Abstract
The mechanical (tensile strength, elongation at break, mechanical work of deformation) and barrier (water vapor permeability and water vapor uptake) properties of chitosan films produced with acetic and lactic acids have been studied as a function of storage time, molecular weight of chitosans, concentration of plasticiser and the storage temperature. It was demonstrated that mechanical properties of chitosan-based films can be improved to a great extent during storage at low temperatures in freezer and refrigerator. Transition of chitosan molecules during storage in the solid state to more extended conformations and free volume changes are considered as mechanisms for the improvement of mechanical and barrier properties of chitosan films. The best mechanical properties are achieved for chitosan films produced with acetic acid and plasticized by the addition of 20% of glycerol. Sharp decrease in water vapor permeability has been demonstrated for thinner chitosan films and related to more dense packing and orientation of linear chitosan macromolecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to some reports on food losses, it has been estimated that 10–80% of the world’s harvested fruits and vegetables is lost due to spoilage and improper handling. It is determined that 2% of the food production in United States is lost; but this value in Europe is about 7–8%. Therefore, the development of active antimicrobial films for food packaging is strategic for Europe in reducing food losses and increase it’s competitiveness against United States.
Over the last decades, there has been growing needs to find alternatives to petroleum-based plastics because of environmental concerns. Plastic waste becomes a serious problem because of its low weight-to-volume ratio and its inalterability. Today, incineration is a common method to get rid of polyolefines, but this unfortunately leads to high emission of CO2. One approach for solving this problem is to use biodegradable materials instead of non-renewable polymers in packaging. These materials have the potential to reduce environmental pollution by lowering solid disposal waste and reducing the need for incineration.
Chitosan is a carbohydrate polymer derived from crustacean seafood wastes such as shells of crabs, shrimps and crawfish. Biodegradable packaging made from chitosan—renewable resource and byproduct of seafood processing—is promising for applications in food industry.
Chitosan has a potential as a packaging polymer and more particularly as an edible packaging.
Films made from chitosan are biodegradable and they have low permeability to oxygen.
At present, the above mentioned beneficial characteristics of chitosan films come at the expense of other desirable properties such as tensile strength. So the improvement of mechanical properties of biobased films, including chitosan, bringing them close to that of synthetics such as polyethylene and polypropylene is vital problem.
This polymer also has interesting bacteriostatic and fungiostatic properties. Also little is known so far about time dependent properties of chitosan films and many scientific and technical questions arise regarding storage stability of chitosan.
Materials and methods
Materials
Chitosan is actually a family of polymers with varying deacetylation levels, size and charge distribution, with different molecular weights (from 50 to 2,000 kDa) [1]. The term “chitosan” does not identify a single unique substance but rather refers to a continuum of copolymers varying in the ratio of anhydro-N-acetyl-d-glucosamine to anhydro-d-glucosamine residues, and with the common property of solubility in dilute organic acids such as acetic acid [2]. Following chitosan samples were kindly supplied by company PRIMEX:
-
Low degree of deacetylation/High molecular weight chitosan (DDA 70%, viscosity 2435cP),
-
High degree of deacetylation/Low molecular weight chitosan (DDA 97%, viscosity 29cP),
Preparation of chitosan films
Chitosan films were prepared by dissolving chitosan in aqueous solutions (2%, w/v) of acetic or lactic acids to a final concentrations 0.5 and 1.0% w/v. Acetic acid (Tachem), analytical grade, concentration 99.9% and lactic acid (Lachema), analytical grade, concentration 80% were used for preparation of chitosan films. All solutions were subsequently filtered and poured into Plexiglas mold at room temperature. The thickness of dried films was 0.010–0.020 mm (with acetic acid) and 0.045–0.060 mm (with lactic acid).
Mechanical tests
Zwick/Roell universal testing machine Z010 was used to measure tensile strength, mechanical work of deformation and elongation at break of chitosan films. Testing was performed at 22 °C and 60% relative humidity. Film strip in dimensions on 10 by 100 mm and free from air bubbles or physical imperfections, was held between two clamps positioned at a distance 2.5 cm. During measurement, the film was pulled by top clamp at a rate 50 mm/min. The force and elongation were measured when the films broke. Measurements were run five times for each film. The tensile strength and elongation at break were calculated as below:
-
Tensile strength (Mpa) = Breaking force/Cross-sectional area of the sample.
-
Elongation at break (%) = Increase in length at breaking point/Initial length × 100%.
Measurements of barrier properties of films
About 15 g of annealed at 140 °C silica gel was placed into cups (diameter-22.7 mm, height-46 mm). Chitosan films were fixed hermetically to the open ends of the cups. The cups and the control films were placed in desiccators at 98% relative humidity. This humidity was achieved due to the presence in desiccators of over saturated solution of K2SO4 in water.
The determination of water vapor penetrated through the film and absorbed by silica gel was carried out by weight change measurements (with accuracy ± 0.00005 g) as a function of time. Five specimens (cup with silica gel and film + control film) were examined for all the samples. Values of water uptake, and water vapor permeability coefficient were calculated from the results of weight measurements.
Results and discussion
The mechanical, biocide and barrier properties of chitosan-based films have been studied and reported in a number of scientific publications but there is still an evident lack of clear understanding of underlying mechanisms responsible for barrier, mechanical and biocide properties of chitosan and its derivatives [3].
And there is also an evident lack of the experimental data on the effect of storage time on the chitosan films properties, especially at low temperatures.
Experimental data show increase of tensile strength with the increase in storage time at room temperature both for high and low molecular weight chitosan films. The tensile strength is higher for chitosan films produced from high molecular weight chitosan than chitosan films produced from low molecular weight chitosan (Fig. 1).
Experimental data also show increase of water vapor permeability over storage time at room temperature both for high and low molecular weight chitosan films. The water vapor permeability is higher for chitosan films produced from high molecular weight chitosan than chitosan films produced from low molecular weight chitosan (Fig. 2).
Water vapor permeability increase with increasing the molecular mass was also found recently for chitosan membranes. Harder and tougher membranes were obtained for chitosans with higher molecular mass [4].
But experimental data show decrease of water vapor uptake with increasing storage time at room temperature both for high and low molecular weight chitosan films. The water vapor uptake is higher for chitosan films produced from high molecular weight chitosan than from low molecular weight chitosan (Fig. 3).
Tensile strength, water vapor permeability coefficient and water vapor uptake are higher for chitosan films with higher molecular weight.
Increase of tensile strength and water vapor permeability coefficient and decrease of water vapor uptake of chitosan films can be explained by conformational changes of chitosan macromolecules and decrease of free volume during storage. Though free volume of polymer decreases and consequently decreases water vapor uptake, the conformations of chitosan macromolecules change from random to more extended. Conformational changes can be responsible for the increase of tensile strength and water permeability coefficient.
Conformational changes during storage can be different for chitosan molecules in solutions and in solid state. It was observed [5] that viscosity decreased significantly during the storage of the chitosan solutions. These changes were also confirmed by dynamic light scattering measurements, which could be interpreted in terms of a decrease in the average hydrodynamic size of the chitosan particles. It was suggested that after rapid breakage of macroscopic particles of the polymer, chitosan in acidic solution is primarily in the form of rigid crystalline rod-like aggregates (bundles) which subsequently disassociate into random single polymer coils.
In general, chitosan solutions before storage also showed higher antibacterial activity than those after 15-week storage [6]. Viscosity of chitosan solutions (1% (w/v) in 1% (v/v) acetic and/or lactic acid) decreased with increased storage time and temperature. After 15-week storage, the decrease in viscosity ranged from 44 to 48% and 81 to 90% of the initial viscosity value, respectively, at 4 and 25 °C.
Our data show that reverse situation is observed in the solid state during storage of chitosan films—transition to more extended molecular conformations.
The similar conclusions can be made from the experiments described in the scientific literature and devoted to chitosan structural changes. Five crystalline polymorphs of chitosan have been found by X-ray diffraction measurements: four hydrated and one anhydrous forms, and transition from one crystalline form to another (from hydrated to anhydrous) with different fiber diffraction patterns after 1 month storage at room conditions has been demonstrated. In the anhydrous polymorph chitosan molecules take up an extended twofold helix by removing acetic acid and water molecules during the storage of the chitosan salt. This transition was accelerated by storage of the chitosan acetate at 100% relative humidity [7]. Proper control of temperature and humidity during film formation and storage is necessary to design the optimum performance of chitosan [8]. Chitosonium acetate films were seen to diminish to a large extent their biocide properties when stored at 23 °C and 75% relative humidity for 2 months or alternatively when stored and 37 °C and 0% relative humidity over the same period of time. Films formed at 37 and 80 °C presented a significant inhibitory effect for the growth of Staphylococcus aureus and Salmonella spp. bacteria; but when cast at 120 °C, the films ceased to exhibit antimicrobial properties probably because of structural changes. The thermally-induced conversion of a water-soluble chitosonium acetate in film form into a water-insoluble chitin film was demonstrated by thermal analysis and by solid state 13C-NMR spectroscopy [9]. The release of bound water and a chemical change similar to acetylation at elevated temperatures was reported for ultrathin chitosan films [10].
Chitosan is not soluble in pure water, but is soluble in aqueous solutions of organic acids, where amine groups of chitosan are protonated to NH3+. Many applications of chitosan are based on the polyelectrolytic nature of the amine groups of the macromolecule. The viscous solutions of chitosan in different acids can be used to make functional films. The properties of these films depend on the type of aqueous solvent. Tensile strengths of chitosan films produced with acetic acid are higher than tensile strengths of chitosan films produced with lactic acid and increase with the increase in storage time for both types of chitosan films (Fig. 4). This can be explained by the fact that lactic acid had hydroxyl group instead of hydrogen in the structure compared with acetic acid [11]. It can be also concluded that when the counter ion, such as lactate, was larger, the film lost its strength [12].
But water vapor permeability and water vapor uptake are higher for chitosan films produced with lactic acid (Figs. 5, 6). Water vapor permeability increases and water vapor uptake decreases for chitosan films at room temperature over the storage time.
The experimental data describe the behavior of chitosan films at room temperature and are in line with results obtained in previous studies [13, 14]. Tensile strength increased with increasing molecular weight.
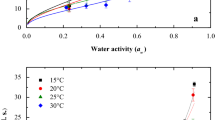
The use of glycerol as plasticizer can essentially improve mechanical properties of chitosan films, especially when content of glycerol is between 10 and 20%, Fig. 7. When a plasticizer is added, the molecular rigidity of a polymer is relieved by reducing the intermolecular forces. Plasticizer molecules interpose themselves between the individual polymer chains, thus, making it easier for the polymer chains to move past each other. The plasticizer improves flexibility and reduces brittleness of the film.
Water vapor uptake of chitosan films decreases during storage at +22 °C probably due to decrease of free volume, but increases during storage at low temperatures in freezer and refrigerator at −24 °C and at +4 °C probably due to increase of free volume and molecular conformational changes (Fig. 8).
Due to the increase of free volume during storage at lower temperatures the transition to more extended molecular conformations is possible. Conformational changes also create channels in the polymeric structure to allow the increase in water vapor permeability. Water molecules interact with cationic chitosan molecules increasing moisture sorption. This increase of water vapor solubility also leads to an increase in water vapor permeability [15].
Improvement of mechanical properties after storage at low temperatures, Fig. 9, can be explained by the transition to more extended molecular conformations.
Treatment at low temperatures affects structure and properties of chitin and chitosan. Freeze-Pump Out-Thaw cycles were proposed [16] as a new route of deacetylation of α- and β- chitins. Although chitosan is a linear polymer, its freeze-drying results in the aggregation of the chains by inter- and intrachain hydrogen bonding network resulting in the formation of particle like structure [17].
It has been recently reported [18] that squeezing very thin polymer layers can cause them to form polymer structures that could make plastic films less permeable to gases. Our data, Fig. 10 confirm this conclusion for water vapor permeability of chitosan films with the thickness less than 20 μm. Such behavior can be related to more dense packing and orientation of linear unbranched chitosan macromolecules.
Conclusions
The mechanical properties of chitosan films increase with the increase of storage time, molecular weight of chitosan, concentration of plasticizer and with the decrease of storage temperature. Water vapor permeability rate increases with the increase of storage time, molecular weight of chitosan, drying temperature and with the decrease of storage temperature. Water vapor uptake of chitosan films decreases during storage at room temperature, but increases during storage at low temperatures in freezer and refrigerator. Thinner chitosan films have lower water vapor permeability. Tensile strengths of chitosan films produced with acetic acid are higher than tensile strengths of chitosan films produced with lactic acid and increase with the increase in storage time. The mechanical properties of chitosan-based films can be improved to a great extent by storage at low temperatures in freezer or refrigerator. The best mechanical properties are achieved for chitosan films produced with acetic acid after the addition of 20% of plasticizer glycerol.
The findings in the present study, based on the tests at room and lower storage temperatures, support the earlier conclusions drawn from other available experimental data regarding the conformational changes of the chitosan molecules.
References
Illum L (1998) Pharm Res 15:1326–1331
Roberts GAF (2007) Adv Chitin Sci 10:3–10
Fernandez-Saiz P, Ocio MJ, Lagaron JM (2010) CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 5(024):1–11. http://www.cabi.org/cabreviews/default.aspx?LoadModule=Review&ReviewID=13087&site=167&page=1486
Santos C, Seabra P, Veleirinho B, Delgadillo I, Lopes da Silva JA (2006) Eur Polym J 42(12):3277–3285
Kampf N (2001) Anomalous behavior of chitosan (polyelectrolyte) solutions. http://www.mrl.ucsb.edu/mrl/events/seminars/show_seminar.php?key=1111102200Kampf
No HK, Kim SH, Lee SH, Park NY, Prinyawiwatkul W (2006) Carbohydr Polym 65(2):174–178
Yamomoto A, Kawada J, Toshifumi Y, Ogawa K (1997) Biosci Biotech Bidchem 61(7):1230–1232
Fernandez-Saiz P, Lagaron JM, Ocio MJ (2009) J Agric Food Chem 57(8):3298–3330
Toffey A, Samaranayake G, Frazier CE, Glasser WG (1996) J Appl Polym Sci 60(1):75–85
Murray CA, Dutcher JR (2006) Biomacromolecules 7(12):3460–3465
Kim KM, Son JH, Kim SK, Weller CL, Hanna MA (2006) J Food Sci E 71(3):119–124
Bégin A, Van Calsteren MR (1999) Int J Biol Macromol 26:63–67
Park SY, Marsh KS, Rhim JW (2002) J Food Sci E 67(1):194–197
Park HJ, Jung ST, Song JJ, Kang SG, Vergano PJ, Testin RF (1999) Chitin Chitosan Res 5(1):19–26
Miranda SP, Garnica O, Lara-Sagahon V, Cardenas G (2004) J Chil Chem Soc 49(2):173–178
Lamarque G, Cretenet M, Viton C, Domard A (2005) Biomacromolecules 6:1380–1388
Tharanathan RN, Kittur FS (2003) Crit Rev Food Sci Nutr 43:61–87
Lemstra PJ (2009) Science 323(5915):725–726
Acknowledgments
This research has been prepared within the framework of the ESF Project “Formation of the research group in food science” Contract Nr. 2009/0232/1DP/1.1.1.2.0/09/APIA/VIAA/122 and European Framework Programme Project CHITOFOOD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kerch, G., Korkhov, V. Effect of storage time and temperature on structure, mechanical and barrier properties of chitosan-based films. Eur Food Res Technol 232, 17–22 (2011). https://doi.org/10.1007/s00217-010-1356-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1356-x