS-1 was examined over a pH range of 2.0–11.0 at 37 °C. The pH stability of the purified YS-1 was examined by pre-incubating YS-1 in buffers of different pH values in the range of 2.0–12.0 for 60 min at 25 °C, and the activity was measured at pH 6.0 and 37 °C.
Substrate specificity
The proteolytic behavior of MCE isolated from Rhizomucor pusillus and B. subtilis YB-3 was examined as described [18]. A solution (200 μl, 0.4%) of α-casein, β-casein, and κ-casein (Sigma, St. Louis, USA) in 0.5 mM phosphate buffer (pH 6.0) was mixed with 10 μl of enzyme solution (300 SU/ml). The samples were then used for SDS-PAGE analysis.
In order to determine the substrate specificity of the purified YS-1 from B. subtilis YB-3, its ability to proteolysis kinds of α-casein, β-casein, κ-casein, cow milk casein, yak milk casein and clot cow milk and yak milk were tested. In addition, calf chymosin and MCE from R. pusillus were considered as controls.
Proteolytic activity of samples was evaluated according to the Twining method using fluorescein isothiocyanate-labeled kinds of caseins (FTC-cow milk casein, FTC-yak milk casein, FTC-α-casein, FTC-β-casein and FTC-κ-casein) as the substrates [19]. One enzymatic unit was defined as the amount of enzyme necessary to degrade 1 μg of FTC-protein in 1 h at 37 °C [20]. Fluorescence intensity was measured using a luminescence microplate reader (Thermo Varioskan Flash 3001, USA) with excitation and emission wavelengths of 490 and 525 nm, respectively.
MCA of samples for different substrates was evaluated by a modified method of Arima et al. [17]. One milliliter of (0.1 mg/ml) purified YS-1 was added to 5 ml 10% skim milk and skim yak milk, respectively. Then the clotting time was recorded.
Statistical analysis
The results were reported as the mean ± SD and were analyzed by one-way analysis of variance (ANOVA), and post hoc comparisons were made using Tukey’s multiple comparison tests (SPSS version 17.0 software, SPSS Inc., Chicago, USA). The significance value (p) <0.05 was statistically different.
Results and discussion
Microbial growth and enzyme production
The time course of cell growth, MCA, due to MCE production, and pH of growth medium during fermentation of B. subtilis YB-3 at small scale (30 ml) is shown in Fig. 1a. The cell reached its maximum at 48 h of cell growth. The pH value increased from 7.0 to 8.4 during the fermentation. The MCA continued to increase and reached a maximum value at 24 h and then decreased precipitously. According to the previous works, this probably attributed to high pH value of culture medium [21] and proteolysis of other proteolytic enzymes [22] producing by B. subtilis YB-3.
Growth, MCA and medium pH of B. subtilis YB-3 strain. a YB-3 strain was inoculated in a shake flask (100 ml). The cell number, MCA and medium pH were measured at various times as indicated. b YB-3 strain inoculated in a 20-L fermenter. The cell number, MCA and medium pH were measured at various times as indicated
Several researchers reported that the optimum fermentation period for the production of MCE in other microorganisms vary from 1 to 6 days. For instance, it takes only 1 day for B. subtilis natto [23], 5 days for Mucor circinelloides [24] and 6 days for Aspergillus oryzae [25]. In comparison, B. subtilis YB-3 took much shorter time to generate MCE activity, making it a better choice as a source of milk coagulant for cheese industry.
The same sets of parameters were then measured in a 20-L fermenter. As shown in Fig. 1b, maximum MCA was observed after 18 h of growth in the fermenter. The growth curve obtained with the fermenter was similar to that with the shaker, except that the logarithmic phase, and consequently the enzyme production, occurred slightly earlier. However, the pH-controlled fermentation allows an increase in enzyme production, from 200 SU/ml (in the case of uncontrolled fermentation) to 800 SU/ml (in the case of the pH-controlled fermentation). This increase is likely due to the following reasons: (1) Culture pH can profoundly affect cell growth, MCE biosynthesis and broth rheology [26], and the control of pH prevented growth suppression of B.subtilis, which occurs at pH above 8.0. (2) The control of pH also prevented the unwanted denaturation of MCE, which occurs at pH above 8.0. It is concluded that pH value in culture medium is a key factor, which significantly influences MCE production by B. subtilis YB-3. The studies on a 20-L fermentor clearly established the scalability of fermentation process using B. subtilis YB-3 for future industrial application.
MCE YS-1 purification and LC–MS/MS analysis of the MCE
The summary of purification of MCE YS-1 from B. subtilis YB-3 is presented in Table 1. The crude enzyme extracts were successively subjected to ethanol fractionation and molecular exclusion chromatography, which yielded a 9.5-fold of purification with a recovery of 19.1% and a specific activity of 8,889 SU/mg. The active fraction from gel filtration step yielded a single band on SDS-PAGE (Fig. 2, lane 2). A zymogram carried out under native condition also showed a single band, indicating that protease is a monomer (Fig. 2, lane 1). MCE from different sources has been purified using a range of chromatographic techniques [7, 12, 18, 27], but only limited numbers can be used as calf chymosin substitutes, in which even few can be used in yak cheese industry. The results suggested that MCE YS-1 has a great potential to be used in yak cheese industry.
SDS-PAGE of proteins after various steps of purification. Lane 1 Zymogram analysis of purified enzyme (0.1% casein as a substrate, 300 SU/ml YS-1); lane 2 fraction after Waters protein Pak i-125 column (0.45 mg/ml); lane 3 ethanol fractionation (1.5 mg/ml); lane 4 enzymatic culture supernatant (4.5 mg/ml); lane 5 standard molecular weight markers
The purified enzyme has molecular weight of 42 kDa as determined by SDS-PAGE (Fig. 2, lane 2) and MALDI-TOF-MS (Data not shown). It is noteworthy that most Bacillus MCE described in literature give molecular size of 34 kDa [7] or 65 kDa [12]. The different size of our MCE YS-1 suggested that it may represent a new proteolytic enzyme. The peptide mass fingerprinting methods was conducted to determine protein identification of the 42 kDa band obtained here (Fig. 3). A database search (the online Mascot MS–MS Ions search database from Matrix Science) identified 8 peptides that represent 44.67% coverage of the metalloproteinase sequence of Bacillus pumilus (gi56405351), which indicated that MCE YS-1 from B. subtilis YB-3 is a member of the metalloproteinase family.
Analysis of the nucleotide sequence and its flanking DNA regions revealed the presence of an open reading frame (ORF) of 1563 bp, and the deduced amino acid sequence shows a pre-pro-protease of 521 amino acids. Sequence alignment with protein databank shows that the metalloproteinase from B. subtilis YB-3 has (518/521) 99% homology with metalloproteinases of Bacillus pumilus (Data not shown).
Effects of metal ions and protease inhibitors on the MCA
On the basis of the peptide fingerprint, YS-1 was likely to be a metalloproteinase. We therefore examined the effects of various metal ions on MCA of YS-1. As shown in Table 2, the addition of Al3+ or Ca2+ significantly increased the enzyme activity at 5 mM and 10 mM. Similar results were reported by Jellouli et al. [28]. Na+, Li+, Mn2+, Mg2+ or Co2+ showed no significant effect at 5 mM and 10 mM. In contrast, Zn2+, Cu2+ and Fe2+ significantly inhibited the enzyme activity at 5 mM and 10 mM. Inactivation of MCE by Cu2+ and Fe2+ has also been reported by Jasmin et al. [29].
Among the four different protease inhibitors tested, ME (for sulfhydryl proteases), PMSF (for serine proteases), pepstatin (for aspartate proteases) and EDTA (for metalloproteinase), only EDTA significantly inhibited MCA of YS-1 (Table 2), further suggesting that YS-1 was a metalloproteinase.
The effects of temperature and pH
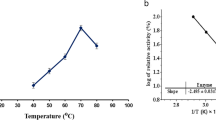
The effects of temperature on MCA of the purified YS-1 were examined at temperatures between 20 and 80 °C. The enzyme was active over a large temperature range (40–80 °C), with an optimum at 70 °C (Fig. 4a). The optimum temperature of the purified enzyme was similar to the values reported earlier for some MCE from other Bacillus strains [30, 31].
Effects of pH and temperature on enzyme activity and stability. a The optimum temperature of the purified YS-1 (pH 7.0) and the activity of the enzyme at 70 °C were taken as 100%; b the thermostability of purified YS-1 (pH 7.0, the incubation time is 60 min) and the nonheated enzyme was considered as 100%; c The optimum pH of the purified YS-1 (37 °C) and the maximum activity, obtained at pH 6.0, were considered as 100%; d The pH stability of the purified YS-1 (37 °C, the incubation time is 60 min) and the activity of the enzyme before incubation were taken as 100%. The following buffer systems were used: 100 mM sodium acetate buffer for pH 5.0–6.0, 100 mM potassium phosphate buffer for pH 7.0–7.5, 100 mM Tris–HCl buffer for pH 8.0–8.5, 100 mM glycine–NaOH buffer for pH 9.0–11.0 and 100 mM KCl–NaOH for pH 12.0. Each point represents the mean ± SD of the results of three independent experiments
In order to investigate thermal stability of the purified YS-1, the time courses inactivation of the enzyme was examined at different temperature levels. The purified YS-1 retained nearly 100% of MCA for 60 min in the temperature range of 0–50 °C. As shown in Fig. 4b, pre-incubation at 60 °C in the absence of calcium ions for 60 min abolished most of the MCA. In contrast, the presence of CaCl2 (2 mM) enabled the enzyme to retain more than 90% of its activity. According to previous works, calcium ions are important for catalysis, and it is presumed that they stabilize the protein through specific and nonspecific binding sites, and may also allow for additional bonding within the enzyme molecule, preventing unfolding at higher temperatures, as has been demonstrated for protease from thermophilic bacteria, particularly thermolysin [32, 33]. Besides, calcium ions were reported to bind to the inner surface and autolytic sites of protein molecule, thereby strengthening the interaction inside the molecule [34].
Compared with MCE from other sources as reported in the literature, the MCE from B. subtilis YB-3 appeared to have outstanding thermostability in the presence of Ca2+. For example, the activities of MCE from Thermomucor [35], Actinidia chinensis [36] and Penicillium oxalicum [27] were all lost rapidly in 55–60 °C. The MCE from B. subtilis natto retains only 20% of its activity after incubating at 60 °C for 30 min [23]. The high optimal temperature of MCE is a major concern in yak cheese industry, as a temperature of 65 °C is required for pre-processing pasteurization for yak milk. It would allow significant shortening of this pre-processing period. In addition, the high optimal temperature of MCE ensures high reaction rate at high temperature, which is also needed to reduce microbial contamination. As most of fungal and bacterial clotting enzymes are catalytically unstable at high temperature [7, 12, 13], MCE YS-1 offers a significant advantage in yak dairy industry because of its high optimal temperature and stability at 60 °C in the presence of Ca2+.
The purified enzyme was highly active between pH 5.0 and 8.0 with an optimum at pH 6.0 (Fig. 4c). This pH profile is quite similar to that of proteases isolated from Rhizopus oryzae [7] and B. subtilis natto [23].
The pH stability study showed that the enzyme is highly stable in the wide pH range of 5.0–9.0 for 60 min (Fig. 4d). Out of this range, the activity dropped precipitously. Under acidic conditions (pH 2.0–4.0), metalloproteinase lost its activity completely, probably due to auto-degradation, whereas under alkaline conditions (pH > 9.0), an irreversible conformational change led to the loss of activity [37]. The enzyme retained about 90% activity in the pH range of 6.0–8.0. The pH of yak milk is usually between 6.5 and 8.5 [38]. The pH stability study and the pH profile showed that YS-1 was most stable and active at this pH range, indicating that YS-1 would be quite suitable to be employed in yak cheese-making process.
Substrate specificity
To further determine whether YS-1 is suitable for yak cheese-making, we investigated the proteolytic behavior and substrate specificity of YS-1. Figure 5 compares the time course of the degradation of α-casein, β-casein and κ-casein by the MCE isolated from R. pusillus and B. subtilis YB-3. Clearly, the rate of κ-casein, β-casein and α-casein hydrolysis by MCE YS-1 was higher than that of MCE from Rhizomucor pusillus. The degradation patterns of the three caseins by MCE YS-1 were different from that of Rhizomucor pusillus. As shown in Fig. 5c, both YS-1 and R. pusillus enzymes digested κ-casein into a short peptide (MW 15,000), but in the case of YS-1, an additional degradation product (MW 10,000) was generated. Fig. 5a, b shows that R. pusillus enzyme appeared to be ineffective in digesting α-casein and β-casein. In contrast, YS-1 completely degraded α-casein within 10 min, generating nonspecific hydrolysis products; similarly, YS-1 completely degraded β-casein within 5 min, generating small peptides of the size of MW 20,000, 16,000 and 13,000).
SDS-PAGE pattern of κ-casein, β-casein and α-casein hydrolysis products by Rhizomucor pusillus MCE at time 0, 5, 10, 20, 30 min (corresponding lanes labeled from 1 to 5) and by MCE YS-1 at time 5, 10, 20, 30 min (corresponding lanes labeled from 6 to 9). Lane 0 standard molecular weight markers. The main peptide is indicated by an arrow; a α-casein and its nonspecific hydrolysis products, b β-casein and its products, c κ-casein and its new product
Table 3 compares the relative proteolytic activity of MCE YS-1 with calf chymosin (Maxiren 1800) and MCE from Rhizomucor pusillus. In addition to three pure caseins, cow milk casein and yak milk casein were included for comparison. Compared with calf chymosin and MCE from Rhizomucor pusillus, MCE YS-1 showed broad substrate specificity with different casein fractions, especially with the yak casein and β-casein. The proteolysis rate of β-casein with MCE YS-1 was about six times higher than that with MCE from Rhizomucor pusillus, and the proteolysis rate of yak milk casein was about 1.7 times and three times higher than that with calf chymosin and MCE from Rhizomucor pusillus, respectively. Furthermore, MCE YS-1 resembles calf chymosin and MCE from R. pusillus in the proteolysis rate of cow milk casein and κ-casein. It is concluded that the purified MCE YS-1 prefers yak milk being rich in β-casein to cow milk being rich in α-casein. The action of B. subtilis neutral protease on β-casein led to the appearance of peptides in the region of κ-casein [39]; hence, κ-casein can be more easily hydrolyzed by MCE YS-1. It is concluded that MCE YS-1 is by far the more potent enzyme for digesting yak milk casein.
As shown in Fig. 6, there was higher clotting activity of calf chymosin (Maxiren 1800) than that of MCE YS-1 and R. pusillus when milk was taken as a substrate. On the contrary, there was significantly higher clotting activity of MCE YS-1 than that of calf chymosin and MCE from R. pusillus when yak milk was taken as a substrate. It is suggested that MCE YS-1 is more suitable for clotting yak milk than the traditional calf chymosin and fungal MCE.
In summary, the purified MCE YS-1 presented high proteolytic activity to β-casein and yak milk casein and led to a 75% more rapid coagulation of yak milk. Therefore, it can be concluded that MCE YS-1 is specific for yak milk casein. The substrate specificity toward yak milk has made MCE YS-1 from B. subtilis YB-3 a good choice for the yak cheese industry. However, the reasons for the distinct substrate preferences should be investigated in our further research.
Conclusion
A novel extracellular MCE YS-1 from B. subtilis YB-3 strain, isolated from Qinghai-Tibet Plateau, was purified to homogeneity and characterized. The enzymatic properties of the MCE YS-1 suggested that it is useful for applications in dairy industry. The key advantages of the purified enzyme are the high yak milk specificity, high optimal temperature (up to 70 °C) and pH stability (5.0–9.0). These characteristics make the MCE YS-1 from B. subtilis YB-3 the ideal choice for food industry, particularly, for yak cheese industry. This is also the first report on B. subtilis, producing a yak milk preferred MCE.
References
Walstra P (1990) On the stability of casein micelles. J Dairy Sci 73(8):1965–1979
Zhang Z, Wang C, Yao Z, Zhao J, Lu F, Yu G, Lan W, Lu Z (2011) Isolation and identification of a fungal strain QY229 producing milk-clotting enzyme. Eur Food Res Technol 232:861–866
Huang X, Chen L, Luo Y, Guo H, Ren F (2011) Purification, characterization, and milk coagulating properties of ginger proteases. J Dairy Sci 94(5):2259–2269
Bruno MA, Lazza CM, Errasti ME, Lopez LMI, Caffini NO, Pardo MF (2010) Milk clotting and proteolytic activity of an enzyme preparation from Bromelia hieronymi fruits. Food Sci Technol 43(4):695–701
Ahmed IAM, Morishima I, Babiker EE, Mori N (2009) Characterisation of partially purified milk-clotting enzyme from Solanum dubium Fresen seeds. Food Chem 116(2):395–400
Dutt K, Meghwanshi GK, Gupta P, Saxena RK (2008) Role of casein on induction and enhancement of production of a bacterial milk clotting protease from an indigenously isolated Bacillus subtilis. Lett Appl Microbiol 46(5):513–518
Kumar S, Sharma N, Saharan M, Singh R (2005) Extracellular acid protease from Rhizopus oryzae: purification and characterization. Process Biochem 40(5):1701–1705
Sampaio PN, Calado CRC, Sousa L, Bressler DC, Pais MS, Fonseca LP (2010) Optimization of the culture medium composition using response surface methodology for new recombinant cyprosin B production in bioreactor for cheese production. Eur Food Res Technol 231(2):339–346
Zi X (2003) Reproduction in female yaks (Bos grunniens) and opportunities for improvement. Theriogenology 59(5–6):1303–1312
Brunner J (1981) Cow milk proteins: twenty-five years of progress. J Dairy Sci 64(6):1038
Li H, Ma Y, Dong A, Wang J, Li Q, He S, Maubois JL (2009) Protein composition of yak milk. Dairy Sci Technol 90(1):111–117
Ageitos JM, Vallejo JA, Sestelo AB, Poza M, Villa TG (2007) Purification and characterization of a milk-clotting protease from Bacillus licheniformis strain USC13. J Appl Microbiol 103(6):2205–2213
Nouani A, Belhamiche N, Slamani R, Belbraouet S, Fazouane F, Bellal M (2009) Extracellular protease from Mucor pusillus: purification and characterization. Int J Dairy Technol 62(1):112–117
Li HY, Li Y, Liu H (2010) A mutant Bacillus subtilis which can produce yak milk preferred milk-clotting enzyme. Chinese patent no. 101665779A (in China)
Troeberg L, Nagase H (2004) Zymography of metalloproteinases. In: Current protocols in protein science, chap 21, unit 15. Wiley, USA
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Arima K, IwasakI S, Tamura G (1967) Milk clotting enzyme from microorganisms. Agric Biol Chem 31(5):540–545
Sato S, Tokuda H, Koizumi T, Nakanishi K (2004) Purification and characterization of an extracellular proteinase having milk-clotting activity from Enterococcus faecalis TUA2495L. Food Sci Technol Res 10(1):44–50
Twining SS (1984) Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem 143(1):30–34
Ageitos J, Vallejo J, Poza M, Villa T (2006) Fluorescein thiocarbamoyl-kappa-casein assay for the specific testing of milk-clotting proteases. J Dairy Sci 89(10):3770–3777
Cheeseman G (1965) Denaturation of rennin; effect on activity and molecular configuration. Nature 205:1011–1012
Martinez DA, Nudel BC (2002) The improvement of lipase secretion and stability by addition of inert compounds into Acinetobacter calcoaceticus cultures. Can J Microbiol 48(12):1056–1061
Shieh C, Phan Thi L, Shih I (2009) Milk-clotting enzymes produced by culture of Bacillus subtilis natto. Biochem Eng J 43(1):85–91
Sathya R, Pradeep B, Angayarkanni J, Palaniswamy M (2009) Production of milk clotting protease by a local isolate of Mucor circinelloides under SSF using agro-industrial wastes. Bioproc Biosystems Eng 14(6):788–794
Vishwanatha KS, Appu Rao A, Singh SA (2010) Production and characterization of a milk-clotting enzyme from Aspergillus oryzae MTCC 5341. Appl Microbiol Biot 85(6):1849–1859
Zou X, Guo X, Sun M (2009) pH control strategy in a shaken minibioreactor for polysaccharide production by medicinal mushroom Phellinus linteus and its anti-hyperlipemia activity. Bioproc Biosystems Eng 32(2):277–281
Hashem A (1999) Optimization of milk-clotting enzyme productivity by Penicillium oxalicum. Bioresour Technol 70(2):203–208
Jellouli K, Ghorbel-Bellaaj O, Ayed HB, Manni L, Agrebi R, Nasri M (2011) Alkaline-protease from Bacillus licheniformis MP1: purification, characterization and potential application as a detergent additive and for shrimp waste deproteinization. Process Biochem 46(6):1248–1256
Jasmin C, Chellappan S, Sukumaran RK, Elyas K, Bhat SG, Chandrasekaran M (2010) Molecular cloning and homology modelling of a subtilisin-like serine protease from the marine fungus, Engyodontium album BTMFS10. World J Microbiol Biotechnol 26(7):1269–1279
El-Bendary MA, Moharam ME, Ali TH (2007) Purification and characterization of milk clotting enzyme produced by Bacillus sphaericus. J Appl Sci Res 3(8):695–699
Esawy MA, Combet-Blanc Y (2006) Immobilization of Bacillus licheniformis 5A1 milk-clotting enzyme and characterization of its enzyme properties. World J Microbiol Biotechnol 22(3):197–200
James PDA, Iqbal M, Edwards C, Miller PGG (1991) Extracellular protease activity in antibiotic-producing Streptomyces thermoviolaceus. Curr Microbiol 22(6):377–382
Zabolotskaya MV, Demidyuk IV, Akimkina TV, Kostrov SV (2004) A novel neutral protease from Thermoactinomyces species 27a: sequencing of the gene, purification, and characterization of the enzyme. Protein J 23(7):483–492
Ghorbel B, Sellami-Kamoun A, Nasri M (2003) Stability studies of protease from Bacillus cereus BG1. Enzyme Microb Tech 32(5):513–518
Merheb-Dini C, Gomes E, Boscolo M, da Silva R (2010) Production and characterization of a milk-clotting protease in the crude enzymatic extract from the newly isolated Thermomucor indicae-seudaticae N31:(milk-clotting protease from the newly isolated Thermomucor indicae-seudaticae N31). Food Chem 120(1):87–93
Lo Piero AR, Puglisi I, Petrone G (2011) Characterization of the purified actinidin as a plant coagulant of bovine milk. Eur Food Res Technol 233:517–524
Wang SL, Kao TY, Wang CL, Yen YH, Chern MK, Chen YH (2006) A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for [beta]-chitin preparation. Enzyme Microb Tech 39(4):724–731
Sheng Q, Li J, Alam MS, Fang X, Guo M (2008) Gross composition and nutrient profiles of Chinese yak (Maiwa) milk. Int J Food Sci Tech 43(3):568–572
Albillos SM, Busto MD, Perez-Mateos M, Ortega N (2007) Analysis by capillary electrophoresis of the proteolytic activity of a Bacillus subtilis neutral protease on bovine caseins. Int Dairy J 17(10):1195–1200
Acknowledgments
The authors wish to thank Drs K.Y. Chen and Alice Liu, Rutgers University for their valuable support and suggestions. This work was supported by Program for Agricultural Biotechnology Research and Development in Gansu (GNSW-2009-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Liang, S., Zhi, D. et al. Purification and characterization of Bacillus subtilis milk-clotting enzyme from Tibet Plateau and its potential use in yak dairy industry. Eur Food Res Technol 234, 733–741 (2012). https://doi.org/10.1007/s00217-012-1663-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1663-5