Abstract
Gliadin and glutenins comprising the prolamins of gluten present in wheat flour are well recognized for allergic properties. There are four kinds of symptoms of adverse reactions to wheat flour: celiac disease, Baker’s asthma, atopic dermatitis and food-dependent-exercise-induced anaphylaxis. Studies have shown that wheat flour becomes hypoallergenic by hydrolyzing peptide bonds near the essential proline residues of the epitope structure. Present study was framed with the main objective to develop modified gluten flours by various bio-processing methods and to study their rheological, biochemical and immunological characteristics. T. durum semolina was incubated with proteolytic enzymes such as pepsin, pancreatin and protease for 4, 6, 8, 10 h and overnight. Similarly, the semolina flour was treated thermally using microwave heating at different wavelength such as 360, 540, 720, 960 W. Apart from this, non-gluten blend was prepared by blending semolina with 40% of non-gluten flours. The resultant samples were subjected to different biochemical and immunochemical studies. Microstructure and Rheological characteristics are also studied. Results revealed affected microstructure of gluten matrix and rheological properties of the microwave- and enzyme-treated flour. The electrophoresis pattern of pepsin-treated flour showed decrease in the intensity of protein bands corresponding to allergen profile than control. The flour treated with protease also showed distinct bands than control. The electrophoresis pattern of non-gluten blends also showed decrease in the intensity of the bands corresponding to allergens. Immunochemical studies confirm the use of proteolytic enzymes is efficient method for reducing wheat allergens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food allergies and other food sensitivities are individualistic adverse reactions to foods [1]. Food intolerance is used to describe non-toxic, non-immune-mediated reactions, and food allergy relates to immunological reactions [2]. Adverse food reactions can include IgE and non-IgE-mediated primary immunological sensitivities, non-immunological food intolerances and secondary sensitivities. These various types of reactions are often considered collectively as food allergies. The components of food that elicit these abnormal immune responses are typically naturally occurring proteins in the foods. Studies on the prevalence of food allergy have shown that the clinical manifestations of food reactions are most commonly observed in the first 3 years of life. In prospective studies, in 80–87 situations, a child once proven reactive to a food can be shown to clinically tolerate that food by 3 years of age. Wheat allergy is most frequent in children and infants, wheat being one among six most commonly implicated allergen [3].
Food allergic reactions to wheat can give way to an array of clinical manifestations that can be immediate or delayed [3]. There are at least four kinds of symptoms of adverse reactions to wheat flour: celiac disease, Baker’s asthma, atopic dermatitis and food-dependent-exercise-induced anaphylaxis [4]. Celiac disease or gluten-sensitive enteropathy is a permanent condition of wheat of gluten intolerance in children and adults [2]. Baker’s asthma is a typical occupational allergic disease caused by the inhalation of wheat flour and is a serious problem in the food industries [5]. IgE-mediated reactions to wheat have been demonstrated as early as the beginning of the twentieth century for “Baker’s asthma” [3]. Baker’s asthma is atypical occupational allergic disease caused by the inhalation of wheat flour, which is a serious problem in the food industries.
The major grain storage proteins in wheat are alcohol-soluble prolamines. In wheat, the polyamines comprise the major components of gluten. The amino acid sequences of individual prolamins fall into three groups namely sulfur-rich (S-rich), sulfur-poor (S-poor) and high molecular weight (HMG) prolamins [6]. α-amylase inhibitor and gliadin are identified as allergens occurring in wheat. Tanabe et al. found that glutenin was allergenic for most patients allergic to wheat and also have elucidated a Gln–Gln–Gln–Pro–Pro motif as the IgE-binding epitope structure [7, 8]. Based on the above-described epitope structure, Gln–Xaa–Xaa–Pro–Pro, it was assumed that wheat flour becomes hypoallergenic by hydrolysis of peptide bonds near the essential proline residues of the epitope [4]. With the above idea, using the food-usable enzymes with high activity to hydrolyze peptide bonds near proline residues and also having less amylase activity to minimize the development of sweetness, Watanabe et al. [9] proposed a novel method for producing hypoallergenic wheat flour. Bromelain and actinase were selected for the purpose. The team also proposed methods for the preparation of different wheat-based products such as hypoallergenic bread, pasta like noodles. It is also reported that hypoallergenic flour could be prepared by washing the soluble allergenic fraction of the wheat flour [10]. Leszczynska et al. [11] studied the effect of microwave heating on the immunoreactivity of gliadin and wheat flour and found that microwave heating of food could not be applied for the elimination of allergic properties of wheat gliadins.
Pasta a traditional food product has become more popular among the consumers for its handling, cooking and storage properties. Among cereal products, pasta possesses unique nutritional features. It is considered as slowly digestible food, hence promoting low plasma glucose response. The annual per capita consumption of pasta varies among the countries around the world. Italy had the highest consumption at 28.3 kg/person/year. Ireland had the lowest at 1.0 kg/person/year, and the quantity of pasta sold in the United States was >404 million kg in 1998.
All modifications by different processing methods to wheat flour can affect the biochemical characteristics and rheological behavior of the dough, which influences the product quality. The first objective of the present work was to develop modified gluten flours with different bio-processing methods with the aim of producing pasta for wheat allergic patients. The second objective was to study the immunochemical, biochemical and rheological characteristics of the modified gluten flours.
Materials and methodology
Raw materials
Triticum durum semolina and other non-wheat flours such as maize oats and sorghum flours were procured from local market. Gliadin was procured from Sigma chemicals, USA. Molecular marker for SDS-PAGE, Anti-rabbit IgG-ALP, BCIP/NBT (5-bromo-4-chloro-3-indoyl phosphate/nitro blue tetra-Zolium) and para-Nitrophenylphosphate were procured from Bangalore Genie, India. All other chemicals used were of analytical grade.
Methodology
Enzyme treatment:
T. durum semolina was treated with 0.5% pepsin and pancreatin in combination and also with 0.5% protease (from Aspergillus orizae) according to the method of Tanabe et al. [12]. Pepsin and pancreatin (from porcine pancreas) were dissolved in 30% water and incubated at 37 °C for 2, 4, 6, 8, 10 h and overnight. Similarly, 0.5% protease was dissolved in 30% water and incubated for 2, 4, 6, 8, and 24 h. The mixture was then freezed at −20 °C and then freeze-dried. Freeze-dried samples were powdered and used for further analysis.
Thermal treatment:
With the idea that microwave heating may produce conformational and chemical changes in the gliadin structure that have important consequences on immunoreactivity of the wheat flour. Semolina samples (each 500 g) were heated using microwave at 360, 540, 720 and 900 W for 5 min, cooled and used for analysis.
Non-gluten blend:
Non-gluten blend was prepared by replacing 40% of the durum flour by other non-wheat cereal flours such as Maize, Sorghum and Oats flour.
Biochemical characterization—electrophoresis
Different flour samples treated with different methods along with T durum semolina flour were analyzed by Electrophoresis. SDS-PAGE was carried out as per the method adopted by Prabhasankar [13]. Twelve percent of acrylamide gel was used to separate the protein fractions of the modified gluten flour. Gels were stained with coomassie brilliant blue R250.
Immunochemical characterization
Production of polyclonal antibodies
Immunization of rabbit with gliadin was carried out according to the previously published reports (Prabhasankar [13]).
Dot-Blot analysis
Dot-Blot analysis was carried out according to the method described in Prabhasankar [13]. The 2.0 mg of treated flour was extracted with 70% ethanol, and 4 μL of extract (10 μg) was spotted on NCP (sigma chemicals, USA) followed by blocking with 2% gelatin in PBS-T (phosphate-buffered saline, pH 7.4 containing 0.05% Tween) followed by treating with anti-gliadin antibodies. Then, the blot was washed with PBS-T and then treated with anti-rabbit IgG-ALP conjugate. Finally, the blot was treated with BCIP/NBT substrate.
Enzyme-linked immunosorbent assay (ELISA)
Flour samples were extracted with buffer containing 6% 1 M Tris–HCl, 50% Glycerol and 20% SDS, and the allergenicity of the extract was tested by ELISA. Microtiter plates were coated with 200 μL of flour sample extracts (10 μg of protein/well) and incubated overnight at 4 °C. Sites in the wells were saturated by incubating 200 μL of 0.5% gelatin in PBS buffer containing 0.05% Tween-20 at 37 °C for 2 h. IgG, obtained from Anti sera of New Zealand white rabbit immunized with gliadin, were used at the dilution of 1:8,000 as primary antibody. This was incubated at 37 °C for 2 h followed by the incubation of Goat Anti-rabbit IgG ALP conjugate, at 37 °C for 2 h. The wells were washed between each addition with PBS buffer containing 0.05% Tween-20. Color development was carried out using substrate pNPP in diethanolamine buffer (1 mg/mL). The OD value was read at 450-nm filter using an ELISA reader.
Dough physical characterization
Rheological characteristics
Pasting characters of all the flour samples treated with different methods were studied using Brabender-visco-amylo-graph (Brabender OHG, Duisburg, Germany), following the AACC methods, and Farinograph was determined by AACC method 54-21 [14].
Microstructural characterization
The modified gluten flour samples were scanned under scanning electron microscope according to the method described in Prabhasankar et al. [15]. The samples were mounted on the specimen holder and sputter-coated with gold. Then, each sample was transferred to electron microscope (LEO 435 VP, USA) and observed under 5,000× magnification.
Results and discussions
The preparation of modified gluten flour was carried out with two different methods along with one non-gluten blend preparation. In first trial, the flour was incubated with proteolytic enzymes such as pepsin in combination with pancreatin and with protease (each 0.5%) for different duration. These enzymes are used because of their low cost and effective proteolysing activity. The resulted mixture was freeze-dried and powdered. Color of the enzyme-treated flour was not affected than control flour. Moisture content of the flour was decreased to 7.70%, whereas protein content was not affected.
In the second method, the flour was heated using microwave oven at 540, 720 and 900 W for 5 min. This was cooled and packed immediately to avoid the moisture absorption. Hence, the moisture content fell down to 4.28%. Protein content of this flour remained same as control flour.
Similarly, to prepare non-gluten blends, 40% of wheat flour was replaced by non-gluten flours like sorghum, maize and oats. The blend was mixed thoroughly to get the uniform distribution. The moisture content of the blend was 8.5%, whereas the control flour contained 9.32% of moisture. The protein content (10.03%) of the flour was not affected by the addition of gluten when compared with control (10.83%).
Rheological properties
Pasting characteristics
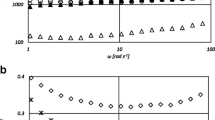
The Fig. 1 and Table 1 show the amylographic characteristics of the flours treated with different methods. The results indicated that the gelatinization temperature of the blends with the non-gluten flours decreased to 75.5 °C than the control, which has 80.9 °C. But the gelatinization temperature of the microwave-treated and enzyme-treated sample was increased to 82.6 and 85.2 °C, respectively. Non-gluten blend showed the increase in maximum viscosity 604–640 BU, hot paste viscosity from 497 to 633 BU and cold paste viscosity from 1,047 to 1,066 BU compared to control. Also, breakdown and setback values were higher than the control. This corroborates well with the findings of Indrani et al. They report the early onset of initial viscosity of wheat starch in the presence of other starch from different grains. They also reported that there was an increase in the viscosity of starch and multigrain system upon heating from 30 to 95 °C, which was caused by the amylose and low molecular weight amylopectin promoting the formation of polymer complexes. Increase in the cold paste viscosity, which represents the cooked paste after cooling, indicates a strong tendency for the retrogradation of starch molecules. Similarly, increased breakdown values represent the resistance of starch granules to thermal treatment and mechanical shearing [16]. The maximum peak viscosity of the microwave-treated flour was decreased to 370 than the control flour, indicating the considerable damage to starch with the degradation of its macromolecules, due to microwave heating. Similarly, hot paste viscosity and cold paste viscosity, breakdown and setback values were decreased than the control. The flour treated with enzyme also followed the similar trend of decrease in maximum viscosity than the control. Hot paste, cold paste setback and breakdown values also were decreased than the control, indicating the damage of starch and protein molecules by enzymatic degradation. Amylographic results suggested that modifications occurred by enzymatic and microwave treatment greatly affected pasting characteristics of wheat flour, which is one of the main rheological characteristics that influence the product quality especially cooking quality of pasta.
Farinograph characteristics
Figure 2 and Table 2 show the farinograph results of the different samples. Figure 2c shows the graph of non-gluten blends. Results indicate the increased percentage of water absorption from 61.1 to 62.6% compared with that of control flour (Fig. 2d). Similarly, the dough development time was increased from 4.5 to 8.0 min. This could be due to delay in hydration and gluten network formation due to the presence of starches and fiber particles coming from the non-gluten flours added in the blend. Indrani et al. [16] also reported the increase in water absorption and dough development time with the use of multigrains in the preparation of bread. Bahnassey and Khan also found the higher water absorption when supplemented semolina with legume flours or their protein concentrates. They also observed higher dough development time and stability for blends containing bean flours [17]. Microwave-treated sample as shown in the Fig. 3b indicates the very high water absorption of 71.9% and the dough development time of 20 min. This could be due to the denaturation of the gluten proteins by microwave heating affects the gluten network formation even at higher water absorption rate. Similarly, the Fig. 3a shows the graph of enzyme-treated flour. It showed very low water absorption of 45.2% but the dough development time of 19.9 min. This clearly shows the degradation of gluten network by enzyme treatment, that made flour very week and unable to form complete dough even after 19 min. Since gluten network was affected, water absorption rate was decreased due to its inability to hold water between gluten matrixes. It is evident from the results that the water absorption rate of dough development was much affected by microwave heating and enzymatic treatment when compared with blend of non-gluten flours.
Rheological studies suggests that pasting and farinograph characteristics were much affected by enzyme treatment and by microwave heating, which makes the flour unsuitable to use as such in the preparation of wheat-based products. Non-gluten blends showed better rheological characteristics compared to microwave- and enzyme-treated flour, suggesting its suitability in product preparation. Since aim of the study is to reduce allergenicity without affecting the product quality, study suggests that microwave-treated flour and non-gluten blends require further processing to reduce antigenic property of flour. The ELISA results confirm that use of proteolytic enzymes is very efficient method for reducing wheat allergens.
Validation of modified gluten flours
Biochemical characterization
Earlier studies by Watanabe et al. and Tanabe et al. [12] have shown that enzymes which have the ability to hydrolyze the peptide bonds near proline residues in IgE-binding epitope structure can make wheat flour hypoallergenic. They used bromelain, actinase and protease for this purpose. With this idea, we selected protease along with pepsin and pancreatin, which are low of cost and effective when used in combination. Figure 4 shows the SDS-PAGE pattern of the flours with different treatment. The lane G and H shows the protein profile of pepsin- and pancreatin-treated flour. In this pattern, bands corresponding to 43 kDa were seen when compared to control flour as shown in the lane C and other high molecular bands were not visible. This could be due to high molecular weight proteins were converted to low peptides. The present study observation was corroborated with the results of Tanabe et al. [12], where they have reported that by treating wheat flour with bromelain, most of the wheat proteins converts to low molecular peptides of nearly 20–40 kDa, which were considered to be non-allergenic peptides. The lane I shows the similar pattern of protein profile of protease-treated flour and confirms the conversion of wheat proteins to low molecular weight peptides. Watanabe et al. [18] also reported that actinase-treated product contained low molecular weight of proteinacious components, but collagenase- and transglutaminase-treated products retained high molecular weight proteins. The lane E and F shows the flour treated with microwave heating at 720 and 900 W, respectively. The protein profile was not much distinct when compared to control, showing the less efficiency of microwave heating in reducing the allergenicity. The lane D shows the low gluten flour prepared by blending 40% of non-gluten cereal flours. The protein profile showed intense bands corresponding to allergen profile than the control.
Immunochemical characterization
The Fig. 5 shows the Dot-blot pattern of all flours with different treatment. The spot B shows control flour. The spots E and F show the pepsin with pancreatin- and protease-treated flour. The colors developed in these were less intense, showing less immunoreactivity against IgG. The spot shows the non-gluten blend with less intensity of color developed, but the spot D, which is microwave-treated flour, shows the similar kind of color development compared to control flour as shown in the spot B. This supports the data of SDS-PAGE pattern confirming the less allergenicity of enzyme-treated flours. Zorzi et al. have studied the allergenicity of durum wheat proteins after in vitro digestion using pepsin and pancreatin as influenced by pasta drying temperature. In this study, immunoblotting analysis with polyclonal antibody reveals that antibody recognized the same bands in all the undigested pasta samples confirming the heat resistance of some components of the wheat prolamines. The same protein fractions were immune-detected after pepsin digestion, but after pancreatin digestion, the binding of the antibody was no longer detectable. Hence, they reported that prolamines that are recognized by the antibody although showing pepsin resistance were degraded by pancreatin to fragments with the molecular weight so low as to allow them to run off the electrophoresis gel [19]. Pasini et al. also observed similar kind of results during in vitro digestion of bread dough, crumb and crust. They observed that during pepsin treatment of bread dough, the HMW prolamins were rapidly converted into smaller number of bands with M r Values between 66 and 31 kDa, which tended to disappear after the addition of pancreatin. Similar results were observed for the bread after baking [20]. Leszczynska et al. studied the effect of microwave treatment on the immunoreactivity of gliadin by immunoblotting method. They reported that immune response decreased in comparison with the level at the maximum energy and for the energy of 90 kJ the immunoreactivity of microwave-treated gliadins fell down to the level comparable with that of the untreated [11]. The data also supported by ELISA pattern, and the Fig. 6 shows the ELISA pattern of allergens extracted from flours with different treatment. The graph reveals that flour treated with enzyme (protease) showed less immunoreactivity against IgG, which was negligible, whereas the antigenic property of flour treated with microwave heating and flour with non-gluten blends was not much significantly reduced compared to control flour. Watanabe et al. also reported less ELISA values in flour treated with collagenase and transglutaminase. And they confirmed that wheat flour can be made hypoallergenic when treated with enzymes like actinase, collagenase and transglutaminase [18]. Leszczynska et al. have also studied the immunoreactivity of microwave-treated gliadins and wheat flour by ELISA. They observed immunoreactivity at lower doses of applied energy. But at the level of 500 W, there was drop in reactivity of gliadins for 2 min and reactivity reached the level of untreated sample after 3 min exposition [11].
Results of the immunological studies (Dot-Blot, ELISA) reveals that the proteolytic enzymes such as pepsin in combination with pancreatin and protease are effective in reducing the antigenic property of wheat flour compared to microwave treated and blend with non-gluten flours. This was supported by SDS-PAGE pattern as it shows the distinct bands corresponding to allergen profile than the control flour. SDS-PAGE pattern of the blend with non-gluten flours showed decrease in the intensity of allergen profile, whereas microwave-treated flour showed not much decrease in the intensity and distinct allergen profile, requiring further processing.
Ultrastructure of hypoallergenic wheat flours
Figure 3a, d shows the images of control flour and blend with non-gluten flours. The micrographs highlight the importance of continuous protein network in the entrapment of starch and good cooking quality. Indrani et al. reported that increase in the level of incorporation of multigrain mixture in the preparation of bread interrupts the continuity of the matrix. They also observed thin protein matrix owing to the disruption of continuity because of higher level of incorporation of the multigrain mixture. Ryu studied the influence of additives on preparation of waxy barley and wheat flour bread. SEM images of bread demonstrated non-continuous, loose protein–starch matrix, where in starch granules were dispersed. But more continuous structure was observed in the dough made with barley flour along with additives like hydroxypropylmethyl cellulose [21]. Findings of Prabhasankar et al. [15] supports the data by reporting that incorporation of seaweed beyond 2.5% level disturbs the microstructure of pasta. The Fig. 3c shows the images of microwave-treated flour. The image clearly indicates the denature of gluten proteins by microwave heating when compared to control that is supported by rheological studies. Similarly, the microstructure of enzyme-treated flour (Fig. 3b) also shows the much affected gluten matrix, which explains the inability to form dough. Image of the enzyme-treated flour clearly shows the digested protein components that affected the formation of gluten network, during dough development that supports the rheological data of decreased water absorption and inability to form dough even after 19 min of mixing.
Ultrastructure of the enzyme-treated flour which showed much disturbed and digested protein matrix also showed less immune reactivity. Similarly, microwave-treated flour did not show significant reduction in the immunoreactivity than the control, and also not much affected gluten matrix in the ultrastructure study. The non-gluten blends also behaved in the similar way, which showed not much significant reduction in the immune reactivity and also not much change in the ultrastructure.
Conclusions
The present study represents an attempt to compare the effectiveness of different processing methods in reducing allergenicity of the wheat flour and their effect on the rheological and microstructure characteristics. Since the proteolytic enzyme treatment and also microwave heating affects the rheological characteristics, the flour as such is not suitable for end-product development. Further modification and addition of additives may improve the quality and makes the flour suitable for products. Further combination of thermal and enzymatic treatment may result in better hypoallergenic or low-gluten wheat flour than that of the individual methods. This also provides suitable flour to use in the preparation of wheat-based hypoallergenic or modified gluten products suitable for the consumption by wheat allergenic or celiac disease patients.
References
Taylor SL, Hefle SL (2001) Food allergies and other food sensitivities. Food Technol 55:68–83
Anderson JA (1996) Allergic reactions to food. Crit Rev Food Sci Nutr 36:S19–S38
Scibilla J, Pastorello EA, Zisa G, Ottolenghi A, Bindslev-Jensen C, Pravettoni V, Scovena E, Robino A, Ostolani C (2006) Wheat allergy: a double blind, placebo-controlled in study in adults. J Allergy Clin Immunol 117:433–439
Tanabe S (2008) Analysis of food allergen structures and development of food for allergenic patients. Biosci Biotechnol Biochem 72:649–659
Amano M, Ogawa H, Kojima K, Kamidaisa T, Suetsugu S, Yoshihama M, Satoh T, Samejima T, Matsumotto I (1998) Identification of the major allergens in wheat flour responsible for responsible for Baker’s asthma. Biochem J 330:1229–1234
Tatham AS, Shewry PR (1995) Mini review: The S-poor prolamins of wheat, barley and rye. J Cereal Sci 22:1–16
Tanabe S, Watanabe J, Oyama K, Fukushi E, Kawabata J, Arai S, Nakajima T, Watanabe M (2000) Isolation and characterization of a novel polysaccharide. As a possible allergen occurring in wheat flour. Biosci Biotechnol Biochem 64:1675–1680
Sturgess R, Day P, Ellis HJ, Luadin KEA, Gjertsen H, Kontakou M, Ciclitira PJ (1994) Wheat peptide challenge in celiac disease. The Lancet 343(8900):758–761
Watanabe M, Watanabe J, Sonoyama K, Tanabe S (2000) Novel method for producing hypoallergenic wheat flour by enzymatic fragmentation of the constituent allergens and its application in food processing. Biosci Biotechnol Biochem 64:2663–2667
Tanabe S, Watanabe M (1999) Production of hypoallergenic wheat flour. Food Sci Technol Res 5:311–322
Leszcznska J, Lacka A, Szemraj J, Lukamowicz J, Zegota H (2003) The effect of microwave treatment on the immunoreactivity of gliadin and wheat flour. Eur Food Res Technol 217:387–391
Tanabe S, Arai S, Watanabe M (1996) Modification of wheat flour with bromelain and baking hypoallergenic bread with added ingredients. Biosci Biotechnol Biochem 60:1269–1272
Prabhasankar P (2002) Electrophoretic and immunochemical characterization of wheat protein fractions and their relationship to chapathy making quality. Food Chem 78:81–87
AACC (2000) Approved Methods of the AACC, 10th edn. American Association of Cereal Chemists, St. Paul
Prabhasankar P, Ganeshan P, Baskar N (2009) Influence of Indian brown seaweed (Sargassum marginatum) as an ingredient on quality, bio-functional and microstructure characteristics of pasta. Food Sci Technol Int 15:0471–0479
Indrani D, Soumya C, Rajiv J, Rao GV (2010) Multigrain bread—its dough rheology, microstructure, quality and nutritional characteristics. J Texture Stud 41:302–319
Bahnassey Y, Khan K (1986) Fortification of spaghetti with edible legumes II rheological processing and quality evaluation studies. Cereal Chem 63:216–219
Watanabe M, Suzuki T, Ikezawa Z, Arai S (1994) Controlled enzymatic treatment of wheat proteins for production of hypoallergenic flour. Biosci Biotech Biochem 58:388–390
Zorzi MD, Curioni A, Simonato B, Giannattario M, Pasini G (2007) Effect of pasta drying temperature on gastrointestinal digestibility and allergenicity of durum wheat proteins. Food Chem 104:353–363
Pasini G, Simonato B, Giannattasio M, Perffo ADB, Curioni A (2001) Modifications of wheat flour proteins in vitro digestion of bread dough, crumb, and crust: an electrophoretic and immunological study. J Agric Food Chem 49(2001):2254–2261
Ryu CH (1999) Study on bread making quality within the microstructure of waxy barley wheat flour. 1. Rheological properties of dough made with waxy barley–wheat flour mixture. J Korean Soc Food Sci Nutr 28:1034–1043
Acknowledgments
The author S. S. thanks Indian Council of Medical Research (ICMR) New Delhi for the grant of senior research fellowship to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Susanna, S., Prabhasankar, P. A comparative study of different bio-processing methods for reduction in wheat flour allergens. Eur Food Res Technol 233, 999–1006 (2011). https://doi.org/10.1007/s00217-011-1589-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1589-3