Abstract
The aim of this study was to assess the influence of different winemaking technologies on the chemical characteristics and, in particular, on the phenolic fraction of Aglianico, Montepulciano, Nero di Troia and Sangiovese wines produced in Apulia, Southern Italy. Four different winemaking technologies were compared: control (traditional, 5 days of maceration at 25 °C with three daily punching-down), prolonged maceration (10 days), addition of ellagic tannins and cryomaceration (24 h at 5 °C using dry ice), without any other oenological treatment. Results showed that the different technologies slightly influenced the phenolic fraction of Aglianico, which is known to be naturally rich of phenols. On the contrary, the prolonged maceration led to an increase of total phenols (TP) in Nero di Troia (2,592 mg/kg vs. 2,115 mg/kg of control) and a decrease in Sangiovese (869 mg/kg vs. 1,013 mg/kg); the addition of tannins led to an increase of TP in Montepulciano (1,358 mg/kg vs. 1,216 mg/kg) and to a decrease in Sangiovese (916 mg/kg vs. 1,013 mg/kg); and cryomaceration led to a decrease of anthocyanins in all cultivars (about 15%). Phenols extraction from grapes was found to be mostly dependent on the grape variety rather than on the applied winemaking technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grapes contain a large amount of different phenolic compounds in skins, pulp and seeds, which are partially extracted during winemaking [1]. These compounds, together with those deriving from the chemical reactions occurring during winemaking and ageing, play a fundamental role in some sensory properties of grapes and wines, such as colour, astringency and taste [2, 3]. Moreover, phenolic compounds show some biological properties, such as antioxidant, anti-atherosclerosis, anti-inflammatory activities, and cardio- and cancer-protective effects [4, 5]. Indeed, a moderate consumption of red wine, maximum two glasses per day, is actually recommended since it appears associated with a decreased incidence of cardiovascular diseases, due to the presence of phenolic compounds. Therefore, the increase of their concentration and intake, by improving their extraction during maceration, is of great interest. Anthocyanins are pigments responsible for the colour of red wines, which are transferred to wine during the earliest stages of maceration that are characterised by absence and/or minimum presence of ethanol. Tannins are extracted from grape skins and seeds during the alcoholic fermentation. The final colour of wine depends on the extraction of phenolics from grape pomace, which is affected by some factors such as grape variety, length and temperature of skin contact, and addition of exogenous tannins. Pérez-Lamela et al. [6] observed that the colour in Sousón, Mencía and Brancellao wines was influenced by the grape variety. In fact, the anthocyanic fingerprint of young wines obtained by classical fermentation on skins is a characteristic of each variety, although their initial evolution follows a general trend [7]. The use of prolonged maceration is a practice that increases anthocyanins, tannins and polymeric pigment concentration [8, 9]. A change in process temperature is another effective method that influences phenolics extraction, because temperature affects cell and membrane permeability in grape berries [10]. The prefermentative cold maceration, the so-called cryomaceration or cold soak, has been widely used in the production of white wines in order to enhance the extraction of aroma compounds from grapes [11, 12]. Recently, this technology has been employed in the production of red wines in order to improve the extraction of pigments, tannins and aromas from grape skins [13–16]. Dry ice (solid carbon dioxide) is the most used cryogen to lower temperature; it causes the freezing of the grape skins leading to cellular breakdown that, in turn, favours pigments release and solubilisation. Moreover, dry ice induces a thermal shock that rapidly cools down must and inhibits polyphenoloxidases enzymes and sublimes to blanket the must, protecting it from oxygen before fermentation. The formation of polymeric pigments between anthocyanins and flavanols allows the preservation of wine colour. Such anthocyanin stabilisation may be improved by the addition of exogenous tannins, deriving from grape skins and seeds and ellagic. Recently, it has been reported that the addition of grape seed tannins increases the phenolic content of Primitivo musts and wines [17], and the colour intensity and antioxidant activity of wine obtained with short time of maceration from Castelão/Tinta Miúda blend cultivars [18].
Aglianico, Montepulciano, Nero di Troia and Sangiovese, widely grown in Southern Italy, are non-aromatic red grape vines (Vitis vinifera L.) from which wines with different phenolic characteristics are produced. To the best of our knowledge, little information is available about phenolic composition of these cultivars as affected by winemaking techniques.
The aim of the present study was the assessment of the influence of four different winemaking technologies on the phenolic fraction of Aglianico, Montepulciano, Nero di Troia and Sangiovese wines produced in Apulia region, Southern Italy.
Materials and methods
Samples and vinifications
The research was conducted on September–October 2008 on grapes from two commercial vineyards located in Minervino Murge (Aglianico and Nero di Troia) and San Severo (Montepulciano and Sangiovese) territories (Apulia region, Southern Italy). Aglianico (a late-maturation cultivar) and Nero di Troia (a medium-late-maturation cultivar) vines were trained on espalier trellis, grafted on S04, planted 0.8 m apart in rows and spaced at 2.0 m (6,250 plants/ha), whereas Montepulciano and Sangiovese (medium-late-maturation cultivars) vines were trained on tendone trellis, grafted on 140 Ru and 1,130 Paulsen, respectively, planted 2.5 m apart in rows and spaced at 2.5 m (1,600 plants/ha).
For each cultivar, about 1,200 kg of grapes picked at the so-called “technological maturity” corresponding to total soluble solids value of 20–24°Brix, previously de-stemmed, was used for each of the winemaking technologies described in Table 1. All vinifications (three replicates) were performed in 100-kg-capacity thermostatic stainless steel microvinificators. A total of 4 × 4 × 3 = 48 vinifications were made in the following order (three times of each technology for each cultivar): Sangiovese, Montepulciano, Nero di Troia and Aglianico. At the end of alcoholic fermentation and after static decantation, wines were racked into dark green Bordeaux bottles without any post-treatment and stored at room temperature for about 3 months until analysed.
Chemical analyses
For each cultivar, a 300-berry sample was picked at vintage, cutting and leaving intact part of the peduncle, from different parts of bunches. For each sample, three lots consisting of 30 berries were taken and submitted to analysis (three replicates). To obtain phenolic skin extracts, berry skins were manually removed from the pulp, dried with filter paper and then macerated in 75 mL of ethanol/water/HCl solution (70/30/1, v/v; ethanol-hydrochloric acid solution) for 20 h in the dark at room temperature; finally, the extracts were filtered on filter paper and subjected to phenol analyses [19]. The pulp was pressed, and the juice was analysed for total soluble solids (TSS, °Brix), pH and titratable acidity (TA, g/L tartaric acid) according to EEC 2676 standard procedure [20]. Chemical characteristics of wines were assessed by determining ethanol (E, % v/v), pH, TA, volatile acidity (VA, g/L acetic acid), malic acid (MA, g/L), lactic acid (LA, g/L), dry reduced extract (DRE, g/L) and ashes (g/L) by means of a FOSS WineScan FT120 FT-MIR spectrometer (FOSS, Padova, Italy).
Phenol analysis
Phenol composition of skin extracts and wines and colour indices of wines were determined according to the methods reported by Di Stefano and Cravero [19] using an UV–visible spectrophotometer (Shimadzu UV 1601, Columbia, Maryland, USA). The detailed procedures for the analysis of flavonoids (F), anthocyanins (A), total polyphenols (TP), proanthocyanidins (P) and flavans reactive with vanillin (FRV) are reported by Baiano et al. [17].
HPLC–DAD anthocyanin analysis
Analysis of anthocyanin compounds was performed by high-performance liquid chromatography (HPLC) according to the protocol reported by Revilla and Ryan in 2000 [21], using an Agilent 1,200 apparatus (Agilent, Palo Alto, CA, USA) with a photodiode array detector (DAD) and an injection valve with a 20-mL loop. The samples, previously filtered on 0.45-μm Nylon membrane, were injected into a Zorbax SP C18 (100 × 4.6 mm, 1.8 μm, Agilent, Palo Alto, CA, USA) column and eluted at a flow rate of 0.5 mL/min with water-acetonitrile (95:5, v/v) (solvent A), and water-acetonitrile (50:50, v/v) (solvent B), both adjusted to pH 1.8 with perchloric acid. The gradient program of solvent A was as follows: 0 to 4.8 min from 95 to 90%, 4.8 to 16.8 min 80%, 16.8 to 21.6 min 70%, 21.6 to 31.2 min 60%, 31.2 to 40.8 min 55%, 40.8 to 48.0 min 0%, 48.0 to 58.0 min 0%, 58 to 60 min 95% and 60 to 80 min 95%. Detection was performed at 520 nm, and quantitative analysis was made according to external standard method on the basis of a calibration curve obtained by the injection of solutions at different concentration of malvidin-3-glucoside (R 2 = 0.9991). Tentative identification of anthocyanin compounds was achieved by combining elution pattern and data found by Revilla and Ryan [21]. Results were expressed as mg/kg berries for skin extracts and as mg/L for wines of malvidin-3-glucoside equivalents.
Statistical analysis
Chemical analyses were repeated three times for each sample. The analysis of variance (ANOVA) and the F test were performed by means of the Statistica 6.0 software (StatSoft, Inc. Tulsa, OK, USA) in order to evaluate the effects of cultivar and technology and their interaction. The least significant difference (LSD) post hoc multiple range test was used to compare means for main effects. Many interactions between cultivar (CV) and technology (TL) were observed, but only those regarding phenol compounds and colour indices (F, flavonoids; A, anthocyans; TP, total polyphenols; FRV, flavans reactive with vanillin; P, proanthocyanidins; CI, colour intensity; and T, tonality), and the most relevant anthocyanins assessed by HPLC (delphinidin-3-glucoside, cyanidin-3-glucoside, malvidin-3-glucoside, total acylated anthocyanins and total anthocyanins) are shown in bar graphs and discussed.
Results and discussion
Qualitative characteristics of grapes
Table 2 shows the technological ripening indices and the phenolic composition of grapes at vintage. Results were related to the different varieties, as discussed hereafter. Aglianico and Montepulciano had the highest TSS and TA contents, showing an optimal ripening status. Nero di Troia showed the lowest TA and, consequently, the highest pH, which are the characteristic of this variety at full maturation stage. The highest values of all phenol parameters were found for Aglianico, followed by Montepulciano, Nero di Troia and Sangiovese for flavonoids and anthocyans, and by Nero di Troia, Montepulciano and Sangiovese for total polyphenols, flavans reactive with vanillin and proanthocyanidins.
Anthocyanin composition of grapes at vintage determined by HPLC is reported in Table 3. As expected, the results were very different among the cultivars, confirming that the anthocyanic profile of the grapes is typical of each cultivar, even if the amount of the different anthocyanins can change as a consequence of environmental and pedoclimatic conditions and vineyard management [22–25]. In Aglianico, anthocyanins concentration was about twice than in the other cultivars. Malvidin forms accounted for about 79% of the total anthocyanins. Non-acylated forms were higher than coumarate and acetate ones. Montepulciano differentiated for the highest content of peonidin forms, estimated to be about 42% of total anthocyanins, and equals to malvidin forms, and for the presence of all acylated anthocyanins with the exception of Pt-Cm. Nero di Troia was characterised by a 58% of malvidin forms, with the prevalence of non-acylated and coumarate compounds, and by the presence of all acylated anthocyanins. The proportion of anthocyanins found in Aglianico and Nero di Troia agrees with the results reported by Lovino et al. [24] and by Tamborra and Esti [25]. Finally, Sangiovese showed a peculiar anthocyanin profile, characterised almost exclusively by non-acylated forms, in agreement with data reported in literature [26, 27].
Chemical characteristics of wines
Chemical characteristics of wines were in agreement with grapes ripening (Table 4). Aglianico showed higher values of ethanol, TA and DRE followed by Montepulciano, Nero di Troia and Sangiovese. Winemaking technologies exerted little influence on the basic characteristics of wines. As compared to control (C), prolonged maceration (PM) led to a decrease in TA and malic acid, due to a prolonged contact of the must with the pomace that induced tartaric precipitation and malolactic fermentation, and to an increase in DRE for the enhanced extraction of solids. The addition of tannins (AT) and cryomaceration (CM) exerted a minor impact with slight decrease in TA, MA and DRE. Interactions between cultivars (CV) and technology (TL) were ascertained for all chemical characteristics of wines, with the exception of pH.
Phenolic composition of wines
Phenolic composition and colour indices of wines are reported in Table 5. As expected, cultivar had a strong impact on phenolics and colour indices, which resulted to be closely related to the raw material. Aglianico and Nero di Troia showed the highest values of phenolics, confirming that these cultivars are particularly rich in phenol compounds; for this reason, they were used in the past for blending with other red wines [28, 29]. However, Nero di Troia wines were poor in A, in agreement with the raw material. Montepulciano and Sangiovese were the poorest in phenolics, in accordance with the lower phenolic content of grapes. Colour indices strongly depended on the cultivar, and the colour intensity (CI) resulted to be almost inversely correlated with the tonality (T). In particular, Aglianico showed the highest value of CI, about four times higher than Sangiovese, and the lowest value of T (less than a third than Sangiovese). These results were in accordance with the A content of the two varieties.
Winemaking techniques exerted a certain influence on the extraction of phenolic substances. As compared to the control, the PM led to a less content of F and A and to an enrichment of TP, FRV and P. The decrease in A could be explained both by different reactions that involved anthocyanins during the more prolonged step of maceration [7, 30–32] and by their adsorption on yeast cell walls [33]. No influence on phenolics was exerted by tannins addition, with the exception of FRV that increased. The application of CM caused a decrease in F and A and an increase in FRV. All technological practices caused a slight decrease in CI, but had no effect on T.
Interactions between CV and TL were found for all parameters. In particular, Aglianico showed the highest values of F, but when PM and CM were applied, F was less than Nero di Troia (Fig. 1a). Montepulciano showed higher values of A than Nero di Troia, except for PM treatment, which improved the extraction of anthocyanins from Nero di Troia grapes (Fig. 1b). PM and CM treatments led to greater amount of TP in Nero di Troia with respect to Aglianico, showing that these technologies could be used to increase the TP content in this wine (Fig. 1c). As compared to the control, the applied technological practices led to higher extraction of FRV in Nero di Troia and Montepulciano and lower FRV extraction in Aglianico and Sangiovese, respectively (Fig. 1d). Concerning P, behaviour similar to TP index was observed (Fig. 1e). PM technique led to a CI value in Montepulciano lower than that in Nero di Troia (Fig. 1f). Finally, all three treatments led to lower values of T in Nero di Troia (Fig. 1g) than in the control. On the whole, the investigated winemaking techniques when applied to Nero di Troia caused enrichment in phenols, in particular for A that plays an important role on sensory characteristics of wine, whereas when applied to Sangiovese determined impoverishment in phenolic substances, probably due to the poor phenols content of the grapes used.
Interactions between cultivar and technology on main phenol compounds of wines. F flavonoids (a); A anthocyanins (b); TP total polyphenols (c); FRV flavans reagent with vanillin (d); P proanthocyanidins (e); CI colour intensity (f); T tonality (g); Df delphinidin-3-glucoside (h); Cy cyanidin-3-glucoside (i); Mv malvidin-3-glucoside (j); TAA total acylated anthocyanins (k); TA total anthocyanins (l)
Anthocyanin profiles of wines
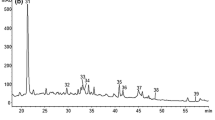
Figure 2 shows the HPLC chromatograms of Aglianico wines obtained by different technologies, whereas in Table 6 are reported the anthocyanic composition of wines as a function of cultivar and technology. As expected, Aglianico wines were characterised by total anthocyanins content 2–3 times higher than the other varieties (Table 6). As already observed for grapes, also the anthocyanic profiles of wines were found cultivar-dependent. Similar results have been reported by García-Beneytez et al. [34] in several cultivars grown in Spain. Moreover, anthocyanic composition of wines was found quite different from the corresponding grapes due to both the different chemical structures of each anthocyanin and their degradation reactions occurring during winemaking [7].
HPLC-UV chromatograms (absorbance at 520 nm) of Aglianico wines obtained by different technologies. a Control; b prolonged maceration; c addition of tannins; d cryomaceration. 1, Df; 2, Cy; 3, Pt; 4, Pn; 5, Mv; 6, Df-Ac; 7, Cy-Ac; 8, Pt-Ac; 9, Pn-Ac; 10, Mv-Ac; 11, Df-Cm; 12, Cy-Cm; 13, Pt-Cm; 14, Pn-Cm; 15, Mv-Cm
As compared to the control, the applied technologies exerted a certain impact on anthocyanin profile, as showed by the high significance found. The extension of maceration time (10 vs. 5 days) led to a decrease of about 10% of total anthocyanins, involving all anthocyanins, except Cy. This result confirms that the maximum extraction of colour during fermentation on skins occurs between the 3th and 6th days of maceration [7, 30, 32, 35]. Moreover, the 5-day extended maceration led to an increase in TP, FRV and P (Table 5), which could promote a greater polymeric pigment formation [36]. The addition of tannins led to a slight increase in most anthocyanins, especially the non-acylated (except Cy) and coumarated (except Pt-Cm and Mv-Cm) ones. Finally, the cold prefermentative maceration with dry ice led to a slight decrease in total anthocyanin, essentially ascribed to the coumarate forms.
Interaction between CV and TL was found for all anthocyanins. As compared to the control, no differences were observed in Df (unstable anthocyanin) content among Montepulciano, Nero di Troia and Sangiovese PM and AT wines (Fig. 1h). Regarding Cy (another unstable anthocyanin), PM caused an increase but only in Aglianico and Montepulciano cultivars (Fig. 1i). Conversely, PM caused a decrease in Mv, the most stable anthocyanin, in Nero di Troia and Sangiovese cultivars, whereas the adding of tannins promoted a Mv increase, but only in Aglianico (Fig. 1j). Regarding the acylated anthocyanins, compounds that are well known to disappear during wine ageing, PM and CM caused a decrease in Aglianico and Nero di Troia, especially for PM technology (Fig. 1k). Finally, total anthocyanins (Fig. 1l) showed a trend similar to Mv (Fig. 1j). These results suggest that tannins could be added during maceration in order to increase anthocyanins extraction in Aglianico, whereas the use of prolonged maceration should be avoided for Sangiovese and Nero di Troia.
Conclusions
The grape cultivars investigated in this work, Aglianico, Montepulciano, Nero di Troia and Sangiovese, showed different phenol contents and composition. The applied winemaking techniques exerted different impacts on the phenol fraction of wines: only a slight influence was observed for Aglianico due to its natural richness in phenols, whereas prolonged maceration and addition of tannins led to enrichment in total phenols of Montepulciano and Nero di Troia. As for Sangiovese, all the applied techniques led to a decrease in phenols. The results obtained in this study support the conclusion that phenol structure of wines is mostly dependent on the grape variety rather than the winemaking technology. Nevertheless, the effect of technology needs to be further investigated, since it seems to be linked to the intrinsic characteristics of grapes in terms of ripening and richness in phenols.
References
Jackson RS (1994) Wine science: principles and applications. Academic Press, New York
Ribéreau-Gayon P, Glories Y, Maujean A, Dubourdieu D (2006) Handbook of enology, vol 2. The chemistry of wine stabilization and treatments, 2nd edn. Wiley, Chichester
Kosir IJ, Lapornik B, Andrensek S, Wondra AG, Vrhovsek U, Kidri J (2004) Identification of anthocyanins in wines by liquid chromatography, liquid chromatography-mass spectrometry and nuclear magnetic resonance. Anal Chim Acta 513:277–282
Fresco P, Borges F, Diniz C, Marques MP (2006) New insights on the anticancer properties of dietary polyphenols. Med Res Rev 26:747–766
Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP (2006) A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem 39:492–497
Pérez-Lamela C, García-Falcón MS, Simal-Gándara J, Orriols-Fernández I (2007) Influence of grape variety, vine system and enological treatments on the colour stability of young red wines. Food Chem 101:601–606
González-Neves G, Gil G, Barreiro L (2008) Influence of grape variety on the extraction of anthocyanins during the fermentations on skins. Eur Food Res Technol 226:1349–1355
Gómez-Plaza E, Gil-Munõz R, López-Andreu FJ, Martínez A, Fernández-Fernández JI (2001) Phenolic compounds and color stability of red wines: Effect of skin maceration time. Am J Enol Vitic 52:266–270
Harbertson JF, Mireles MS, Harwood ED, Weller KM, Ross CF (2009) Chemical and sensory effects of saignée, water addition, and extended maceration on high brix must. Am J Enol Vitic 60:450–460
Koyama K, Goto-Yamamoto N, Hashizume K (2007) Extraction of phenolics from berry skins and seeds of grape (Vitis vinifera) during red wine maceration and influence of temperature profile. Biosci Biotechnol Biochem 71:958–965
Peinado RA, Moreno J, Bueno JE, Moreno JA, Mauricio JC (2004) Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem 84:585–590
Esti M, Tamborra P (2006) Influence of winemaking techniques on aroma precursors. Anal Chim Acta 563:173–179
Parenti A, Spugnoli P, Calamai L, Ferrari S (2004) Effects of cold maceration on red wine quality from Tuscan Sangiovese grape. Eur Food Res Technol 218:360–366
Álvarez I, Aleixandre JL, García MJ, Lizama V (2006) Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal Chim Acta 563:109–115
Gil-Munõz R, Moreno-Pérez A, Vila-López R, Fernández-Fernández JI, Martínez-Cutillas A, Gómez-Plaza E (2009) Influence of low temperature prefermentative techniques on chromatic and phenolic characteristics of Syrah and Cabernet Sauvignon wines. Eur Food Res Technol 228:777–788
Heredia FJ, Escudero-Gilete ML, Hernanz D, Gordillo B, Meléndez-Martínez AJ, Vicario IM, González-Miret ML (2010) Influence of the refrigeration technique on the colour and phenolic composition of Syrah red wines obtained by pre-fermentative cold maceration. Food Chem 118:377–383
Baiano A, Terracone C, Gambacorta G, La Notte E (2009) Phenolic content and antioxidant activity of Primitivo wine: comparison among winemaking technologies. J Food Sci 74:C258–C267
Neves AC, Spranger MI, Zhao Y, Leandro MC, Sun B (2010) Effect of addition of commercial seed tannins on phenolic composition, chromatic characteristics, and antioxidant activity of red wine. J Agric Chem 58:11775–11782
Di Stefano R, Cravero MC (2001) Methods for grape phenolic compound study. Riv Vitic Enol 2:37–45
EEC (1990) European communities. Commission regulation No 2676/90 on “Community analysis methods to use in wine sector”. Off J Eur Commun No. L272/3.10.90
Revilla E, Ryan LM (2000) Analysis of several phenolic compounds with potential antioxidant properties in grape extracts and wines by high-performance liquid chromatography-photodiode array detection without sample preparation. J Chromatogr A 881:461–469
Revilla E, Garcia-beneytez E, Cabello F, Martin-Ortega G, Ryan JM (2001) Value of high performance liquid chromatographic analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. J Chromatogr A 915:53–60
González-Neves G, Charamelo D, Balado J, Barreiro L, Bochicchio R, Gatto G, Gil G, Tessore A, Carbonneau A, Moutounet M (2004) Phenolic potential of Tannat, Cabernet-Sauvignon and Merlot grapes and their correspondence with wine composition. Anal Chim Acta 513:191–196
Lovino R, Baiano A, Pati S, Faccia M, Gambacorta G (2006) Phenolic composition of red grapes grown in Southern Italy. Ital J Food Sci 18:177–186
Tamborra P, Esti M (2010) Authenticity markers in Aglianico, Uva di Troia, Negramaro and Primitivo grapes. Anal Chim Acta 660:221–226
Mattivi F, Guzzon R, Vrhovsek U, Stefanini M, Velasco R (2006) Metabolite profiling of grape: flavonols and anthocyanins. J Agric Food Chem 54:7692–7702
La Notte E, Antonacci D, la Gatta M, Pati S, Coletta A, Gambacorta G (2008) Influence of irrigation and grape production on the phenolic and volatile fraction of black wine grape Sangiovese. Proceeding XXXIth OIV World Congress, Verona
Lovino R (1987) Survey on the chemical composition of the V.Q.P.R.D. wines mostly produced with “Uva di Troia”. Riv Vitic Enol 40:3–23
Moio L, Romano S, Cirella A, Fuschino G (1999) Influenza di alcuni fattori viticoli ed enologici sulla qualità del vino rosso prodotto dall’uva “Aglianico” di Taurasi. Vignevini 1–2:79–92
Sims C, Bates R (1994) Effects of skin fermentation time on the phenols, anthocyanins, ellagic acid sediment, and sensory characteristics of a red Vitis rotundifolia wine. Am J Enol Vitic 45:56–62
Gao L, Girard B, Mazza G, Reynolds AG (1997) Changes in anthocyanins and color characteristics of Pinot noir wines during different vinification processes. J Agric Food Chem 45:2003–2008
Gómez-Míguel M, Heredia F (2004) Effect of the maceration technique on the relationships between anthocyanin composition and objective color of Syrah wines. J Agric Food Chem 52:5117–5123
Morata A, Gómez-Cordovés MC, Suberviola J, Bartolomé B, Colombo B, Suárez J (2003) Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J Agric Food Chem 51:4084–4088
García-Beneytez E, Revilla E, Cabello F (2002) Anthocyanin pattern of several red grape cultivars and wines made from them. Eur Food Res Technol 215:32–37
Kelebek H, Canbas A, Selli S, Saucier C, Jourdes M, Glories Y (2006) Influence of different maceration times on the anthocyanin composition of wines made from Vitis vinifera L. cvs. Bogazkere and Öküzgözü. J Food Eng 77:1012–1017
Reynolds A, Cliff M, Girard B, Kopp TG (2001) Influence of fermentation temperature on composition and sensory properties of Semillon and Shiraz wines. Am J Enol Vitic 52:235–240
Acknowledgments
This work was financially supported by Ministero dell’Economia e delle Finanze, Ministero dell’Istruzione, dell’Università e della Ricerca Scientifica e Tecnologica e l’Assessorato Bilancio e Programmazione Regione Puglia by the program ‘‘Accordo di Programma Quadro in Materia di Ricerca Scientifica della Regione Puglia—Progetto Strategico—Title: ‘Improvement and valorisation of variety expression of Apulian oenological production”’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gambacorta, G., Antonacci, D., Pati, S. et al. Influence of winemaking technologies on phenolic composition of Italian red wines. Eur Food Res Technol 233, 1057–1066 (2011). https://doi.org/10.1007/s00217-011-1613-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1613-7