Abstract
In order to develop an entirely food-grade enterocin P expression system for the food industry, the enterocin P structural gene (entP) with or without the enterocin P immunity gene (entiP) was cloned in plasmid pLEB590 under control of the lactococcal constitutive promoter P45. Introduction of the recombinant vectors in L. lactis MG1614 resulted in production of biologically active enterocin P in the supernatants of recombinant L. lactis MG1614. Moreover, coexpression of the entP and entiP genes could increase the production of enterocin P in all L. lactis MG1614 hosts. Recombinant enterocin P from L. lactis MG1614 (pLEB590-entP2) was purified by a three-step procedure involving ammonium sulfate precipitation, SP-Sepharose Fast Flow cation exchange, and hydrophobic adsorption chromatography. The purified bacteriocin protein concentration from recombinant L. lactis MG1614 (pLEB590-entP2) was 3.9-fold greater than that of E. faecium LM-2, and the final recovery of enterocin P activity from the supernatant of L. lactis MG1614 (40.2%) was dramatically improved compared with that of the native host strain (19.9%). Bacteriocin activity and Tricine-SDS–PAGE analysis revealed that purified recombinant enterocin P is biologically active and has a molecular mass corresponding to the native enterocin P from E. faecium LM-2, suggesting that the synthesis, process, and secretion of enterocin P progresses efficiently in recombinant L. lactis MG1614 hosts. The enterocin P was expressed successfully in this food-grade system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriocins are ribosomally synthesized peptides or proteins with antimicrobial activity, and considerable research interest has focused on the bacteriocins produced by lactic acid bacteria (LAB) because of their potential applications in food, pharmaceuticals, nutraceuticals, and veterinary and human medicine [1–3]. Their presence in foods would be, in general, safe for consumers because they are inactivated by pancreatic or gastric enzymes. Among bacteriocins, nisin is the most studied and is commercially available and its use as a food preservative is accepted in more than 50 countries [4]. Among the LAB, the enterococci produce a diverse and heterogenous group of bacteriocins, termed enterocins, which differ with respect to their antimicrobial activities, structures, processing, and secretion mechanisms [5–7]. The enterocins are generally active against Listeria monocytogenes and some bacteriocin-producing enterococci have been evaluated for use as biopreservatives in fermented dairy and meat products [6, 8]. However, enterococci may transfer antibiotic resistance genes and possess virulence factors leading to illness [9], thus, interest in the heterologous production and functional expression of enterocins in other microbial hosts is growing rapidly. Moreover, the production of enterocins in other hosts could lead to safer production of bacteriocins, increased bacteriocin production, and provide antimicrobial capabilities to LAB that are useful as starters and protective or probiotic cultures in food [10].
As described by Franz et al. [6], the enterocins are divided into class I (lantibiotic), class II (non-lantibiotic), class III (cyclic), and class IV (large proteins). Class II enterocins can be further sub-divided into class IIa (pediocin-like), class IIb (leaderless), and class IIc (non-pediocin-like). The absence or presence (and type) of an N-terminal extension determines the secretion mechanism of class II enterocins. Class IIa enterocins are synthesized as biologically inactive precursors or prepeptides containing an N-terminal extension. Two different N-terminal extensions have been identified within class IIa bacteriocin precursors [11]. The first one is the so-called double-glycine-type leader peptides that are cleaved off concomitantly with export across the cytoplasmic membrane by dedicated adenosine triphosphate-binding cassette transporters (ABC-transporters) and their accessory proteins [12, 13]. The second is the so-called sec-type signal peptides, which are proteolytically cleaved concomitantly with bacteriocin externalization by the general secretory pathway or sec-dependent pathway [14–16].
Several enterocins have been heterologously produced in Lactococcus lactis using the expression vectors associated with antibiotic resistance genes [12–17]; however, antibiotic resistance has become a major clinical and public health problem [1] and antibiotic resistance genes cannot be present in food-grade systems, so food-grade selective markers should be developed for enterocins expression. Compared with the antibiotic resistance selection markers, use of a nisin resistance selection marker for developing bacteriocin-resistant food-grade starters or probiotics for the food industry is preferred.
Enterocin P is a sec-dependent class IIa bacteriocin produced by E. faecium LM-2 isolated from a traditional cheese produced in Inner Mongolia, China [18], and by other E. faecium strains of diverse origin [19, 20]. It is synthesized as a 71-amino acid pre-peptide consisting of a 44-amino acid mature bacteriocin and a 27-amino acid signal peptide. The mature enterocin P shows a broad antimicrobial spectrum against L. monocytogenes and a wide range of gram-positive spoilage and foodborne pathogenic bacteria, suggesting its potential application as a natural food antimicrobial agent.
In this work, we report heterologous production and functional expression of enterocin P in L. lactis using a food-grade expression vector with the nisin immunity gene nisI as a selection marker.
Materials and methods
Bacterial strains, plasmids, and growth conditions
The LAB strains and plasmids used in this work are listed in Table 1. E. faecium LM-2, the enteriocin P producer, was previously isolated from “Byaslag”, a traditional cheese of Inner Mongolia in China [18]. It was grown in MRS broth (BD Biosciences, Shanghai, China) and incubated at 37 °C. L. monocytogenes 54002 was used as the indicator strain in the bacteriocin activity assay. Both bacteria were stored at −80 °C in MRS and Trypticase Soy Broth supplemented with 0.6% Yeast Extract (BD Biosciences, Shanghai, China), respectively, containing 15% (v/v) glycerol. L. lactis MG1614 was propagated at 30 °C in M17 broth (Oxoid) supplemented with 0.5% (wt/vol) glucose (GM17). Transformants of L. lactis were selected with 200 IU/mL nisin (Sigma, Shanghai, China). The food-grade expression vector pLEB590 was supplied by Dr. T. M. Takala, Department of Applied Chemistry and Microbiology, University of Helsinki, Finland [21].
Construction of the recombinant plasmids pLEB590-entP1 and pLEB590-entP2
Total genomic DNA from E. faecium LM-2 was isolated by using the Genomic DNA Isolation Kit (Bio-Rad Laboratories, Hercules, CA, USA) and was used as a template for PCR amplification of a 311-bp BamHI-XhoI fragment (P1) carrying the structural gene entP with its signal sequence and putative ribosome binding site (RBS) and another 529–bp BamHI-XhoI fragment carrying the structural-plus-immunity gene (P2, entP, and entPi). Oligonucleotide primers are shown in Table 2 and were obtained from Laboratory Service Division, University of Guelph. Plasmid DNA isolation was performed with a QIAprep Spin miniprep kit (Qiagen, Mississauga, Canada) or a High Pure plasmid isolation kit (Roche Molecular Biochemicals, Mississauga, Canada), as described by the manufacturer, with the addition of lysozyme (20 mg/mL). All DNA restriction enzymes were from New England BioLabs (Mississauga, Canada) or Roche Molecular Biochemicals and were used as recommended by the supplier. The PCR products were digested by BamHI/XhoI and inserted in digested pLEB590. Ligations were performed with T4 DNA ligase (Roche) and competent L. lactis MG1614 cells were obtained according to the method of Holo and Nes [22]. Ligation mixtures were used to transform electrocompetent cells with a Gene Pulser and a pulse controller apparatus (Bio-Rad Laboratories, Mississauga, Canada). Firstly, to verify the size of inserts, a part of the mini-prepared recombinant plasmid was used as template DNA for PCR amplification, and another part was doubly digested by BamHI/XhoI. And then the proper recombined plasmids with inserts were confirmed by nucleotide sequencing. Furthermore, the proper clones containing plasmids pLEB590-entP1 and pLEB590-entP2 were confirmed by a direct antimicrobial test.
Heterologous production of enterocin P in L. lactis
Recombinant L. lactis MG1614 (pLEB590-entP1) and L. lactis MG1614 (pLEB590-entP2) were inoculated (1%, v/v, respectively) into 100 mL GM17 broth and incubated at 37 °C for 48 h. Samples were taken at appropriate intervals to determine the optical density (at 600 nm) of the culture and the antimicrobial activity of the recombinant bacteriocin produced.
Purification of recombinant enterocin P
The enterocin P produced by L. lactis (pLEB590-entP2) cells was purified according to the method of Liu et al. [23] with slight modification. Briefly, supernatants from 24 h cultures grown in GM17 broth at 30 °C were subjected to precipitation with ammonium sulfate (80%, w/v). The sample was kept at 4 °C with stirring for 2 h. After centrifugation at 12,000 g for 30 min at 4 °C, the pellet and floating materials were harvested and dissolved in 50 mM sodium phosphate buffer pH 6.5 and dialyzed using a 1.0 kDa cut-off membrane against the same buffer at 4 °C overnight. This mixture was then injected into a SP-Sepharose Fast Flow cation exchange column (GE Healthcare, Mississauga, Canada) equilibrated with 50 mM of sodium phosphate buffer (pH 6.5) in AKTA FPLC system (GE Healthcare, Mississauga, Canada). The column was washed with the same buffer and the absorbed proteins were then eluted by the application of a linear salt gradient (0–1 M of NaCl) in the same buffer for 30 min. The flow rate was 1 mL/min and the absorbance was recorded at 220 and 280 nm. Fractions of 5 mL were collected and the antimicrobial activity was determined. The active fractions from the cation exchange column were further subjected to hydrophobic interaction chromatography using Octyl-Sepharose CL-4B (GE Healthcare, Mississauga, Canada) in AKTA FPLC system. The column was equilibrated with 1.7 M of (NH4)2SO4 and then eluted with a linear increasing gradient using H2O and ethanol at a flow rate of 1 mL/min. The absorbance was recorded at 280 nm and bacteriocin activity of each fraction (3 mL) was determined. Enterocin P produced by E. faecium LM-2 grown in MRS was purified by the same procedure and used as a control.
Protein electrophoresis
Expression of the target genes was confirmed by tricine-sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Tricine-SDS–PAGE) according to the method of Zhang et al. [24] with minor modification. Protein electrophoresis was performed on 10–20% precast gradient Tris–Tricine gel (Bio-Rad) in a Mini-PROTEAN electrophoresis unit (Bio-Rad). The running buffer (100 mM Tris, 100 mM Tricine, 0.1% SDS, pH 8.3) was poured into the buffer tank and 21 μL samples were loaded in wells. A low molecular mass protein marker with size ranging from 1.4 to 26.6 KDa (Bio-Rad) was used as standard. The unit was run at 100 V for 2 h and the gel was stained with the Silver Stain Plus reagent (Bio-Rad). To confirm the bacteriocin band, a bioassay was performed at the same time. The identical gel, which was run in the same unit, was fixed in 20% isopropanol–10% acetic acid in water for 30 min at room temperature and washed six times (15 min each time) with deionized water to remove SDS. And then the gel was used for direct detection of antimicrobial activity by overlaying with soft agar (0.75%) seeded with indicator strain (L. monocytogenes 54002) and incubated at 37 °C overnight.
Antimicrobial activity and protein concentration assays
Antimicrobial activity of individual colonies was examined by the stab-on-agar test, as described by Gutierrez et al. [15]. The activity of the supernatant or purified bacteriocin was assayed by agar well diffusion, as described by Mayr-Harting et al. [25]. The bacteriocin activity, expressed as AU (arbitrary unit) per milliliter, was defined as the reciprocal of the highest serial twofold dilution showing a clear zone of growth inhibition of the indicator strain. Protein concentrations were measured by using the RC DCTM protein assay kit (Bio-Rad).
Results
Construction and identification of the recombinant plasmids pLEB590-entP1 and pLEB590-entP2
Cloning of PCR fragments containing the enterocin P structural gene (entP) with or without the immunity gene (entiP) into the vector pLEB590, resulted in the plasmids pLEB590-entP1 and pLEB590-entP2. The proper recombinant plasmids were constructed, as evidenced by plasmid isolation, restriction enzyme analysis, and nucleotide sequencing. Results from an antimicrobial test showed recombinant L. lactis MG1614 cultures containing either pLEB590-entP1 or pLEB590-entP2 displayed antimicrobial activity against L. monocytogenes 54002, while the supernatant of L. lactis MG1614 (pLEB590) control strain did not display any antagonistic effect (data not shown).
Heterologous production of enterocin P in L. lactis
As shown in Fig. 1, the highest production and antagonistic activity of enterocin P in the supernatants of the recombinant L. lactis cultures were observed during the stationary phase (18–32 h), after which the concentration of enterocin P and the activity of the supernatants decreased. Furthermore, the antimicrobial activity of the supernatant from L. lactis MG1614 (pLEB590-entP2) was twofold higher than that found in the supernatant of L. lactis MG1614 (pLEB590-entP1). Thus, L. lactis MG1614 with plasmid pLEB590-entP2 was selected as the recombinant host strain in subsequent studies.
Purification of recombinant enterocin P
The results of the purification of enterocin P from E. faecium LM-2 and L. lactis MG1614 supernatants are shown in Table 3. Compared with the native strain (E. faecium LM-2), coexpression of entP and entiP from pLEB590 did not result in a dramatically increase of specific activity of enterocin P in L. lactis MG1614. However, the purified bacteriocin protein concentration from recombinant L. lactis MG1614 with pLEB590-entP2 (5.66 mg) was 3.9-fold greater than that of E. faecium LM-2 (1.45 mg), and the total activity (4.10 × 105) of purified recombinant bacteriocins was fourfold higher than that of the host strain (1.02 × 105). The final recovery of enterocin P activity was 40.2% of the initial activity in L. lactis MG1614, whereas the corresponding value for E. faecium LM-2 was 19.9%.
Tricine-SDS–PAGE
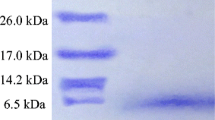
Further characterization of enterocin P from E. faecium LM-2 and L. lactis MG1614 was performed by electrophoresis and an overlay assay. Results from Tricine-SDS–PAGE showed the size of the expressed recombinant protein corresponded to the expected size of mature enterocin P (between 3.5 and 6.4 kDa). Both the crude and purified recombinant enterocin P was shown to be biologically active, which was confirmed by the inhibitory zone observed when the gel was half overlaid with an indicator strain (Fig. 2).
Tricine-SDS–PAGE of the purified recombinant enterocin P and detection of antimicrobial activity on the gel. a Silver-stained gel (1 low molecular mass protein marker with size ranging from 1.4 to 26.6 KDa; 2 crude recombinant enterocin P; 3 purified recombinant enterocin P); b The gel was overlaid with L. monocytogenes 54002 for determining antimicrobial bacteriocins (4 crude recombinant enterocin P; 5 purified recombinant enterocin P)
Discussion
Class IIa bacteriocins are considered promising antimicrobial agents for use in medicine and food preservation [11]. Heterologous expression systems for the production and secretion of class IIa bacteriocins are being developed. However, an entirely food-grade class IIa bacteriocin expression system for the food industry has not been exploited. In this study, we studied the cloning, production, and secretion of enterocin P, using a food-grade expression vector with the nisin immunity gene nisI as a selection marker and food-grade microorganism L. lactis as the production host. This is the first report on heterologous extracellular production of enterocin P in L. lactis by an entirely food-grade expression system.
In our study, all recombinant L. lactis strains carrying lactococcal vectors with the enterocin P structural gene (entP with the signal peptide) in the presence or absence of the enterocin P immunity gene (entiP) displayed extracellular inhibitory activity, as evidenced by halos of inhibition against L. monocytogenes 54002 by agar well diffusion. This suggested that presence of entP is the minimum requirement for the production of biologically active enterocin P. L. lactis strains carrying vectors containing entP gene plus the entiP gene showed greater inhibitory activity, which indicated that the coexpression of the entP and entiP genes increased the production of enterocin P in all L. lactis MG1614 hosts. This is consistent with the findings of Gutierrez et al. [15]. Similarly, Sanchez et al. [17] reported that coexpression of the hirJM79 and hiriJM79 genes increased the production of sec-dependent bacteriocin HirJM79 by all lactococcal hosts. Increased bacteriocin production may be explained by assuming that L. lactis is relatively resistant to enterocin P, but it may endure more of the bacteriocin when it expresses the entiP product. Generally, bacteriocin producers are protected from their own bacteriocin by the concomitant expression of a cognate immunity protein. These proteins act either by affecting bacteriocin aggregation and pore formation or by disturbing the interaction between the bacteriocin and the membrane-located receptor of the mannose-phosphotransferase system for class IIa bacteriocins [15, 17, 26].
The production of bacteriocins by heterologous hosts may be based on the expression of native biosynthetic genes, the exchange of leader peptides, and/or the dedicated ABC secretion and processing systems, or adding signal peptides recognized by general secretary pathways [10, 15, 16]. The higher production of enterocin P by recombinant L. lactis MG1614 strains as compared to that of native strain may be ascribed to the heterologous expression systems facilitating the transcriptional/translational control of bacteriocin gene expression [10]. These results indicate that extracellular production of enterocin P by the sec-dependent pathway is an efficient process in L. lactis and the signal peptide of enterocin P could drive the processing and secretion of bacteriocin in L. lactis. Similar observations had been reported by Gutierrez et al. [15] and Sanchez et al. [17].
As reported in this work, the production and functional expression of enterocin P in L. lactis, using the food-grade expression vector pLEB590, have been achieved. The L. lactis MG1614 (pLEB590-entP2) cells would merit consideration as an alternative experimental model for heterologous production and functional expression of enterocin P. This expression system provides a more stable enterocin P production platform, without the need for selective antibiotic pressure, and thus, may be considered safer for the production of enterocin P as a natural preservative or ingredient for the food industry. Additionally, this expression system can be used to establish new bacteriocinogenic starter cultures or probiotics for application in food processing to control both foodborne pathogens and spoilage organisms.
References
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Hancock REW, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557
Wu J, Hu S, Cao L (2007) Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob Agents Chemother 51:3131–3135
Delves-Broughton J, Blackburn P, Evans RJ, Hugenholtz J (1996) Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek 69(2):193–202
Cintas LM, Casaus P, Herranz C, Nes IF, Hernandez PE (2001) Bacteriocins of lactic acid bacteria. Food Sci Technol Int 7:281–305
Franz CM, van-Belkum MJ, Holzapfel WH, Abriouel H, Galvez A (2007) Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev 31:293–310
Sanchez J, Basanta A, Gomez-Sala B, Herranz C, Cintas LM, Hernandez PE (2007) Antimicrobial and safety aspects, and biotechnological potential of bacteriocinogenic enterococci isolated from mallard ducks (Anas platyrhynchos). Int J Food Microbiol 117:295–305
Foulquie-Moreno MR, Rea MC, Cogan TM, De-Vuyst L (2003) Applicability of a bacteriocin-producing Enterococcus faecium as a co-culture in Cheddar cheese manufacture. Int J Food Microbiol 81:73–84
Martin M, Gutierrez J, Criado R, Herranz C, Cintas LM, Hernandez PE (2006) Genes encoding bacteriocins and their expression, and potential virulence factors of enterococci isolated from wood pigeons (Columba palumbus). J Food Prot 69:520–531
Rodriguez JM, Martinez MI, Horn N, Dodd HM (2002) Heterologous production of bacteriocins by lactic acid bacteria. Int J Food Microbiol 80:101–116
Ennahar S, Sashihara T, Sonomoto K, Ishizaki A (2000) Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev 24:85–106
Horn N, Martinez MI, Martinez JM, Hernandez PE, Gasson MJ, Rodriguez JM, Dodd HM (1998) Production of pediocin PA-1 by Lactococcus lactis using the lactococcin A secretory apparatus. Appl Environ Microbiol 64:818–823
Moon GS, Pyun YR, Kim WJ (2006) Expression and purification of a fusion-typed pediocin PA-1 in Escherichia coli and recovery of biologically active pediocin PA-1. Int J Food Microbiol 108:136–140
Herranz C, Driessen AJM (2005) Sec-mediated secretion of bacteriocin enterocin P by Lactococcus lactis. Appl Environ Microbiol 71:1959–1963
Gutierrez J, Larsen R, Cintas LM, Kok J, Hernandez PE (2006) High-level heterologous production and functional expression of the sec-dependent enterocin P from Enterococcus faecium P13 in Lactococcus lactis. Appl Microbiol Biotechnol 72:41–51
Martin M, Gutierrez J, Criado R, Herranz C, Cintas LM, Hernandez PE (2007) Cloning, production and expression of the bacteriocins enterocin A produced by Enterococcus faecium PLBC21 in Lactococcus lactis. Appl Microbiol Biotechnol 76:667–675
Sanchez J, Borrero J, Gomez-Sala B, Basanta A, Herranz C, Cintas LM, Hernandez PE (2008) Cloning and heterologous production of hiracin JM79, a sec-dependent bacteriocin produced by Enterococcus hirae DCH5, in lactic acid bacteria and Pichia pastoris. Appl Environ Microbiol 74:2471–2479
Liu G, Griffiths MW, Wu P, Wang H, Zhang X, Li P (2010) Enterococcus faecium LM-2, a multi-bacteriocinogenic strain naturally occurring in “Byaslag”, a traditional cheese of inner Mongolia in China. Food Control 22:283–289
Cintas LM, Casaus P, Havarstein LS, Hernandez PE, Nes IF (1997) Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol 63:4321–4330
Moreno MRF, Leisner JJ, Tee LK, Ley C, Radu S, Rusul G, Vancanneyt M, De-Vuyst L (2002) Microbial analysis of the Malaysian tempeh, and characterization of two bacteriocins produced by isolates of Enterococcus faecium. J Appl Microbiol 92:147–157
Takala TM, Saris PE (2002) A food-grade cloning vector for lactic acid bacteria based on the nisin immunity gene nisI. Appl Microbiol Biotechnol 59:467–471
Holo H, Nes IF (1989) High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol 55:3119–3123
Liu G, Lv Y, Li P, Zhou K, Zhang J (2008) Pentocin 31–1, an anti-listeria bacteriocin produced by Lactobacillus pentosus 31–1 isolated from Xuan-Wei Ham, a traditional China fermented meat product. Food Control 19:353–359
Zhang J, Liu G, Shang N, Cheng W, Chen S (2009) Purification and partial amino acid sequence of pentocin 31–1, an anti-listeria bacteriocin produced by Lactobacillus pentosus 31–1. J Food Prot 72:2524–2529
Mayr-Harting A, Hedges AJ, Berkeley RCW (1972) Methods for studying bacteriocins. In: Norris JR, Ribbons DW (eds) Methods in microbiology. Academic Press, New York
Kjos M, Nes IF, Diep DB (2009) Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology 155:2949–2961
Acknowledgments
This research was funded by Natural Science Foundation of China (30671482) and Special Fund for Agro-scientific Research in the Public Interest of China (200903012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, G., Wang, H., Griffiths, M.W. et al. Heterologous extracellular production of enterocin P in Lactococcus lactis by a food-grade expression system. Eur Food Res Technol 233, 123–129 (2011). https://doi.org/10.1007/s00217-011-1494-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1494-9