Abstract
The aims of this study were to purify and characterize a bacteriocin produced by a strain of Enterococcus faecalis TG2 and to test the safety of the strain. In this work, the active peptide was purified through precipitation with 70% saturated ammonium sulfate, cation-exchange chromatography, and gel filtration. The specific activity of purified bacteriocin was 30,073.42 AU/mg of protein, which corresponded to a 33.34-fold increase. The molecular mass of the purified bacteriocin was 6.3362 kDa determined by LC-MS/MS. The ten amino acid of N-terminal was MTRSKKLNLR and the ten amino acid of C-terminal was ATGGAAGWKS. The activity of the bacteriocin was unaffected by pH 2–10 and thermostable but was sensitive to proteolytic enzymes. The antimicrobial activity of the bacteriocin was not affected by metal ions. Tween-20, Tween-80, Triton X-100, and EDTA did not affect the bacteriocin activity and SDS was able to increase the activity of bacteriocin. Bacteriocin activity was not lost after treatment by < 8% NaCl. Inhibitory spectrum of the bacteriocin showed a wide range of activities against other lactic acid bacteria, food-spoilage, and food-borne pathogens. Ent. faecalis TG2 was sensitive to tetracycline and erythromycin but resistant to ampicillin, gentamicin, kanamycin, and chloramphenicol. Results from PCR indicated that Ent. faecalis TG2 did not harbor any virulence genes. The study suggests that Ent. faecalis TG2 and its bacteriocin might be used as bio-preservatives in food products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mankind has been involuntarily used lactic acid bacteria (LAB) that is a diverse group of beneficial bacteria for thousands of years. The starter cultures not only provide peculiar taste to fermented products, but also serve to extend the shelf-life of the product [1]. LAB has been used as starter cultures for preparation of a large number of fermented dairy products which include yogurt, cheese, buttermilk, kefir, and many products indigenous to various regions of the world. The safety and quality of fermented products may be increased by some strains of LAB due to production of different antimicrobial substances that can inhibit the growth of pathogenic and spoilage bacteria [2]. Antimicrobial metabolites of LAB include organic acids, hydrogen peroxide, diacetyl, and also additional metabolites such as small proteins or peptides named bacteriocins. Bacteriocins are secondary metabolite products secreted to prevent the growth of strains which have a close relationship related to the bacteriocin-producing strain. Bacteriocins can also inhibit the growth of competitive bacterial strains [3, 4]. Currently, many works report that bacteriocins produced by some LAB are studied extensively as to their generally recognized as safe (GRAS) status [5,6,7]. In food preservation, more and more bacteriocins have been tested as natural antimicrobials due to their strong antibacterial properties [8, 9]. Bacteriocin can prevent the growth of many food-borne pathogens and spoilage bacteria such as Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Pseudomonas spp., Bacillus spp., and Clostridium spp. They can also be used in different food products in order to enhance their shelf life [10]. Most of bacteriocins are small, cationic (a net positive charge at neutral or slightly acidic pH) heat-stable, amphiphilic, wide antimicrobial spectrum, and varying mode of activities. Bacteriocins also show different molecular structures, molecular masses, pH ranges of activity, and genetic determinants [10,11,12,13,14].
Many works have reported that Enterococci have been found in different habitats, especially in the human and animal gastrointestinal tracts [15, 16]. Although Enterococci have been used as artisanal cultures for preparation of various types of cheeses for a long time, they are sometimes associated with pathogenicity such as endocarditis, bacteremia, and urinary tract infections [17, 18].The pathogenic strains harbor multiple antibiotic resistances and virulence factors, for example, adhesins, invasins, and hemolysin [19,20,21]. Clinical isolated strains have the highest incidence of virulence and pathogenicity, followed by the animal and food isolated strains. However, no reports have been reported that enterocins secreted by Enterococci have any risk in food preservation. Many enterocins produced by Enterococci have been reported including enterocins L50A and L50B [22], enterocin B [23], enterocin P [24], enterocin A [25], and many others [10]. However, no works have been reported that they were purified and characterized enterocins secreted by Ent. faecalis isolated from stinky tofu brine, which is a traditional Chinese fermented food.
In a previous study, bacteriocin-like antimicrobial substance produced by LAB, named TG2 isolated from stinky tofu brine and identified as Ent. faecalis. The aim of this work was to purify and characterize bacteriocin produced by Ent. faecalis TG2 and evaluate the safety of the strain, which might be used as potential natural preservatives inhibiting the growth of food-borne pathogens and food-spoilage bacteria, improving the shelf life of food.
Materials and Methods
Bacterial Strain and Cultivation
Ent. faecalis TG2 was isolated from stinky tofu brine collected from a bazzar in Changde County, Hunan Province, People’s Republic of China. This strain was preserved in Key Laboratory of Food Science, School of Chemical Engineering and Technology, Tianjin University. For the seed culture, one colony was inoculated into 200 mL/250 mL MRS liquid culture medium (20.0 g/L glucose, 10.0 g/L tryptone, 10.0 g/L beef extract, 5.0 g/L yeast extract, 2.0 g/L K2HPO4, 5.0 g/L anhydrous sodium acetate, 2.0 g/L ammonium citrate, 0.58 g/L MgSO4·7H2O, 0.25 g/L MnSO4·H2O, 1 g/L Tween-80) and incubated at 30 °C for 24 h. For purification of the bacteriocin produced by TG2, 2 mL seed liquid of strain TG2 was inoculated into 1 L MRS liquid culture medium. The incubation lasted 24 h under the conditions at 30 °C. The crude bacteriocin from TG2 could be obtained by centrifugation (4000 rpm for 30 min at 4 °C). Lactobacillus sakei DSMZ 20017T was used as indicator strain and was grown at 30 °C in MRS for 12 h.
Determination of Bacteriocin Activity by Agar Well Diffusion Assay
Determination of bacteriocin activity was tested by agar well diffusion assay. An arbitrary unit (AU) was defined as 50 μL of the highest dilution of the twofold serial dilution which showed a minimal visible inhibition zone against the sensitive cells of indicator strain [26].
Where D is the dilution factor and V is the volume of cell-free supernatants (CFS).
Purification of Bacteriocin
The CFS produced by Ent. faecalis TG2 was obtained as described above, and the bacteriocin activity in it was tested by agar well diffusion assay. The crude bacteriocin was purified to homogeneity by using 70% (w/v) saturated ammonium sulfate, cation-exchange chromatography (SP-Sepharose Fast Flow, GE, USA), and gel filtration (Superdex 30 pg, Sigma, USA) [27]. The final purified bacteriocin was subsequently used for further characterization. Tricin-SDS-PAGE was performed to confirm the homogeneity and rough molecular mass of the purified bacteriocin. The purified bacteriocin band was cut and placed on plate containing indicator strain for activity determination and sent to Gene Great Company (People’s Republic of China) for protein determination. The amino acid sequence was determined by liquid chromatograph-mass spectrometer/mass spectrometer (LC-MS/MS) (ekspertTMnanoLC; AB Sciex TripleTOF 5600-plus).
Effect of Enzymes on Antimicrobial Activity
Fifty microliters (~ 640 AU/mL) of the partially purified bacteriocin (PPB) from gel filtration was added to the 50 μL of each enzyme solution which has an ultimate concentration of 1 mg/mL and shaken well (lipase, α-amylase or catalase in 50 mmol/L potassium phosphate buffer, pH 7.0; α-chymotrypsin, pronase E, proteinase K, or trypsin in 50 mmol/L potassium phosphate buffer, pH 7.8; pepsin in 50 mmol/L glycinee HCl buffer, pH 2.2) [26]. Reaction mixtures were incubated for 4 h at 37 °C. In this step, PPB in buffer (50 mmol/L phosphate buffer, pH 7.0) without enzyme, enzyme solutions and buffers alone were used as controls. All the enzymes were purchased from Solarbio (Beijing, China). The pH of all samples was readjusted to 7.0 with sterile phosphate buffer (pH 7, 100 mmol/L). Then enzymes activity was stopped by heating at 100 °C for 10 min. After cooling, bacteriocin activity was assayed against L. sakei DSMZ 20017T by agar well diffusion assay. This step was tested in duplicate.
Effect of Temperature and pH on Antimicrobial Activity
To evaluate the effect of temperature and pH on bacteriocin activity, the experiments were performed as follows: 100 μL PPB solution (~ 2560 AU/mL) were incubated at 4, 37, and 100 °C for 0, 15, 30, 60, 90, and 120 min and at 121 °C by autoclaving for 20, 40, and 60 min. The residual bacteriocin activity was tested as described previously. PPB solution (100 μL) (~ 640 AU/mL) was added to the aliquots (4 mL) buffer solution (pH 2–12), respectively. After 4 h incubation at 37 °C, the pH was adjusted to 7.0 and bacteriocin activity was tested as described earlier. All described experiments were tested in duplicate.
Effects of Reagents, Metal Ions, and Salt on Antimicrobial Activity
PPB solution (~ 640 AU/mL) was treated with sodium dodecyl sulfate (SDS) at a final concentration of 1, 2, and 5% (w/v), Tween-20, Tween-80, and Triton X-100 at a final concentration of 1, 2, and 5% (v/v), and ethylene diamine tetra acetic acid (EDTA) at a final concentration of 1, 2, and 5 mmol/L [28]. Untreated PPB solution and surfactants were served as controls. These samples were incubated for 2 h at 37 °C, and then the activity was tested by agar well diffusion assay.
PPB solution (~ 640 AU/mL) was mixed with Fe2+, Mg2+, Zn2+, Cu2+, Ca2+, Mn2+ solution at a final concentration of 1, 2, and 5 mmol/L, respectively. Untreated PPB solution and the same solutions of cation were served as controls. After 3 h at 37 °C, the residual activity was tested as described above.
PPB (~ 640 AU/mL) solution was added to sterile microcentrifuge containing 6, 7, 8, 9, and 10% (w/v) NaCl. Untreated PPB solution and the same concentration of NaCl were served as controls. These solutions were incubated at 37 °C for 2 h, and then tested activity. All described experiments were tested in duplicate.
Antimicrobial Activity Assay
The antibacterial activity spectrum of bacteriocin was assessed against food-spoilage and food-borne pathogens bacteria by agar well diffusion assay. Soft agar of each medium was inoculated with 106 CFU/mL of each indicator strain (Table 1) and mixed well. The mixture was poured into sterile Petri dish and then left to solidify and wells of 5 mm in diameter were cut. PPB (50 μL) (2560 AU/mL) solution was placed in wells. At the optimum temperature, the inoculated mediums were incubated for 24 h and the inhibition zones were observed and recorded.
Antibiotic Sensitivity Testing
By disk diffusion antibiotic sensitivity testing, the antibiotic sensitivity of Ent. faecalis TG2 was assessed against ampicillin (≤ 4 mg/mL), gentamicin (≤ 32 mg/mL), kanamycin (≤ 512 mg/mL), tetracycline (≤ 2 mg/mL), chloramphenicol (≤ 8 mg/mL), and erythromycin (≤ 4 mg/mL). All antibiotics were dissolved with sterile distilled water, except that tetracycline was dissolved in ethanol + distilled water (1/1, v/v). On the inoculated agar plate surface, disks (6 mm) were placed and 30 μL of the antibiotic solutions were added. The plates were left for 1 h at room temperature before incubating at 37 °C for 24 h [29]. A clear zone was taken as positive. The minimum inhibitory concentration (MIC) breakpoint value that was described by the European Food Safety Authority [30] was measured with the purpose of classifying Ent. faecalis TG2 as sensitive or resistant toward those antibiotics.
Screening for Virulence Genes
In order to evaluate the safety of Ent. faecalis TG2 as bio-preservatives for controlling the pathogens in food products, the presence of virulence genes in Ent. faecalis TG2 was tested by PCR which used total DNA extracted by the commercial kit Bacterial DNA (Tiangen Biotech, Beijing, China) and specific primers were showed in Table 2. The target genes were as follows: ace (adhesin of collagen protein), asa1 (aggregation substance), cylA/B (cytolysins), efaAfs (cell wall adhesin), and esp. (enterococcal surface protein). The amplifications were performed according to a previous method [26]. The amplified fragments were analyzed by electrophoresis for 30 min at 80 V in 1.5% (w/v) agarose gel with 5 μL goldview buffer (pH 8.0) and observed under UV light. In this step, ace obtained from Ent. faecalis BEF2480, asa1 obtained from Ent. faecalis MMH594, cylA obtained from Ent. faecalis DS16, cylB obtained from Ent. faecalis DS16, esp. obtained from Ent. faecium Ec2594, and efaAfs obtained from Ent. faecalis EBHI were used as positive controls. Ent. faecalis 217 without asa1, cylA, and esp. and Pediococcus acidilactici PA003 not having ace, cylB, and efaAfs were treated as negative control strains.
Results and Discussion
Purification of the Bacteriocin Produced by Ent. faecalis TG2
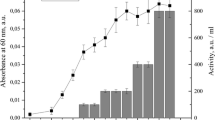
The crude extract of bacteriocin produced by Ent. faecalis TG2 was purified by three steps as shown in Table 3. Precipitation with 70% saturated ammonium sulfate was the first step, which increased the specific activity about 3.6-folds. In the second step, the active sample was purified by cation-exchange chromatography. The fraction eluted with 1 mol/L NaCl had the antimicrobial activity against the indicator strain L. sakei DSMZ 20017T. This step increased the specific activity about18-folds. The third purification was gel filtration, and the final purified bacteriocin was 30,073.42 AU/mg of protein, which corresponded to a 48.10-fold increase.
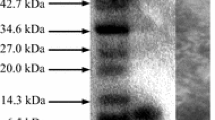
Bacteriocin band cut from gel of Tricine-SDS-PAGE (Fig. 1) appeared antimicrobial activity against L. sakei DSMZ 20017T. According to the results of Tricine-SDS-PAGE, the bacteriocin sample occurred as a single band with a molecular weight ranging about 6 kDa. From the amino acid sequence (Fig. 2), bacteriocin produced by TG2 had a precursor cleavage site after the double glycine residues and the highly conserved YGNGX motif in the N terminus, both of which are typical of class IIa bacteriocins. The ten amino acid of N-terminal was MTRSKKLNLR and the ten amino acid of C-terminal was ATGGAAGWKS. There was seem homology with other reported antimicrobial peptide when the bacteriocin was searched in the National Center for Biotechnology Information (NCBI), which had 88% identity with enterocin CRL35 and 86% identity with mundticin L precursor. The fragment was input into and analyzed with ExPASy (Expert Protein Analysis System) to predict the secondary structure of the bacteriocin, and the predicted structure was shown in Fig. 2. The α-helix was 41.0%, the β-sheet was 14.7%, and the random coil was 44.3%. The content of α-helix and β-sheet reached 55.7% and random coil remained high level. The α-helix and β-sheet might provide energy for bacteriocin stability in extreme environments. Thus, the activity of the bacteriocin was unaffected by pH 2–10 and thermostable. But antimicrobial activity of bacteriocin was lost or partially lost under extreme conditions.
The molecular weight of many enterocin is within the range of 3.5–6.5 kDa, for example, the partially purified bacteriocins produced by Ent. faecium 130 that was isolated from mozzarella cheese [34]. The molecular mass of the bacteriocin produced by TG2 is higher than other bacteriocins such as enterocin 1071A (4.284 kDa) and enterocin 1071B (3.897 kDa) produced by Ent. faecalis BFE 1071 isolated from the feces of minipigs in Gottingen [35]. Abriouel et al. [36] reported that enterocin AS-48 RJ, a variant of enterocin AS-48 produced by Ent. faecium RJ16, is isolated from goat cheese with a molecular weight of 7.125 kDa, which is higher than our bateriocin.
Effect of Enzymes on Antimicrobial Activity
The effects of enzymes were presented in Table 4. The activity of bacteriocin was totally lost by the treatment with α-chymotrypsin, trypsin, pepsin, pronase E, and proteinase K. However, catalase, lipase, and α-amylase had no effect on the antimicrobial activity. These characteristics also appear in bacteriocin produced by Enterococcus strains isolated from pig feces, which were inactivated by proteinase K, α-chymotrypsin, trypsin, pronase, papain, and pepsin, but lipase, lysozyme, and catalase had no effect on the antimicrobial activity [37]. The crude bacteriocin produced by Ent. faecium AQ71 isolated from Azerbaijani Motal cheese was inactivated by protease type X, pronase E, protease type VII, and a-chymotrypsin typeII, but not affected by catalase, lipase type II, and a-amylase [28]. Bayoub et al. [38] reported that proteinase K, pepsin, and trypsin could inactivate bacteriocins 3 Da and 3 Db produced by Ent. faecium isolated from fermented traditional Moroccan dairy product; however, catalase did affect the activity. Protease sensitivity assay indicated proteinaceous nature of bacteriocin produced by TG2.
Effect of Temperature and pH on Antimicrobial Activity
The effects of temperature and pH on antimicrobial activity were showed in Table 4. The bacteriocin activity of Ent. faecalis TG2 was stable after heating 120 min at 100 °C and at 121 °C for 20 min. The antimicrobial activity was lost 50% after autoclaving at 121 °C for 40 min. The bacteriocin produced by TG2 was thermostable after heating 60 min at 121 °C. Therefore, there is a broad application prospects in thermal processing of food. Bacteriocin activity was stable at pH 2–10 and most of activity was disappeared at pH 11. At pH 12, antimicrobial activity was completely eliminated.
Many reports reported that most of the bacteriocin produced by enterococcus strains was thermostable and had a wide range of pH. For example, partially purified bacteriocins produced by Ent. faecalis and Ent. faecium were heat stable and had the highest antimicrobial activity at pH 7 [37]. In a similar study, the crude bacteriocin produced by Ent. faecium AQ71 isolated from the Azerbijani Motal cheese was stable at pH ranging from 3 to 10 with no loss of activity after heating at 121 °C for 15 min [28].
Effects of Reagents, Metal Ions, and Salt on Antimicrobial Activity
The results were presented in Table 4. Effect of different surface active agents on antimicrobial activity demonstrated that different concentrations of Tween-20, Tween-80, Triton X-100, and EDTA had no effect on bacteriocin activity. Within this concentration range, there was no correlation between concentration and antimicrobial activity. However, bacterocin modified by different concentrations SDS had a higher antimicrobial activity. No inhibition was observed by surfactants. Some other bacteriocins showed similar but not absolutely same result. Bacteriocin activity produced by Ent. faecalis CV7 was found to increase with the addition of detergents such as Tween-20, Tween-80, and urea [39]. Different concentrations of metal cations did not change the activity of bactriocin compared with the controls. Metal cations had no effect against the L. sakei DSMZ 20017T. Effect of different concentrations NaCl on bacteriocin activity revealed that no loss of activity was observed after treatment by 6, 7, and 8% NaCl, and 50% of antimicrobial activity was lost after treatment by 9 and 10% NaCl. In a similar study, effect of different chemicals on bacteriocin activity in cell-free supernatants revealed that Triton X-20, Triton X-80, Triton X-100, β-mercaptoethanol, Na-EDTA, and SDS did not modify the inhibition of the test organism with the treated supernatant, and CFS treated with NaCl at a final concentration of 6.5% (w/v) had the antimicrobial activity [28]. These results state that the bacteriocin is resistant to surface-active compounds and salt, demonstrating a good potential to survive in the high-salt food processing.
Antimicrobial Spectrum of Bacteriocin
The antimicrobial spectrum of bacteriocin produced by TG2 (2560 AU/ml) was assessed by the agar well diffusion assay. The bacteriocin showed a wide spectrum of inhibition (Table 1). PPB could inhibit the growth of closely related species of Enterococcus and Lactobacillus and showed activity against Bacillus spp., Pediococcus pentassaceus, and Leuconostos citreum NM105. Bacteriocin produced by TG2 had a strong activity against Listeria, which could inhibit the growth of all tested Listeria spp. No activity was observed against strains of Escherichia coli, Salmonella, fungus, and other tested bacterial strains. The inhibitory spectrum displayed a wide range of antimicrobial activity against closely related strains, food-spoilage bacteria, and food-borne pathogens, indicating it might be used as a suitable biopreservative to increase the safety and extend the storage life of different food products. In a similar study, H-Kittikun et al. [26] characterized a bacteriocin produced by Ent. faecalis KT2W2G isolated from the mangrove forests in southern Thailand. They found that the most of sensitive strains were gram-positive especially all tested Listeria spp. In addition, one of gram-negative indicator bacteria S. montevideo was also inhibited. Moreover, the results obtained in this study consistently well with previous studies demonstrating that bacteriocin produced by Enterococci strains are having an antibacterial activity against closely related gram-positive bacteria, food-borne pathogens, and food-spoilage bacteria such as Enterococcus spp., L. monocytogenes, and Bacillus spp. [10, 28, 40].
Antibiotic Sensitivity of the Strain TG2
The antibiotic resistance of TG2 was described in Table 5. Ent. faecalis TG2 was resistant to ampicillin, gentamicin, kanamycin, and chloramphenicol, but sensitive to tetracycline and erythromycin. A similar profile of sensitivity to antibiotics was tested in the study of Nueno-Palop and Narbad [41] who reported that Ent. faecalis CP58 isolated from human gut was sensitive to vancomycin, tetracycline, rifampicin, and erythromycin but resistant to kanamycin and chloramphenicol. Additionally, Ahmadova et al. [28] reported that Ent. faecium AQ71 isolated from Azerbaijani Motal cheese was susceptible to ampicillin, gentamicin, tetracycline, ciprofloxacin, vancomycin, and penicillin. A different description of sensitivity to antibiotics was also studied in the study of Koo et al. [42] and Rams et al. [43].
Enterococci belong to the LAB, and they are important in foods due to their involvement in food spoilage and fermentations, as well as to their utilization as probiotics in humans and slaughter animals. However, they are also important nosocomial pathogens causing bacteremia, endocarditis, and other infections. Some of their strains are resistant to many antibiotics and possess virulence factors. The role of Enterococci in disease has raised questions of the safety of their use in foods or as probiotics [19].
Screening for the Presence of Virulence Genes
Safety of use of Ent. faecalis TG2 was also assayed by the screening for the presence of genes which could encode different virulence factors. Results from PCR amplification revealed that Ent. faecalis TG2 did not harbor any of these virulence genes (Fig. 3). In the negative control test, the bands did not found. In a similar study, Ahmadova et al. [28] characterized a bacteriocin-producing strain of Ent. faecium AQ71 isolated from Azerbaijani Motal cheese. This study reported that strain AQ71 does not harbor the genes encoding cytolysin (cylA, cylB, cylM, cylLL, cylLS), enterococcal surface protein (esp), cell wall adhesins (efaAfm), aggregation substance (asa1), and adhesion of collagen protein (ace). The absence of cytolysin coding genes (cylA and cylB) is a good characteristic for food application. Generally, cytolysin is a bacterial toxin expressed by some isolates of Ent. faecalis that display both hemolytic and bactericidal activities [3]. The absence of enterococcal surface protein (esp) gene in Ent. faecalis TG2 agrees well with results of H-Kittikun et al. [26] who detected frequent presence of the esp. gene only in medical Ent. faecium strains. However, many reports had reported that Ent. faecalis strains mostly contain virulence genes. Nueno-Palop and Narbad [41] reported probiotic assessment of Ent. faecalis CP58 isolated from human gut. The strain harbors efaAfs, gelE, agg, cpd, cob, ccf, and cad, a profile which is similar to many Ent. faecalis isolated from food sources. Ent. faecalis TG2 which does not harbor any virulence factors may maintain its application within safety framework for human and animal consumption.
PCR screening for the presence of virulence genes in Ent. faecalis TG2. Lanes 1, 3, 5, 7, 9, and 11: amplification products of corresponding genes obtained for Ent. faecalis TG2; lanes 2, 4, 6, 8, 10, and 12: amplification products of corresponding genes obtained for positive controls (Ent. faecalis BEF2480, Ent. faecalis MMH594, Ent. faecalis DS16, Ent. faecalis DS16, Ent. faecium Ec2594, and Ent. faecalis EBHI, respectively). Lane M: 100 bp and 1 kb marker
Conclusion
Results presented in this work indicated that bacteriocin produced by Ent. faecalis TG2 could be applied in thermally processed foods because the bacteriocin has activity after heating 60 min at 121 °C. Moreover, the Ent. faecalis TG2 did not possess virulence factors, so the bacteriocin produced by the strain may be used as a potential biopreservative which can inhibit food-pathogens and food-spoilage bacterias to increase the safety and extend the shelf life of food. The strain Ent. faecalis TG2 can resist to ampicillin, gentamicin, kanamycin, and chloramphenicol. However, whether the strain could be used for industrial production of food need too further study. In the future, the safety of the strain should be tested by some further appropriate experiment, such as a hemolytic experiment, before being put into use.
References
Cleveland, J., Montville, T. J., Nes, I. F., et al. (2010). Bacteriocins: safe, natural antimicrobials for food preservation. International Journal of Food Microbiology, 71, 1–20.
Drider, D., Fimland, G., Héchard, Y., et al. (2006). The continuing story of class IIa bacteriocins. Microbiology and Molecular Biology Reviews, 70, 564–582.
Hwanhlem, N., Biscola, V., El-Ghaish, S., et al. (2013). Bacteriocin-producing lactic acid bacteria isolated from mangrove forests in Southern Thailand as potential bio-control agents: purification and characterization of bacteriocin produced by Lactococcus lactis subsp. lactis KT2W2L. Probiotics and Antimicrobial Proteins, 5, 264–278.
Kjos, M., Snipen, L., Salehian, Z., et al. (2010). The Abi proteins and their involvement in bacteriocin self-immunity. Journal of Bacteriology, 192, 2068–2076.
Balciunas, E. M., Martinez, F. A. C., Todorov, S. D., et al. (2013). Novel biotechnological applications of bacteriocins: a review. Food Control, 32, 134–142.
Deegan, L. H., Cotter, P. D., Hill, C., et al. (2006). Bacteriocins: biological tools for bio-preservation and shelf-life extension. International Dairy Journal, 16, 1058–1071.
Pinto, A. L., Fernandes, M., Pinto, C., et al. (2006). Characterization of anti-Listeria bacteriocins isolated from shellfish: potential antimicrobials to control non-fermented seafood. International Journal of Food Microbiology, 129, 50–58.
Malini, M., & Janakiraman, S. (2012). Detection of heat stable bacteriocin from Lactobacillus acidophilus NCIM5426 by liquid chromatography/mass spectrometry. Indian Journal of Science and Technology, 5, 2325–2332.
Sezer, Ç., Güven, A., Oral, N. B., et al. (2013). Detoxification of aflatoxin B1 by bacteriocins and bacteriocinogenic lactic acid bacteria. Turkish Journal of Veterinary and Animal Sciences, 37, 594–601.
Javed, A., Masud, T., ul Ain, Q., et al. (2011). Enterocins of Enterococcus faecium, emerging natural food preservatives. Annals of Microbiology, 61, 699–708.
Cintas, L. M., Casaus, M., Herranz, C., et al. (2001). Bacteriocins of lactic acid bacteria. Revista de Agaroquimicay Tecnologia de Alimentos, 7, 281–305.
Nes, I. F., Diep, D. B., Håvarstein, L. S., et al. (1996). Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek, 70, 113–128.
Parada, J. L., Caron, C. R., Medeiros, A. B. P., et al. (2007). Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Brazilian Archives of Biology and Technology, 50, 512–542.
Zacharof, M., & Lovitt, R. (2012). Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia, 2, 50–56.
Khan, H., Flint, S., & Yu, P.-L. (2010). Enterocins in food preservation. International Journal of Food Microbiology, 141, 1–10.
Klibi, N., Jouini, A., Rojo-Bezares, B., et al. (2008). Phenotypic and genotypic characterization of bacteriocins in clinical enterococcal isolates of Tunisia. World Journal of Microbiology and Biotechnoloy, 24, 653.
Franz, C. M., Holzapfel, W. H., & Stiles, M. E. (1999). Enterococci at the crossroads of food safety? International Journal of Food Microbiology, 47, 1–24.
Kayser, F. (2003). Safety aspects of enterococci from the medical point of view. International Journal of Food Microbiology, 88, 255–262.
Franz, C. M., Huch, M., Abriouel, H., et al. (2001). Enterococci as probiotics and their implications in food safety. International Journal of Food Microbiology, 151, 125–140.
Hall, L., Duke, B., Urwin, G., et al. (1992). Epidemiology of Enterococcus faecalis urinary tract infection in a teaching hospital in London, United Kingdom. Journal of Clinical Microbiology, 30, 1953–1957.
Jett, B. D., Huycke, M. M., & Gilmore, M. S. (1994). Virulence of enterococci. Clinical Microbiology Reviews, 7, 462–478.
Cintas, L. M., Casaus, P., Holo, H., et al. (1998). Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. Journal of Bacteriology, 180, 1988–1994.
Casaus, P., Nilsen, T., Cintas, L. M., et al. (1997). Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology, 143, 2287–2294.
Cintas, L. M., Casaus, P., Håvarstein, L. S., et al. (1997). Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium with a broad antimicrobial spectrum. Applied and Environmental Microbiology, 63, 4321–4330.
Aymerich, T., Holo, H., Håvarstein, L. S., et al. (1996). Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Applied and Environmental Microbiology, 62, 1676–1682.
H-Kittikun, A., Biscola, V., El-Ghaish, S., et al. (2015). Bacteriocin-producing Enterococcus faecalis KT2W2G isolated from mangrove forests in southern Thailand: purification, characterization and safety evaluation. Food Control, 54, 126–134.
Ge, J. P., Du, R. P., Zhao, D., et al. (2016). Bio-chemical characterization of a β-mannanase from the Bacillus licheniformis HDYM-04 isolated from flax water-retting liquid and its decolarization ability of dyes. RSC Advances, 6, 23612–23621.
Ahmadova, A., Todorov, S. D., Choiset, Y., et al. (2013). Evaluation of antimicrobial activity, probiotic properties and safety of wild strain Enterococcus faecium AQ71 isolated from Azerbaijani Motal cheese. Food Control, 30, 631–641.
Andrews, J. M. (2001). Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 48, 5–16.
EFSA, European Food Safety Authority. (2008). Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance. EFSA Journal, 732, 1–15.
Ben Omar, N., Castro, A., Lucas, R., et al., (2004) Functional and safety aspects of enterococci isolated from different Spanish foods. Systematic and Applied Microbiology; 27: 118–130.
Vankerckhoven, V., Van Autgaerden, T., Vael, C., et al., (2004). Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. Journal of Clinical Microbiology; 42: 4473–4479.
Eaton, T. J., Gasson, M. J. (2001) Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Applied and Environmental Microbiology; 67: 1628–1635.
Tulini, F. L., Gomes, B. C., & de Martinis, E. C. P. (2011). Partial purification and characterization of a bacteriocin produced by Enterococcus faecium 130 isolated from mozzarella cheese. Food Science and Technology (Campinas), 31, 155–159.
Balla, E., Dicks, L. M., Du Toit, M., et al. (2000). Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalis BFE 1071. Applied and Environmental Microbiology, 66, 1298–1304.
Abriouel, H., Lucas, R., Omar, N. B., et al. (2005). Enterocin AS-48RJ: a variant of enterocin AS-48 chromosomally encoded by Enterococcus faecium RJ16 isolated from food. Systematic and Applied Microbiology, 28, 383–397.
Du Toit, M., Franz, C. M., Dicks, L. M., et al. (2000). Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. Journal of Applied Microbiology, 88, 482–494.
Bayoub, K., Mardad, I., Ammar, E., et al. (2011). Isolation and purification of two bacteriocins 3D produced by Enterococcus faecium with inhibitory activity against Listeria monocytogenes. Current Microbiology, 62, 479–485.
Perumal, V., & Venkatesan, A. (2017). Antimicrobial, cytotoxic effect and purification of bacteriocin from vancomycin susceptible Enterococcus faecalis and its safety evaluation for probiotization. LWT-Food Science and Technology, 78, 303–310.
Nascimento, M. d. S. d., Moreno, I., & Kuaye, A. Y. (2010). Antimicrobial activity of Enterococcus faecium FAIR-E 198 against gram-positive pathogens. Brazilian Journal of Microbiology, 41, 74–81.
Nueno-Palop, C., & Narbad, A. (2011). Probiotic assessment of Enterococcus faecalis CP58 isolated from human gut. International Journal of Food Microbiology, 45, 390–394.
Koo, M., Cho, A. R., Jeong, A. R., et al. (2013). Antibiotic susceptibility and molecular typing of Enterococcus faecalis from retail pork meat products in Korea. Journal of the Korean Society for Applied Biological Chemistry, 56, 295–299.
Rams, T. E., Feik, D., Mortensen, J. E., et al. (2013). Antibiotic susceptibility of periodontal Enterococcus faecalis. Journal of Periodontology, 84, 1026–1033.
Funding
This work was financially supported by the financial aid from the National Science-Technology Support Program of China (2015BAD16B01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xi, Q., Wang, J., Du, R. et al. Purification and Characterization of Bacteriocin Produced by a Strain of Enterococcus faecalis TG2. Appl Biochem Biotechnol 184, 1106–1119 (2018). https://doi.org/10.1007/s12010-017-2614-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2614-1