Abstract
This research focuses on biochemical changes related to quality losses observed in Atlantic mackerel (Scomber scombrus) muscle stored under commercial frozen storage conditions (9 months, −18 °C) when subjected to high-hydrostatic pressure (HHP) treatments (125, 150, 175, and 200 MPa for 0 min) before freezing. After freezing, free fatty acid (FFA) formation (lipid hydrolysis assessment) showed a marked inhibition in HHP-treated fish and during frozen storage of samples treated at 175 MPa. Fluorescence ratio (FR) assessment of tertiary lipid oxidation showed a partial inhibitory effect during the 0–9-month period for samples treated at 175 and 200 MPa. After a 3-month storage of samples treated at these pressure levels, one-dimensional SDS-PAGE analysis of the sarcoplasmic protein fraction revealed the disappearance of a band; additionally, samples treated at 150 MPa showed the same effect at month 9. After gel excision, trypsin digestion, tandem mass spectrometry (MS/MS), and sequence database analysis, the band was identified as phosphoglycerate mutase 2 (28.7 kDa). On the other hand, HHP processing did not show a significant effect on trimethylamine (TMA) values, primary and secondary lipid oxidation, PUFA levels, 1-D myofibril protein pattern, and the activity of acid phosphatase and cathepsins B and D. Biochemical quality indices such as FFA, TMA, and FR and the activity of acid phosphatase and cathepsin B showed a progressive increase throughout the frozen storage of all samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During frozen fish storage, changes in chemical constituents may lead to marked quality losses as a result of texture, flavor, and color deterioration and the breakdown of nutritional components. Factors such as the freezing and frozen storage conditions and the quality of the raw material have been reported as responsible for such quality losses. Several hypotheses have been proposed to explain the degradation of fish proteins caused by freezing and frozen storage including partial dehydration, interaction with deteriorative molecules (oxidized lipids, free fatty acids, formaldehyde, etc.), and alteration of the microenvironment (Mackie 1993; Sikorski and Kolakowska 1994). Lipid oxidation is the most important quality loss factor in fatty fish species since the presence of highly unsaturated fatty acids and pro-oxidant molecules can lead to substantial enzymatic and non-enzymatic rancidity (Harris and Tall 1994; Sikorski and Kolakowski 2000).
High-hydrostatic pressure (HHP) processing is a non-thermal technology applied commercially in the 100–700-MPa range allowing sensory and nutritional retention of food while inactivating microbial populations and endogenous enzymes (Ashie et al. 1996; Norton and Sun 2008; Mújica-Paz et al. 2011). Although covalent bonds are not broken by HHP processing, weak hydrogen and hydrophobic bonds can be irreversibly modified. Consequently, low-molecular weight food components such as vitamins are not affected, whereas high-molecular weight molecules such as enzymes and other functional proteins can be modified (Groβ et al. 1993; Campus 2010). Previous research has shown a wide range of practical benefits when HHP is applied prior to subsequent processing or storage such as products to be further refrigerated (Chéret et al. 2006), chilled (Ortea et al. 2010), or cold-smoked stored (Lakshmanan et al. 2005). In the case of fish freezing, frozen storage, and thawing, pressure-shift technology has shown improvements in protein denaturation, water-holding capacity, and toughening (Chevalier et al. 2000; Tironi et al. 2010). Concerning frozen fish storage, recent studies have focused on the potential of HHP treatment prior to freezing. Thus, sensory, physical (Aubourg et al. 2013), chemical (Vázquez et al. 2013), and enzymatic (Fidalgo et al. 2014) changes were analyzed in Atlantic mackerel (Scomber scombrus) muscle HHP-treated (150, 300, and 450 MPa for 0, 2.5, and 5 min) prior to freezing and then stored under accelerated frozen storage conditions (−10 °C). As a result, sensory acceptance and nutritional quality retention throughout the frozen storage showed to be improved when the treatment applied was 150 MPa for 0 min.
Atlantic mackerel is a small pelagic fish species captured in large amounts during periods of relatively low demand. Previous research has shown an important endogenous pro-oxidant activity and significant quality loss during its frozen storage (Saeed and Howell 2001; Aubourg et al. 2005). Consequently, Atlantic mackerel remains underutilized reflecting mainly its poor frozen shelf-life. HHP treatment of Atlantic mackerel has been reported to inactivate Anisakis simplex larvae in raw fish (Brutti et al. 2010). Gelification conditions of mackerel muscle by HHP processing were optimized by addition of natural antioxidants (Montero et al. 2005). The effect on the physico-chemical quality parameters of refrigerated (4 °C) mackerel subjected to HHP processing (200, 300, and 400 MPa for 5 and 15 min at 5, 10, and 15 °C) was recently evaluated also (Senturk and Alpas 2013). This research focuses on the quality retention of Atlantic mackerel under commercial frozen conditions (9-month period at −18 °C). Based on a previous work (Aubourg et al. 2013), HHP treatments at 125, 150, 175, and 200 MPa for 0 min were applied prior to sample freezing. Changes in lipids (hydrolysis and oxidation), proteins (myofibril and sarcoplasmic fractions), and enzymes activity (acid phosphatase, cathepsin B, and cathepsin D) were evaluated during frozen storage of mackerel muscle.
Materials and Methods
Chemicals
Bicinchoninic acid (BCA), sodium dodecyl sulfate (SDS), dithiothreitol (DTT), Tris–HCl, and the protease inhibitor phenylmethylsulphonyl fluoride (PMSF) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Acrylamide and bis N,N′-methylene-bis-acrylamide were provided by Bio-Rad Laboratories, Inc. (Hercules, CA). Glycerol was obtained from Merck KGaA (Darmstadt, Germany). Ammonium persulfate (APS), N,N,N′,N′-tetramethylethylenediamine (TEMED) and bromophenol blue were purchased from GE Healthcare Science (Uppsala, Sweden). Sequencing grade bovine trypsin was purchased from Promega Corp. (Madison, WI). Trizma hydrochloride (Tris–HCl), acetic acid, sodium hydroxide, citric acid, 2-bis-(2-hydroxyethyl)-amino-2-(hydroxymethyl)-1,3-propanediol (Bis-Tris), ethylenediamine-tetraacetic acid (EDTA), p-nitrophenol, thymolphtalein, trichloracetic acid (TCA), trisodium citrate, and L-tyrosine were also obtained from Sigma-Aldrich Co. LLC (Steinheim, Germany). The enzymes substrates p-nitrophenylphosphate (p-NPP), p-nitrophenol (p-NP), Z-arginine-arginine-7-amido-4-methylcoumarin hydrochloride (Z-Arg-Arg-7-AMC HCl, #C5429), hemoglobin from bovine blood, and olive oil were purchased from Sigma-Aldrich Co. LLC. All other chemicals were of analytical grade (Panreac Quimica S.L.U., Barcelona, Spain) and water was purified using a Millipore Milli-Q system (Billerica, MA).
Raw Fish, Processing, Frozen Storage, and Sampling
The response to HHP treatments of marine species has been reported to vary with species, composition, and size at capture (Ortea et al. 2010; Campus 2010). Previous research on HHP-treated Atlantic mackerel stored under accelerated storage conditions (−10 °C) had shown that 150 MPa for 0 min yielded the highest sensory scores (Aubourg et al. 2013), while lowering lipid damage development (Vázquez et al. 2013) and enzyme activity (Fidalgo et al. 2014). Accordingly, this pressure level and a lower (125 MPa) and two higher (175 and 200 MPa) values were included in this study. Atlantic mackerel (156 individuals) caught near the Bask coast in Northern Spain was obtained at the Ondarroa harbor (Bizkaia, Spain) and transported under ice to the AZTI Tecnalia (Derio, Spain) pilot plant for HHP treatment within 6 h after catch. Whole mackerel individuals were placed in flexible polyethylene bags (three individuals per bag) and vacuum-sealed at 400 mbar. The length and weight of the specimens ranged 30–34 cm and 245–295 g, respectively. Fish individuals were randomly distributed. Samples were treated in a 55-L high-pressure unit (WAVE 6000/55HT; NC Hyperbaric, Burgos, Spain) at 125, 150, 175, and 200 MPa and 0 min holding time. Water used to pressurize samples at a rate of 3 MPa/s yielded 41.7, 50, 58.3 and 66.7 s as the corresponding come up times while decompression time was less than 3 s in all cases. Cold pressurizing water was used to maintain temperature conditions during HHP treatment at room temperature (20 °C). After HHP treatment, mackerel (120 individuals) were kept at −18 °C and analyzed after 48 h (month 0) and after storage for 1, 3, 6 and 9 months at −18 °C. Fish without HHP treatment and subjected to the same freezing and frozen storage conditions (30 individuals) were used as 0.1-MPa controls. Three batches or replicates (n = 3) for each processing condition were analyzed independently. Analyses were carried out on the fish white muscle pooled from two individual fishes. Similarly, six initial individuals were distributed into three batches (two individuals per batch) and analyzed as initial fish material.
Assessment of Lipid and Trimethylamine Contents
Lipids in fish muscle were extracted with a chloroform-methanol (1:1) mixture following the Bligh and Dyer (1959) method and expressed as g lipid/100 g muscle. Trimethylamine-nitrogen (TMA-N) values expressed as mg TMA-N/100 g muscle were obtained following the picrate method (Tozawa et al. 1971) requiring the preparation of a 5 % (w/v) trichloroacetic acid extract of the mackerel muscle.
Lipid Damage Analysis
Free fatty acids (FFA) in lipid extracts, determined by the Lowry and Tinsley (1976) method, were expressed as milligram FFA/100 g muscle and as gram FFA/100 g lipids. Peroxide values (PVs in lipid extracts, determined following the Chapman and McKay (1949) method, were expressed as milliequivalent active oxygen/kilogram lipids. The thiobarbituric acid index (TBA-i) was determined as described by Vyncke (1970). Content of TBA reactive substances (TBARS) spectrophotometrically measured at 532 nm was expressed as milligram malondialdehyde/kilogram muscle. Formation of fluorescent compounds was determined in the aqueous phase obtained during the lipid extraction by measurements at 393/463 and 327/415 nm (Aubourg 1999). A relative fluorescence (RF) was defined as the F/Fst ratio where F is the fluorescence measured at each excitation and emission maximum and Fst is the fluorescence intensity of a quinine sulfate solution (1 μg/mL in 0.05 M H2SO4) at the corresponding wavelength. To quantify the formation of fluorescent compounds, a fluorescence ratio (FR) was calculated as the ratio between the two RF values: FR = RF393/463 nm/RF327/415 nm. Lipid extracts were converted into fatty acid methyl esters (FAME) and then analyzed using a Perkin-Elmer 8700 gas chromatograph (Waltham, MA) equipped with a fused silica capillary column SP-2330 (0.25 mm i.d. ×30 m, 0.20 μm film, Supelco Inc., Bellefonte, PA) and using nitrogen at 10 psi as carrier gas (linear flow rate of 1.0 mL/min), a flame ionization detector (FID) set at 250 °C, and 19:0 fatty acid as internal standard (Aubourg et al. 1996). Peaks corresponding to fatty acids were identified by comparison of the retention times of two standards mixtures (Qualmix Fish, Larodan, Malmo, Sweden; FAME Mix, Supelco Inc.). Content of each fatty acid was calculated as grams/100 g total fatty acids. The polyene index (PI) was calculated as the following fatty acids ratio: (C 20:5ω3 + C 22:6ω3)/C 16:0.
Protein Changes: Analysis and Identification
Sarcoplasmic (low-salt-soluble) and myofibril (high-salt-soluble) protein extractions from the mackerel muscle were performed by modification of the protocol developed by Pazos et al. (2011). Briefly, 0.5 g muscles were homogenized using an Ultra-Turrax high-performance disperser in ten volumes of Tris buffer (10 mM Tris–HCl, pH 7.2), containing 5 mM protease inhibitor (PMFS). The sarcoplasmic protein fraction was obtained from the supernatant after centrifugation (IKA®-Werke GmbH & Co., Staufen, Germany) of the homogenate at 40,000g (4 °C, 20 min). The pellet was then homogenized with a saline solution (0.6 M NaCl, 10 mM Tris buffer, 5 mM PMFS, pH 7.2), and myofibril proteins were isolated from the supernatant obtained after centrifugation at 4500g (4 °C, 20 min). Sarcoplasmic and myofibril proteins were stored at −80 °C until use. Protein concentrations were determined in both fractions by the BCA assay (Smith et al. 1985) and expressed as grams/100 g muscle.
One-dimensional (1-D) laboratory-made 10 % polyacrylamide gels (v/v; acrylamide/N,N′-ethylene-bis-acrylamide, 200:1) with an upper stacking gel (4 % polyacrylamide) were loaded with 20 or 30 mg of protein per lane. 1-D gels were run in a Mini-PROTEAN 3 cell (Bio-Rad) with an aqueous running buffer containing 1.44 % (w/v) glycine, 0.67 % Tris-base, and 0.1 % SDS (Laemmli 1970). Gels were stained overnight with the Coomassie dye PhastGel Blue R-350 (GE Healthcare). Protein bands of interest were manually excised from 1-D gels and in-gel digested with trypsin as previously described (Pazos et al. 2014). Tryptic peptides were analyzed by liquid chromatography (LC) (Model 1260, Agilent, Palo Alto, CA) coupled to a linear ion trap (LIT) mass spectrometer model LTQ Velos Pro with electrospray interface (Thermo Fisher Scientific Inc., Rockford, IL). Nitrogen was used as nebulizing and drying gas and helium as collision gas. The chromatographic separation was performed on a BioBasic-18 column (5 μm particle size, 150 × 0.18 mm RP; Thermo Scientific, Waltham, MA) at a flow rate of 1.5–1.7 μL/min with a 90-min linear gradient of mobile phases A (0.5 % acetic acid in water) and B (0.5 % acetic acid in 100 % acetonitrile) from 5 to 35 % B. Peptides were monitored using MS survey scans from 350 to 1600 Da (2 μscans), followed by MS/MS scans (2 μscans) of the six more intense m/z peaks using a 1-Da isolation width and a normalized collision energy of 35 %. Singly charged ions were directly excluded from MS/MS analysis, and a dynamic exclusion was set at 30 s after the second fragmentation event of the same m/z peak. Protein identification was performed using the database searching function (PEAKS 7, Bioinformatics Solutions Inc., Waterloo, Ontario, Canada), to compare experimental MS/MS spectra with those on the UniProtKB/Swiss-Prot database, which included their respective decoy sequences. The following search limitations were used: tryptic cleavage, up to two missed cleavage sites, and tolerances ±1.2 Da for precursor ions and ±0.8 Da for MS/MS fragments ions. The variable modifications allowed were cysteine carbamidomethylation and methionine oxidation. The false discovery rate (FDR) was kept below 1 %.
Enzymatic Activity Analysis
Enzymatic extracts were prepared by homogenization of fish muscle (10 g) with 50 mL of ice-cold distilled water for 2 min, using an IKA Ultra-Turrax T25 homogenizer for 2 min at 8000g and then kept in ice with occasional stirring during 30 min. Thereafter, extracts were centrifuged at 14,600g for 20 min at 4 °C (Laboratory Centrifuge 3 K30, Sigma Laborzentrifugen GmbH, Osterode, Germany). Supernatant were filtered through a Whatman no. 1 filter and stored at −20 °C prior to enzymatic activity quantifications.
Acid phosphatase activity was determined following the methodology described by Ohmori et al. (1992) with only minor modifications. Enzymatic extracts (0.250 mL) were mixed with 0.225 mL of substrate solution (4 mM p-NPP in 0.1 mM acetate buffer and 1 mM EDTA, pH 5.5). After 15 min of incubation at 37 °C, the reaction was stopped by adding 1 mL of 100 mM KOH and measuring spectrophotometrically at 400 nm (Lambda 35 UV/vis spectrometer, Perkin-Elmer Instruments, Waltham, MA) the release of p-nitrophenol (p-NP). Enzyme activity was expressed as nanomole p-NP/minute/gram muscle.
Cathepsin B activity evaluated by the method described by Lakshmanan et al. (2005) was expressed as fluorescence units (FU)/minute/gram muscle. With few modifications, cathepsin D activity was determined following the protocol described by Buckow et al. (2010). Enzyme extracts (0.2 mL) were mixed with 0.6 mL of substrate solution (2 % denatured hemoglobin (w/v) in 200 mM citrate buffer, pH 3.7). After 3 h of incubation at 37 °C, the reaction was stopped by adding 0.6 mL of 10 % TCA (w/v). After vigorous stirring, the precipitate was removed by 15 min centrifugation at 18,000g (Elmi Micro Centrifuge CM-50, Porvoo, Finland). Soluble peptides were spectrophotometrically measured at 280 nm (Lambda 35 UV/vis spectrometer, Perkin-Elmer Instruments) and converted to tyrosine equivalents using a previously built calibration curve. Cathepsin D activity was expressed as microgram tyrosine/minute/gram muscle.
Statistical Analysis
Biochemical measurements for fish samples after 0, 1, 3, 6, and 9 months of frozen storage time were subjected to one-way ANOVA (p < 0.05) to explore differences in pressure level and storage time effects. Comparison of means was performed using a least-squares difference (LSD) method (Statsoft, Statistica, version 6.0, Tulsa, OK). Correlation analysis indicating the type of fitting was also performed.
Results and Discussion
Assessment of Lipid Hydrolysis
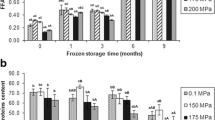
Lipid hydrolysis determined as FFA formation in mackerel muscle (Fig. 1a, mg/100 g muscle) was calculated on the basis of lipid content values (6.5–9.0 g lipids/100 g muscle; data not shown). After freezing (month 0 frozen samples), a marked FFA content increase was observed in all samples; however, this increase was lower in samples treated at 175 and 200 MPa. During frozen storage, FFA formation in all samples showed a progressive increase (r 2 = 0.88–0.94). Compared with control fish, all HHP-treated samples showed lower values at time 0 and 3 months and thereafter, the 175-MPa treatment yielded the lowest mean values. Comparison among HHP-treated samples did not provide evidence of a definite effect of pressure level throughout the frozen storage period under study. Similar conclusions were obtained when FFA content was expressed on the basis of lipid content (g/100 g lipids) showing also a good correlation with storage time for all treatments (r 2 = 0.86–0.94).
Free fatty acid content (mg/100 g muscle) (a) and fluorescence ratio (b) assessment in frozen mackerel muscle previously processed under different high-pressure conditions. Mean values of three replicates (n = 3) with standard deviations indicated by bars. For each frozen storage time, values accompanied by different letters (A–D) denote significant differences (p < 0.05) as a result of the pressure treatment prior to freezing and frozen storage at −18 °C for up to 9 months. Initial values: 11.99 ± 5.88 mg/100 g muscle (free fatty acid content) and 0.56 ± 0.10 (fluorescence ratio)
Previous research has detected an important FFA formation in Atlantic mackerel throughout frozen storage (Aubourg et al. 2005; Vázquez et al. 2013). Accumulation of FFA in fish muscle has no nutritional significance but undesirable secondary effects include muscle texture changes (Sikorski and Kolakowska 1994), lipid oxidation acceleration (Mackie 1993), and off-odor development (Refsgaard et al. 2000). Research reported concerning the effect on FFA formation after HHP treatment followed by a frozen storage is scarce. Ohshima et al. (1992) found that enzymatic degradation of phospholipids in cod (Gadus morhua) muscle was successfully inhibited during storage at −2 °C for 6 days when previously treated at pressures above 400 MPa for 15 and 30 min; however, no effect was observed when applying a pressure of 200 MPa. Chevalier et al. (2000) showed that FFA formation during storage at −20 °C for 75 days did not show differences between turbot (Scophthalmus maximus) filets subjected to pressure shift freezing at 140 MPa or air-blast freezing (−20 °C). Finally, Vázquez et al. (2013) observed a marked reduction in FFA content in Atlantic mackerel (S. scombrus) subjected to HHP treatments (150, 300, and 450 MPa for 0.0, 2.5, and 5.0 min) prior to freezing at −20 °C and frozen storage at ‑10 °C for 3 months. The reduction effect increased with the pressure level and holding time employed.
Assessment of Lipid Oxidation Development
The formation of primary and secondary lipid oxidation compounds was evaluated by means of peroxide and TBA values, respectively (Tables 1 and 2). Values for both parameters showed a relatively low lipid oxidation development as reflected by values in the 0.07–4.99 meq active oxygen/kg lipids and 0.35–1.08 mg malondialdehyde/kg muscle range, respectively. Such low values are in agreement with previous research on frozen (−10 °C for 3 months) mackerel previously treated under HHP conditions (Vázquez et al. 2013). An inhibitory effect on peroxide formation was observed in HHP-treated fish after freezing (comparison of month 0 samples), particularly at 200 MPa. Although some significant differences were detected during frozen storage, a definite pressure level effect on the presence of primary oxidation compounds was not identified.
No effect of pressure treatment on TBARS values was observed after freezing and during frozen storage. Samples of Atlantic mackerel muscle previously subjected to more intense HHP treatments than the ones here reported (200, 300, and 400 MPa for 5 and 15 min at 5, 10, and 15 °C) were recently analyzed by Senturk and Alpas (2013) showing in TBA-i values an increase and decrease with pressure level and holding time, respectively. In the present study, although PV and TBA-i scores showed some significant differences effect of frozen storage time, definite trends could not be determined. For 175- and 200-MPa-treated samples, the highest peroxide mean values were obtained at month 6, while control and 125- and 150-MPa fish samples showed the highest mean values at month 9. All samples yielded the highest mean TBARS values at the end of storage. In addition, PV and TBA-i values showed poor correlations with frozen storage time.
Data on the formation of tertiary lipid oxidation compounds (namely, fluorescent interaction molecules) is presented in Fig. 1b. Immediately after freezing, a higher mean value was obtained for control samples when compared with HHP-treated fish. This inhibitory effect on fluorescent compound formation was observed throughout frozen storage, i.e., a higher mean value was observed for control fish for all sampling times. Values corresponding to samples treated at 175 MPa were significantly lower than control fish when sampled at months 1–9, while 200-MPa-treated samples were also significantly lower than controls after 1, 3, and 9 months of frozen storage. For 125- and 150-MPa-treated samples, lower values were also found for 1 and 3 months when compared with control samples. These results show that HHP treatments caused an inhibitory effect on lipid oxidation development (tertiary lipid oxidation compounds). Concerning the effect of storage time, a progressive increase with time was observed in most cases showing correlation values in the 0.88–0.93 range (quadratic fitting). Lipid oxidation is a complex process involving the formation of different classes of compounds, most of them unstable and thus susceptible to breakdown and formation of lower molecular weight compounds or to react with other molecules, mostly nucleophiles, present in fish muscle. This is the case of peroxides and TBARS, widely reported to breakdown and give rise to tertiary lipid oxidation compounds after interacting with protein-type molecules (Aubourg 1999; Tironi et al. 2002). According to data obtained in this study, formation of fluorescent compounds seems to be the most adequate index to follow the progress of lipid oxidation.
Two opposite mechanisms can be considered to explain lipid changes observed in this study, i.e., the inactivation effect of pressure on endogenous enzyme activity (Torres et al. 2013; Vázquez et al. 2013) and the pressure-denaturation of iron-containing proteins which has been reported to increase free metal iron (Lakshmanan et al. 2003). These two effects would account for the opposite effects of HHP treatment observed when lipid oxidation development is evaluated. During 6 days of storage at −2 °C, Ohshima et al. (1992) found a TBARS formation increase in frozen cod (G. morhua) and mackerel (S. scombrus) muscle previously pressure treated at 616, 408, and 204 MPa for 15 and 30 min. A comparison of TBARS values observed during a 75-day storage at −20 °C of turbot (S. maximus) filets subjected to pressure shift freezing at 140 MPa or to air-blast freezing at −20 °C could not identify a definite advantage of the pressure treatment (Chevalier et al. 2000). In a work closely related to this study, Vázquez et al. (2013) observed a marked inhibition of tertiary lipid oxidation compounds formation in frozen (−10 °C up to 3 months) mackerel (S. scombrus) previously subjected to 150, 300, 450 MPa for 0.0, 2.5, and 5.0 min. The effect observed was greater when increasing the pressure level or the holding time.
Interaction of lipid oxidation and lipolysis is a particularly intriguing area of study, as triglyceride hydrolysis has been shown to lead to an oxidation increase, while phospholipid hydrolysis would produce the opposite effect (Shewfelt 1981; Sikorski and Kolakowski 2000). In the present study, correlation values in the 0.76–0.95 range were observed between the FR score and the development of lipid hydrolysis, while poor correlation values were determined for PV and TBA-i scores with FFA content.
The effect of pressure level and subsequent frozen storage on the PUFA content was also analyzed in this study. PI values (data not shown) were in the 0.85–1.03 range for all pressure treatments in agreement with previous research on frozen mackerel also pressure-treated before freezing and frozen storage (Vázquez et al. 2013). In spite of changes observed in lipid hydrolysis (FFA content) and oxidation (FR values), pressure treatment and frozen storage had no significant effect on PI scores. Previous research (Ortiz et al. 2009; Tironi et al. 2010) has shown an important detrimental effect of lipid oxidation on the PUFA content expressed as a PI decrease.
Trimethylamine Formation
TMA is one of the most commonly employed quality methods to assess microbial activity in marine species kept under refrigerated conditions. However, if freezing and frozen storage are encountered, microbial activity is expected to be mostly inhibited so that TMA formation would not be expected. Consequently, the conversion of trimethylamine oxide (TMAO) into TMA observed in this study may be caused by non-enzymatic processes, native tissue enzymes, or enzymes produced by microorganisms before the HHP and freezing process. Values summarized in Table 3 showed that after freezing, TMA-N content increased for all samples with significant differences (p < 0.05) resulting from the pressure treatment prior to freezing. Although some significant differences among samples can be pointed out throughout frozen storage, a definite effect of the pressure pre-treatment could not be determined during the 1–9-month fish-sampling period. TMA-N content showed a progressive formation during frozen storage (r 2 = 0.84–0.93). Additionally, good correlation values were obtained for TMA values with FFA values (r 2 = 0.90–0.93) and FR scores (r 2 = 0.86–0.93). These results show that significant conversion of TMAO to TMA occurred during the frozen storage of all samples. In previous research by Senturk and Alpas (2013), the combined effect on TMA formation of pressure level (200, 300, and 400 MPa), pressure holding time (5 and 15 min) and temperature (5, 10, and 15 °C) were determined in mackerel (S. scombrus) muscle; as a result, a marked increase in most cases after the HHP treatment was observed, but with no significant differences among the different HHP condition tested.
Analysis of Protein Changes
Figure 2 shows the SDS-PAGE profile of myofibril proteins for all frozen mackerel samples. After freezing, myofibril proteins from pressure-treated and control samples exhibited the same 1-D SDS-PAGE profile pattern (Fig. 2a). Moreover, the protein pattern obtained throughout frozen storage (months 3 and 9; Fig. 2b, c, respectively) did not reveal differences with the protein profile observed after freezing. This means that HHP processing in the 150–200-MPa range and frozen storage for up to 9 months at −18 °C did not significantly affect the 1-D electrophoretic pattern of myofibril proteins. In a previous work by Pazos et al. (2014), the effect on Atlantic mackerel myofibril proteins of treatments at 150, 300, and 450 MPa for 0, 2.5, and 5 min was analyzed after frozen storage at −10 °C for 3 months. In agreement with this study, myofibril proteins showed no solubility and electrophoretic gel profiles changes throughout the whole frozen storage period. The SDS-PAGE profile of sarcoplasmic proteins showed no differences among frozen fish samples at month 0 (Fig. 3a). However, a band of approximately 30 kDa disappeared during frozen storage. This band, labeled as 1S, could not be detected after 3 months of frozen storage in samples treated at 175 and 200 MPa (Fig. 3b). Fish treated under 150 MPa showed the loss of this band at month 9 (Fig. 3c). It can be concluded that the HHP treatments studied in this work induce this targeted degradation only in combination with frozen storage. Values of total sarcoplasmic protein content in the 2.74–4.22 g/100 g muscle range showed no effect of pressure treatment and frozen storage time. Previous research concerning mackerel showed a decrease on sarcoplasmic protein content when higher pressures (300 and 450 MPa) than in the present case were applied (Pazos et al. 2014).
Effect of high-pressure level and frozen storage time on the 1-D SDS-PAGE profile of myofibril proteins (MW, molecular weight). Atlantic mackerel was treated at 150, 175, and 200 MPa for 0 min and subsequently stored at −18 °C for 0 (a), 3 (b), and 9 (c) months. Profiles for control fish (0.1-MPa condition) are also expressed
Effect of high-pressure level and frozen storage on the 1-D SDS-PAGE profile of sarcoplasmic proteins (MW, molecular weight). Atlantic mackerel was treated at 150, 175, and 200 MPa for 0 min and subsequently stored at −18 °C for 0 (a), 3 (b), and 9 (c) months. The band labeled 1S denotes the protein band affected by high-pressure processing and frozen storage. Profiles for control fish (0.1-MPa condition) are also expressed
Over the last decade, proteomics has been successfully applied to evaluate quality in food systems including meat, fish, milk, and transgenic plants (Gallardo et al. 2013). Proteomics analysis based on one- and two-dimensional PAGE and tandem mass spectrometry (MS/MS) is a particularly powerful technology to identify global changes in protein constituents (Han and Wang 2008). In spite of its potential, proteomics tool applications to HHP-treated fish are still very limited. A marked content decrease in 94-, 50-, and 43-kDa bands was noticed in mackerel (Scomber japonicus) muscle treated at 200 MPa or higher, although the identity of these proteins was not reported (Ohshima et al. 1992). Chevalier et al. (1999) also found a disappearance of an unidentified 48-kDa protein band in turbot (S. maximus) muscle when treated at pressures higher than 150 MPa. More recently, SDS-PAGE analysis of Coho salmon (Oncorhynchus kisutch) sarcoplasmic fraction showed a partial loss of a band corresponding to 29 kDa that was identified by MS/MS analysis as phosphoglycerate mutase (Ortea et al. 2010), in agreement with the identification carried out in the present research. Finally, Pazos et al. (2014) analyzed the selective-targeted effect of HHP processing (150, 300, and 450 MPa for 0.0, 2.5, and 5.0 min) on proteins by identifying sarcoplasmic protein bands of frozen (−10 °C) Atlantic mackerel that were modified by the treatment. In agreement with their work, the present proteomics study showed that sarcoplasmic proteins are more liable to HHP-freezing-frozen storage processing than the myofibril fraction. The 1S band excised from the gel was digested with trypsin, and the resulting peptides were subjected to MS analysis by means of an electrospray ion trap mass detector. Peptides were fragmented and non-interpreted fragmentation spectra (MS/MS) and de novo-inferred sequences were searched against the protein sequence databases using the SEQUEST software and the BLAST tool, respectively. As a result, the protein band was assigned to the glycolytic enzyme phosphoglycerate mutase 2 (PGAM2) based on the following identification parameters: UniProtKB/Swiss-Prot Code (Q32DV0), Mass (28,685 Da), −10IgP Score (106.99), #Peptides/#Unique Peptides (6/5), Sequence (K.AMEAVAAQGK.A*; R.KAMEAVAAQGK.A*; R.ALPFWNDEIAPQIK.A*; R.HYGGLTGLNK.A*; R.FCGWFDADLSEK.G*; K.HGEEQVK.I) and sequence coverage (21 %). Phophoglycerate mutase is a transferase enzyme responsible for transferring a phosphate group from the C-3 carbon of 3-phosphoglycerate to the C-2 carbon forming 2-phosphoglycerate, acting in the final part of the glycolysis pathway. In agreement with previously mentioned research (Ortea et al. 2010), results from this study show that the assessment of this enzyme could be an effective tool to study the fish quality loss during storage.
Enzymatic Activity Analysis
A general behavior of the acid phosphatase activity data summarized in Table 4 showed for all samples a progressive increase with storage time (r 2 = 0.79–0.87; quadratic fitting). An inhibitory effect of HHP treatments at 150 and 200 MPa was observed in month 0 samples by comparison with their counterpart controls. Comparisons throughout frozen storage of pressure treatments did not show a general pattern; however, HHP-treated samples showed a lower activity at the end of the experiment when compared with their counterpart control samples. In previous work, Fidalgo et al. (2014) observed that acid phosphatase from mackerel (S. scombrus) was strongly affected by frozen storage time (3 months at −10 °C) in samples untreated or HHP-treated (150, 300, and 450 MPa; 0.0, 2.5, and 5.0 min). An important effect of pressure could also be observed with lower values obtained by increasing the pressure level. Fair correlation values were obtained in the present study for the acid phosphatase activity when compared with chemical quality indices such as FFA content (0.79–0.89), TMA formation (0.75–0.94), PV (0.74–0.90), and FR value (0.76–0.93).
Cathepsin B activity assessments (Table 4) showed also a progressive increase (r 2 = 0.88–0.91; quadratic fitting) during the frozen storage of all samples. The highest values (p < 0.05) for each sample type were also observed at the end of storage. A comparison of samples after freezing showed an inhibitory effect of treatments at 125, 150, and 175 MPa but not at 200 MPa. Although some significant differences were observed during frozen storage, a definite effect of the HHP treatment was not possible to conclude. It should be mentioned that in the 3–9-month period, a lower mean activity was observed in samples previously treated at 150–200 MPa when compared with their counterpart control samples. In previous related research by Fidalgo et al. (2014), cathepsin B activity in frozen mackerel (3 months at −10 °C) was also affected by HHP treatments (150, 300, and 450 MPa for 0.0, 2.5, and 5.0 min) before freezing and frozen storage, being the pressure effect higher than that for frozen storage and pressure holding time. A decrease in cathepsin B activity was evident with a pressure level increase, although a recovery effect was observed during frozen storage. In the present study, cathepsin B activity showed fair correlation values with chemical quality indices such as FFA content (0.77–0.89), TMA formation (0.67–0.88), PV score (0.42–0.94), and FR value (0.78–0.94).
A higher cathepsin D activity (Table 4) was observed at month 0 in 125-MPa samples. Throughout the 3–6-month period, controls showed higher mean values than HHP-treated fish samples. Finally, 200-MPa-treated samples had the highest (p < 0.05) cathepsin D activity at the end of storage. A progressive activity increase in control samples was observed throughout frozen storage reaching 71 % activity increase. In a previous work by Fidalgo et al. (2014), 300-MPa treatment before freezing caused an activity increase when compared with control samples while 150- and 450-MPa-treated samples showed the highest activity value after 3 months of storage at −10 °C. Cathepsin D activity showed poor correlation values with any of the chemical quality indices studied in this work.
Although HHP treatment may inhibit the activity of hydrolytic enzymes, the effects on fish muscle of 125–200-MPa treatments depend on several factors causing activation or inactivation of muscle enzymes. Consequently, the enzyme activity values observed in this study can be considered the result of different and opposite effects. While HHP treatments may inactivate enzymes by disrupting intramolecular bonds determining their secondary, tertiary, and quaternary conformation (Ashie et al. 1996; Campus 2010), pressure can also disrupt lysosomal membranes releasing proteases leading to an increase in hydrolytic activity (Ohmori et al. 1992; Chéret et al. 2005). Cathepsins B (a cysteine protease) and D (an aspartic acidic protease) have been reported to be released from the lysosomal matrix into both the cytoplasm and the intracellular spaces as a consequence of the breakdown of lysosomes (Chéret et al. 2006).
Conclusions
Research carried out during the latest decades has shown that HHP treatment can lead to an important quality enhancement in seafood on the basis of its ability for inactivating the microbial development and the endogenous enzyme activity. However, if relatively strong conditions are employed, HHP treatment has shown to induce detrimental modifications in valuable nutritional constituents in seafood.
On the basis of the results obtained in a previous study, the present research has focused on the biochemical changes produced in frozen Atlantic mackerel stored under commercial conditions (9 months at −18 °C) that was previously treated under optimized HHP conditions (125, 150, 175, and 200 MPa). As a result, previous HHP treatment led to valuable biochemical changes such as inhibition of lipid hydrolysis (FFA formation) and oxidation (tertiary compounds) in the frozen mackerel muscle that were accompanied by the modification of the sarcoplasmic protein profile (partial disappearance of the phosphoglycerate mutase 2 band) when the strongest pressure conditions (namely, 175 and 200 MPa) were applied. Meantime, no effect of the HHP treatment was observed on other biochemical indicators of quality loss in frozen fish including TMA and PUFA content, 1-D myofibril protein fraction, and the activity of acid phosphatase, and cathepsins B and D.
Present research proves the need of optimizing the HHP treatment conditions for each marine species and processing in order to guarantee the highest retention of chemical constituents. Additionally, this work provides the first attempt focused on the quality enhancement of a frozen marine species stored under commercial conditions by means of a previous HHP treatment.
References
Ashie, I., Smith, J., & Simpson, B. (1996). Spoilage and shelf-life extension of fresh fish and shellfish. Critical Reviews in Food Science and Nutrition, 36, 87–121.
Aubourg, S. (1999). Review: recent advances in assessment of marine lipid oxidation by using fluorescence. Journal of the American Oil Chemists’ Society, 76, 409–419.
Aubourg, S, Medina, I., & Pérez-Martín, R. (1996). Polyunsaturated fatty acids in tuna phospholipids: distribution in the sn-2 location and changes during cooking. Journal of Agricultural and Food Chemistry, 44, 585-589.
Aubourg, S., Rodríguez, A., & Gallardo, J. (2005). Rancidity development during frozen storage of mackerel (Scomber scombrus): effect of catching season and commercial presentation. European Journal of Lipid Science and Technology, 107, 316–323.
Aubourg, S., Torres, J. A., Saraiva, J., Guerra-Rodríguez, E., & Vázquez, M. (2013). Effect of high-pressure pretreatments applied before freezing and frozen storage on the functional and sensory properties of Atlantic mackerel (Scomber scombrus). Food Science and Technology (LWT), 53, 100–106.
Bligh, E., & Dyer, W. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Brutti, A., Rovere, P., Cavallero, S., D’Amelio, S., Danesi, P., & Arcangeli, G. (2010). Inactivation of Anisakis simplex larvae in raw fish using high hydrostatic pressure treatments. Food Control, 21, 331–333.
Buckow, R., Truong, B., & Versteeg, C. (2010). Bovine cathepsin D activity under high pressure. Food Chemistry, 120, 474–481.
Campus, M. (2010). High pressure processing of meat, meat products and seafood. Food Engineering Reviews, 2, 256–273.
Chapman, R., & McKay, J. (1949). The estimation of peroxides in fats and oils by the ferric thiocyanate method. Journal of the American Oil Chemists’ Society, 26, 360–363.
Chéret, R., Chapleau, N., Delbarre-Ladrat, C., Vérrez-Bagnis, V., & De Lamballerie, M. (2005). Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. Journal of Food Science, 70, E477–E483.
Chéret, R., Hernández-Andrés, A., Delbarre-Ladrat, C., de Lamballerie, M., & Vérrez-Bagnis, V. (2006). Proteins and proteolytic activity changes during refrigerated storage in sea bass (Dicentrarchus labrax L.) muscle after high-pressure treatment. European Food Research and Technology, 222, 527–535.
Chevalier, D., Le Bail, A., Chourot, J., & Chantreau, P. (1999). High pressure thawing of fish (whiting): influence of the process parameters on drip losses. Lebensmittel-Wissenschaft und -Technologie, 32, 25–31.
Chevalier, D., Sequeira-Muñoz, A., Le Bail, A., Simpson, B., & Ghoul, M. (2000). Effect of pressure shift freezing, air-blast freezing and storage on some biochemical and physical properties of turbot (Scophthalmus maximus). Lebensmittel-Wissenschaft und -Technologie, 33, 570–577.
Fidalgo, L. G., Saraiva, J. A., Aubourg, S. P., Vázquez, M., & Torres, J. A. (2014). Effect of high-pressure pre-treatments on enzymatic activities of Atlantic mackerel (Scomber scombrus) during frozen storage. Innovative Food Science & Emerging Technologies, 23, 18–24.
Gallardo, J. M., Carrera, M., & Ortea, I. (2013). Proteomics in food science. In A. Cifuentes (Ed.), Foodomics: advanced mass spectrometry in modern food science and nutrition (pp. 125–165). Hoboken: John Wiley & Sons, Inc.
Groβ, M., Auerbach, G., & Jaenicke, R. (1993). The catalytic activities of monomeric enzymes show complex pressure dependence. FEBS Letters, 321, 256–260.
Han, J.-Z., & Wang, Y.-B. (2008). Proteomics: present and future in food science and technology. Trends in Food Science and Technology, 19, 26–30.
Harris, P., & Tall, J. (1994). Rancidity in fish. In J. Allen, & R. Hamilton (Eds.), Rancidity in foods (pp. 256–272). London: Chapman and Hall.
Laemmli, U. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Lakshmanan, R., Pigott, J., & Paterson, A. (2003). Potential applications of high pressure for improvement in salmon quality. Trends in Food Science and Technology, 14, 354–363.
Lakshmanan, R., Miskin, D., & Piggott, J. R. (2005). Quality of vacuum packed cold-smoked salmon during refrigerated storage as affected by high-pressure processing. Journal of the Science of Food and Agriculture, 85, 655–661.
Lowry, R., & Tinsley, I. (1976). Rapid colorimetric determination of free fatty acids. Journal of the American Oil Chemists’ Society, 53, 470–472.
Mackie, I. M. (1993). The effects of freezing on flesh proteins. Food Reviews International, 9, 575–610.
Montero, P., Giménez, B., Pérez-Mateos, M., & Gómez-Guillén, M. C. (2005). Oxidation stability of muscle with quecetin and rosemary during thermal and high-pressure gelation. Food Chemistry, 93, 17–23.
Mújica-Paz, H., Valdez-Fragoso, A., Tonello Samson, C., Welti-Chanes, J., & Torres, J. A. (2011). High-pressure processing technologies for the pasteurization and sterilization of foods. Food and Bioprocess Technology, 4, 969–985.
Norton, T., & Sun, D.-W. (2008). Recent advances in the use of high pressure as an effective processing technique in the food industry. Food and Bioprocess Technology, 1, 2–34.
Ohmori, T., Shigehisa, T., Taji, S., & Hayashi, R. (1992). Biochemical effects of high hydrostatic pressure on the lysosome and proteases involved in it. Bioscience, Biotechnology, and Biochemistry, 56, 1285–1288.
Ohshima, T., Nakagawa, T., & Koizumi, C. (1992). Effect of high hydrostatic pressure on the enzymatic degradation of phospholipids in fish muscle during storage. In E. Bligh (Ed.), Seafood science and technology, chapter 8 (pp. 64–75). Oxford: Fishing News Books.
Ortea, I., Rodríguez, A., Tabilo-Munizaga, G., Pérez-Won, M., & Aubourg, S. (2010). Effect of hydrostatic high-pressure treatment on proteins, lipids and nucleotides in chilled farmed salmon (Oncorhynchus kisutch) muscle. European Food Research and Technology, 230, 925–934.
Ortiz, J., Larraín, MªA., Vivanco, J., & Aubourg, S. (2009). Rancidity development during the frozen storage of farmed coho salmon (Oncorhynchus kisutch): effect of antioxidant composition supplied in the diet. Food Chemistry, 115, 143-148.
Pazos, M., Pereira da Rocha, A., Roepstorff, P., & Rogowska-Wrzesinska, A. (2011). Fish proteins as targets of ferrous-catalyzed oxidation: identification of protein carbonyls by fluorescent labeling on two-dimensional gels and MALDI-TOF/TOF mass spectrometry. Journal of Agricultural and Food Chemistry, 59, 7962–7977.
Pazos, M., Méndez, L., Gallardo, J. M., & Aubourg, S. (2014). Selective-targeted effect of high pressure processing on proteins related to quality: a proteomics evidence in Atlantic mackerel (Scomber scombrus). Food and Bioprocess Technology, 7, 2342–2353.
Refsgaard, H., Brockhoff, P., & Jensen, B. (2000). Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. Journal of Agricultural and Food Chemistry, 48, 3280–3285.
Saeed, S., & Howell, N. (2001). 12-lipoxygenase activity in the muscle tissue of Atlantic mackerel (Scomber scombrus) and its prevention by antioxidants. Journal of the Science of Food and Agriculture, 81, 745–750.
Senturk, T., & Alpas, H. (2013). Effect of high hydrostatic pressure treatment (HHPT) on quality and shelf life of Atlantic mackerel (Scomber scombrus). Food and Bioprocess Technology, 6, 2306–2318.
Shewfelt, R. (1981). Fish muscle lipolysis—a review. Journal of Food Biochemistry, 5, 79–100.
Sikorski, Z., & Kolakowska, A. (1994). Changes in protein in frozen stored fish. In Z. Sikorski, B. Sun Pan, & F. Shahidi (Eds.), Seafood proteins (pp. 99–112). New York: Chapman and Hall.
Sikorski, Z., & Kolakowski, E. (2000). Endogenous enzyme activity and seafood quality: influence of chilling, freezing, and other environmental factors. In N. Haard, & B. Simpson (Eds.), Seafood enzymes (pp. 451–487). New York: Marcel Dekker.
Smith, P., Krohn, R., Hermanson, G., Mallia, A., Gartner, F., Provenzano, M., Fujimoto, E., Goeke, N., Olson, B., & Klenk, D. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150, 76–85.
Tironi, V., Tomás, M., & Añón, M. C. (2002). Structural and functional changes in myofibrillar proteins of sea salmon (Pseudopercis semifasciata) by interaction with malondialdehyde (RI). Journal of Food Science, 67, 930–935.
Tironi, V., de Lamballerie, M., & Le Bail, A. (2010). Quality changes during the frozen storage of sea bass (Dicentrarchus labrax) muscle after pressure shift freezing and pressure assisted thawing. Innovative Food Science & Emerging Technologies, 11, 565–573.
Torres, A., Vázquez, M., Saraiva, J., Gallardo, J., & Aubourg, S. (2013). Lipid damage inhibition by previous high pressure processing in white muscle of frozen horse mackerel. European Journal of Lipid Science and Technology, 115, 1454–1461.
Tozawa, H., Erokibara, K., & Amano, K. (1971). Proposed modification of Dyer’s method for trimethylamine determination in codfish. In R. Kreuzer (Ed.), Fish inspection and quality control (pp. 187–190). London: Fishing News Books Ltd.
Vázquez, M., Torres, J. A., Gallardo, J., Saraiva, J., & Aubourg, S. (2013). Lipid hydrolysis and oxidation development in frozen mackerel (Scomber scombrus): effect of a high hydrostatic pressure pre-treatment. Innovative Food Science & Emerging Technologies, 18, 24–30.
Vyncke, W. (1970). Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichmittel, 72, 1084–1087.
Acknowledgments
The Xunta de Galicia and the European Social Fund are thankfully recognized for the financial support of the postdoctoral “Isidro Parga Pondal” contract to M. P. The Spanish Ministry of Science and Innovation is also gratefully acknowledged for the doctoral fellowship to L. M. The authors thank Dr. María Lavilla (AZTI Tecnalia, Derio, Spain), Dr. Barbara Teixeira (IPMA, Lisbon, Portugal), Mr. Marcos Trigo, and Mrs. Lorena Barros for their help in carrying out the present study. This work was supported by the Secretaría Xeral de I + D from the Xunta de Galicia (Galicia, Spain) through the Research Project 10TAL402001PR (2010–2012), by Fundação para a Ciência e a Tecnologia (FCT Portugal), European Union, QRN, FEDER, COMPETE through founding of the Organic Chemistry Research Unit (QOPNA) (project PEst-C/QUI/UI0062/2013; FCOMP-01-0124-FEDER-037296), and by Formula Grants no. 2011-31200-06041 and 2012-31200-06041 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pazos, M., Méndez, L., Fidalgo, L. et al. Effect of High-Pressure Processing of Atlantic Mackerel (Scomber scombrus) on Biochemical Changes During Commercial Frozen Storage. Food Bioprocess Technol 8, 2159–2170 (2015). https://doi.org/10.1007/s11947-015-1567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1567-z