Abstract
Immunoassays are the most commonly used quantitative techniques to determine the gliadin content of food aimed at coeliac patients. Though the minimal amount of gliadins inducing the typical histopathological changes at the intestinal mucosa in coeliacs is still a matter of debate, current research is focussed on the development of methods having higher sensitivities. One of the main drawbacks in gliadin analysis is the low efficiency of the conventional extraction procedure using 60% ethanol. The use of reducing (2-mercaptoethanol) and denaturing (guanidinium chloride) agents has been recommended to improve the extraction efficiency. Owing to the well-known effects of these agents on native conformation of proteins, and their widely reported interference on the antigen/antibody interaction in other systems, we assessed whether gliadin detection by immunoassays is affected by the presence of those agents. Using two ELISA formats with a panel of polyclonal and monoclonal antibodies, we found that recognition by specific antibodies of partially or totally denatured gliadins is severely impaired. The magnitude of the interference depends on the antibodies used and the ELISA format. The impact of such interference was analysed for each step of the immunoassays. 2-mercaptoethanol had a stronger effect than guanidinium chloride, and the antigen became almost undetectable for some assays when both reagents were used in combination. Remarkably, since quantitative results are obtained by comparison with a calibration curve using a native antigen, there is no equivalence between the antigen/antibody interaction occurring in the sample and that in the standard gliadin, leading to underestimation of the actual gliadin content. Therefore, we suggest that not only the effects of reducing and denaturing agents on the antigen during the extraction procedure, but also the effects of residual amounts of these agents on the antigen/antibody interaction should be considered when a quantitative immunoassay is performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coeliac disease is a permanent gastrointestinal disorder characterised by a cellular response of both the adaptive and the innate immune systems against certain gluten peptides in the small intestine [1–3]. Prevalence of coeliac disease in general Western populations is close to 1%, but remains under-diagnosed in many situations [4]. While several therapeutic approaches have been proposed based on the knowledge of the molecular pathogenic mechanism of the disease [5], the gluten-free diet (GFD), excluding wheat, barley, rye and related cereals continues to be the cornerstone of treatment. Good dietary compliance usually results in a rapid and good clinical response and reduces the risk of further long-term complications observed in untreated patients [6]. A follow-up study of paediatric coeliac patients in Canada showed that 89% of children showed a significant improvement in health after starting the gluten-free diet, while accidental consumption triggered reactions in 54% of them [7]. Abdulkarim et al. [8], reported that around half of treated patients still have symptoms because of inadvertent, even minimal, gluten ingestion. Adherence to the gluten-free diet implies a change in eating habits, which may alter the social behaviour and have psychological consequences [9, 10]. In addition, strict adherence to the diet has been associated with nutritional deficiencies because gluten-free products are often poor in vitamins, minerals and fibre [11, 12]. Consequently, successful management of coeliac disease requires the intervention of a dietician, a physician and a coeliac support group [13].
Surveys performed in different groups of patients have indicated that patients claim for better labelling of gluten-containing ingredients and for a broad spectrum of commercially available gluten-free food in the supermarket [7]. Therefore, the use of reliable methods to certify food products intended to be consumed by coeliac patients is mandatory. Codex Alimentarius Committee on Nutrition and Food for Special Dietary Use, states that gluten-free products should not exceed a gluten level of 20 ppm for naturally gluten-free products and 200 ppm for rendered gluten-free foods, though these definitions are under revision (CL 2006/5-NFSDU, March 2006).
The complexity of toxic prolamins and the individual variation, clinical heterogeneity, and ethical concerns of carrying out protocols enrolling a high number of patients and controls make it difficult to reach a definite safe threshold value for a daily gluten intake. Notwithstanding, it is well known that a daily gluten intake of small amounts can be enough to trigger histopathological changes in the intestinal mucosa in coeliacs [6, 14]. By evaluating the clinical signs and histology of adult coeliac patients consuming products containing gluten, Collin et al. [15] found that a daily intake of 30 mg of gluten is safe. A microchallenge study showed that 50 mg gliadins/day produces measurable damage at the small intestinal mucosa [16].
Gluten, the best characterised exogenous antigen driving an autoimmune disorder, is defined as the dough-forming proteins that remain after washing the wheat flour to remove starch and is essentially formed by gliadins and glutenins [17]. The complexity of this protein system, particularly its biochemical and immunochemical properties, low solubility in aqueous solvent, and high homology and cross reactivity, poses difficulty for the quantitative determination of gliadins by immunoassays and also for the assessment of toxicity by biological tests. By ex vivo studies using organ culture and in vitro assays assessing the activation of T cell clones, the most commonly used techniques to identify toxic sequences, a small number of causative peptides have been identified in the gliadin fraction but also in glutenins [18–23].
Extraction from wheat flour with 60% ethanol (after elimination of the albumin/globulin fraction) renders the gliadin fraction, which comprises more than a hundred of monomeric proteins, classified into α/β, γ and ω-gliadins according to their electrophoretic migration at acid pH and their amino acid sequences. Except for ω-gliadins, the other groups contain cysteins able to form disulphide bonds. By contrast, glutenins occur as interchain cross-linked proteins. Barley and rye present similar components with high degree of homology [17].
Most of the commercial foodstuff consists of highly processed products, which could contain proteins modified as a consequence of heat treatments, changes in pH, chemical and enzymatic hydrolysis and high-pressure procedures during manufacturing. In the case of gluten-containing food, the interaction among prolamins themselves, or with other components of the food matrix, occur by covalent (disulphyde bridges) and non-covalent interactions established during dough formation. This causes a substantial reduction in protein recovery from samples and poses an additional drawback in gluten-free food certification. Wieser et al. [24] showed that the extractability of gliadin from bread with 60% ethanol (the conventional extraction solvent) was strongly reduced in comparison with flour. α and γ-gliadins were much more affected than ω-gliadins, and less gliadin was extracted from the crust than from the crumbs. A complete extraction of gliadins from bread required both the use of reducing and denaturing agents (50% 1-propanol, 2 M urea, 1% w/v dithiotreitol) and increased temperature (60 °C), allowing a gliadin recovery of 98%.
Though RP-HPLC proved to be a robust technique to determine gliadins [25], immunochemical assays are the most widely used methods for the control of food aimed for coeliac patients. In the last 20 years several analytical strategies have been proposed to increase the reliability of the ELISA used in the certification of gluten-free food [26–33]. More recently, the use of reducing and/or disaggregating agents in the extraction procedure has been recommended for quantitative ELISA [31].
Immunochemical quantification requires protein recognition by specific antibodies and also equivalent antigen/antibody interaction in samples and standard. The use of denaturing agents during the extraction procedure, allows a better prolamin recovery but causes changes in antigen and antibody conformation, and, consequently, can affect gliadin quantification.
Recently, a capture ELISA test using a monoclonal antibody (named R5) with a detection limit of 1.5 ppm of gliadin was developed [31, 33]. The R5 antibody recognises mainly the QQPFP sequence, and others (QQQFP, LQPFP and QLPFP), which occur in gliadins, secalins and hordeins [34]. Since a combination of 250 mmol/L 2-ME and 2 mol/L GuHCl was able to extract 98–100% of gliadins from heated samples, and the residual amount of 2-ME and GuHCl after the 1/100 dilution of samples did not affect the antigen/antibody interaction in the R5 capture ELISA, the use of reducing and denaturing agents to increase the gliadin recovery during the extraction procedure in heat-treated samples was recommended [31, 33, 35]. However, Ellis et al. [28] described that the use of reducing agents for gliadin extraction, even after 100-fold dilution, proved unsuccessful due to interference in the immunoassay, probably due to antigen and/or capture antibody denaturation.
Preliminary studies using a panel of anti-gliadin monoclonal antibodies produced by our group showed that reducing agents such as 2-ME and dithiotreitol [36] or 2-ME and GuHCl [37], alter antigen–antibody interactions. Since these additives can modify protein conformation, the aim of this study was to investigate the effect of reducing and denaturing agents on the interaction between gliadins and specific antibodies, and to examine how disruption of antigen/antibody interaction can alter gliadin determination by immunoassays. By studying different assay formats and antibody systems, we were able to assess the effect of reducing and denaturing agents on different steps of the quantitative immunoassays. Our results showed that sample extraction with denaturing/disaggregating agents could impair gliadin determination by quantitative immunoassays, producing in some cases a severe underestimation in gliadin quantification.
Methods
Samples
Flour of a local wheat variety (cv. Oasis) and wheat flour-based cookies were extracted using 60% aqueous ethanol, or 60% aqueous ethanol containing 2% 2-mercaptoethanol (2-ME)(ICN Biomedicals, Ohio, USA), 2 M guanidine hydrochloride (GuHCl) (Biomedicals, Ohio, USA) or both. Samples were homogenised in Omnimixer (Omni International, Waterbury, CT, USA) during 3 min with solvent at ratio 1:10 (300 mg sample: 3 ml solvent) [27], incubated for 40 min at 50 °C according to Valdés et al. [31], and supernatants from centrifugation (3 min, 12,000 rpm) were analysed at the appropriate dilution.
Gliadin standard solutions were prepared using commercial gliadin (Sigma, St Louis MO, USA) and were quantified as described elsewhere [27].
Protein determination
Protein content in extracts obtained from wheat flour and cookie samples was determined by BCA protein assay kit (Pierce, Rockford, USA). To overcome the interference of free thiol groups, samples were previously incubated with 50 mM sodium iodoacetate (Sigma, St Louis, MO, USA) for 15 min to block reducing sulfhydryl groups.
Quantitative ELISAs
Two different formats were used: sequential competitive ELISA and capture ELISA.
Sequential competitive ELISA
Sequential competitive ELISA was performed using anti-gliadin polyclonal antibodies as described previously [27]. Briefly, plates were coated overnight at 4 °C with 1 μg/ml gliadin standard in PBS pH 7.4. All subsequent incubations were performed at 37 °C. After washing once with PBS-0.05% Tween 20, plates were blocked for 2 h with 3% (w/v) skim milk powder prepared in PBS. Standard gliadin or sample extracts were preincubated in 1.5 ml plastic tubes for 2 h with an appropriate dilution of anti-gliadin rabbit polyclonal antibody in 1:1 ratio, diluted in 1% (w/v) skim milk powder in PBS-0.05% Tween 20 (buffer A). Samples were then transferred into gliadin-coated wells and incubated for 30 min. Plates were then washed three times and incubated for 1 h with goat anti-rabbit IgG horseradish peroxidase conjugate (BioRad, Hercular CA, USA) diluted in buffer A. Plates were washed three times and the enzyme substrate (1 mg/ml OPD; 1 μl/ml H2O2 in citrate buffer pH 5) was added. The reaction was stopped after 15 min with 2 M H2SO4, and Optical density (OD) was read at 492 nm.
Capture ELISAs
Capture ELISAs were developed using pairs of different anti-gliadin monoclonal antibodies (mAbs) prepared by our group. 1B4E9 or 3B4H1 mAbs were used for antigen capture and biotinylated 2A1C4 or 1B4E9 mAbs were used as detection antibodies following the procedure described elsewhere [38]. Briefly, plates were coated overnight at 4 °C with 1B4E9 or 3B4H1 mAb in PBS. All subsequent incubations were performed at 37 °C. After washing, plates were blocked for 2 h with 3% (w/v) skim milk powder in PBS. Then samples diluted in buffer A were transferred into antibody-coated wells and incubated for 1 h. Plates were then washed three times and incubated for 1 h with a biotinylated mAb (2A1C4 or 1B4E9). After washing, plates were incubated 1 h with avidin–alkaline phosphatase conjugate (Sigma, Skeinheim, Germany). After washing with PBS-0.05% Tween 20 and then once with diethanolamine buffer (1 M diethanolamine, 2 mM MgCl2, pH 9,8) the enzyme substrate (1 mg/ml p-nitrophenyl phosphate in diethanolamine buffer) was added and the reaction stopped with 0.1 M EDTA after 30 min. OD was read at 405 nm.
Results
Analysis of the interference of denaturing and reducing agents on the specific binding of anti-gliadin polyclonal and monoclonal antibodies
Indirect ELISA is one of simplest tests to be performed to evaluate the effect of 2-ME and GuHCl on the antigen/antibody interaction. We have previously shown interference with the binding of anti-gliadin monoclonal and polyclonal antibodies when indirect ELISA was performed in the presence of either dithiotreitol or 2-ME [36]. To extend these studies, the combined effect of 2-ME and GuHCl was evaluated. To this end, a dilution of anti-gliadin polyclonal or monoclonal antibody was prepared in buffer containing different concentrations of 2-ME and GuHCl, and then incubated in gliadin-coated wells. Results were expressed as OD ratio relative to the same antibody dilution without agents (ratio = 1).
Compared with controls, the increase in the 2-ME concentration caused a reduction in antibody binding (Fig. 1a). At 0.5% 2-ME, the binding of anti-gliadin polyclonal antibody decreased to about 60% of the maximum, while the remaining activity at 2% 2-ME was about 20%. Binding of polyclonal antibody was not affected at GuHCl concentrations below 0.1 mol/L, but it was reduced to 67% of maximum by 2 mol/L GuHCl (Fig. 1b). To study the combined effect of 2-ME and GuHCl, antibody solutions were diluted in mixtures containing both agents at different concentrations and incubated in gliadin-coated wells. Interference with antibody binding was more evident when both the denaturing and reducing agents were present together (Fig. 1c). Antibody binding was 77% at 0.1% 2-ME–0.1 mol/L GuHCl, and dropped to 27% for 0.5% 2-ME–0.5 mol/L GuHCl.
Interference of antibody binding in the presence of 2ME and GuHCl. Antibody binding was evaluated by Indirect ELISA in gliadin-coated wells. Polyclonal antibody was diluted in buffer containing 2-ME at concentrations ranging from 0 up to 2% (a), in buffer containing GuHCl at concentrations ranging from 0 up to 2 mol/L (b) or buffer containing both reagents (c). Anti-gliadin 1B4E9 mAb was diluted in buffer containing similar combinations of 2-ME and GuHCl concentrations (d). Results are expressed as Mean value ± SD of OD ratio relative to the same antibody dilution without agents (ratio = 1). Duplicate results from one representative experiment are shown
A similar analysis for the 1B4E9 monoclonal antibody showed that 2-ME had a stronger effect, since antibody binding was 17% of maximum in the presence of 0.5% 2-ME. A slight increase in antibody binding was observed for 1 mol/L GuHCl (Fig. 1d).
Though the interference with antibody binding at 0.5% 2-ME–0.5 mol/L GuHCl was similar for both polyclonal and monoclonal antibodies, a stronger interference was found for monoclonal antibodies when the whole range of combinations was analysed. Polyclonal antibodies appeared to be more resistant to the combined effect of 2-ME and GuHCl (Fig. 1c, d).
Interference of denaturing and reducing agents on quantitative immunoassays
To evaluate the effect of 2-ME and GuHCl on the immunochemical quantification of gliadin, standard gliadin solutions were prepared in buffer containing 2-ME, GuHCl or both at different final concentrations and were then analysed by competitive and capture ELISA.
Sequential competitive ELISA
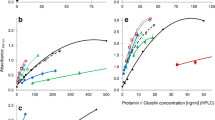
2-ME and GuHCl were added, at different final concentrations, to the mixture of polyclonal antibody and standard gliadin and incubated for 2 h (preincubation step) prior to transfer to the gliadin-coated well. The addition of 2-ME resulted in an overestimation of the actual gliadin concentration. By comparison with a control sample, a threefold increase was observed for 2% 2-ME. The presence of GuHCl at 0.02 mol/L or higher concentrations caused an underestimation of the gliadin content (Fig. 2a, b). Therefore, the addition of these agents can have opposite effects. The plot in Fig. 2c shows that at some conditions there is a balance between these positive and negative effects, which results in a good, but artificial, correlation with the expected gliadin concentration in the sample.
Interference of 2-ME and GuHCl in competitive ELISA using polyclonal antibodies. Standard gliadin solutions at 100 ng/ml were diluted in buffer containing different concentrations of 2-ME (0–2%) and GuHCl (0–2 mol/L). Gliadin concentration was then determined by competitive ELISA using polyclonal antibodies. a Interference of 2-ME (0–2%) in the presence of 0–2 mol/L GuHCl. b Interference of GuHCl (0–2 mol/L) in the presence of 0–2% 2-ME. c 3D plot for combinations of GuHCl and 2-ME concentrations. Results are expressed as Mean value ± SD of OD ratio relative to the same antibody dilution without agents (ratio = 1). Duplicate results from one representative experiment are shown
Capture ELISA
Gliadin samples were prepared with the addition of 2-ME and GuHCl at different final concentrations and the interference was evaluated by capture ELISA using different pairs of anti-gliadin monoclonal antibodies for capture and detection.
Addition of 2-ME significantly interfered with antibody interactions in a capture ELISA using 3B4H1 mAb for antigen capture and 1B4E9 biotinylated mAb for detection (Fig. 3). When gliadin samples were diluted in the presence of 0.1% 2-ME, the concentration determined by capture ELISA was less than 40% of the expected value, while antigen became undetectable for 0.5% or higher 2-ME concentrations (Fig. 3a). GuHCl concentrations below 0.5 mol/L had no appreciable effect, while at 2 mol/L GuHCl the measured gliadin concentration was 50% of the actual value (Fig. 3b). At 0.5% 2-ME–0.5 mol/L GuHCl, the measured gliadin concentration was 5% of the actual value (Fig. 3c).
Interference of 2-ME and GuHCl in capture ELISA using monoclonal antibodies. Standard gliadin solutions at 500 ng/ml were diluted in buffer containing different concentrations of 2-ME (0–2%) and GuHCl (0–2 mol/L). Gliadin concentration was then determined by capture ELISA using the 3B4H1 mAb for antigen capture and the biotinylated 1B4E9 mAb as secondary antibody. a Interference of 2-ME (0–2%) in the presence of 0–2 mol/L GuHCl. b Interference of GuHCl (0–2 mol/L) in the presence of 0–2% 2-ME. c 3D plot for combinations of GuHCl and 2-ME concentrations. d Similar analysis using 1B4E9 mAb for antigen capture and biotinylated 2A1C4 mAb. Results are expressed as Mean value ± SD of OD ratio relative to the same antibody dilution without agents (ratio = 1). Duplicate results from one representative experiment are shown
The results for 2-ME interference were similar when capture ELISA was performed with other monoclonal antibodies (1B4E9 mAb as capture antibody and biotinylated 2A1C4 for detection), but the effect of GuHCl was more pronounced (Fig. 3d). When gliadin was measured in the presence of 0.5 mol/L GuHCl the measured gliadin concentration was 40% of the expected value. In the presence of both agents (0.5% 2-ME–0.5 mol/L GuHCl) the gliadin concentration was 2% of the expected value.
Though some variations in the magnitude of the interference were observed for different pairs of monoclonal antibodies, capture ELISA underestimated the concentration of the standard gliadin in all tested combinations of 2-ME and GuHCl concentrations.
Effect of different concentrations of denaturing and reducing agents on gliadins
To study the effect of denaturing and reducing agents on antigen conformation and consequently on antigen/antibody interactions, gliadin standard solutions were treated as a model of food sample. To this end, these solutions were incubated for 40 min at 50 °C in the presence of different concentrations of 2-ME, GuHCl or both. To avoid any effect of 2-ME and GuHCl on antibody binding, prior to be analysed by ELISA, samples were diluted 1,000-fold [37].
Treatment of gliadin samples with 2-ME at concentrations higher than 0.02% produced a severe reduction in antigen recognition by polyclonal antibodies in competitive ELISA (Fig. 4a). Samples treated with GuHCl alone did not present appreciable differences when compared with controls, but the addition of higher 2-ME concentrations in combination with GuHCl did cause a noticeable decrease in detection (Fig. 4b). Consequently, when gliadin solution was incubated in the presence of 2% 2-ME and 2 mol/L GuHCl (conditions proposed for the extraction solvent [31]) the determined gliadin concentration was 30% of the expected value.
Effect of treatment with 2-ME and GuHCl on gliadin determination by competitive ELISA using polyclonal antibodies. Standard gliadin solutions at 100 μg/ml were incubated for 40 min at 50 °C in buffer containing different concentrations of 2-ME (0–2%) and GuHCl (0–2 mol/L). After 1,000-fold dilution in buffer without denaturing agents, concentration was determined by competitive ELISA using polyclonal antibodies. a Interference of GuHCl (0–2 mol/L) in the presence of 0–2% 2-ME. b Interference of 2-ME (0–2%) in the presence of 0–2 mol/L GuHCl. c 3D plot for combinations of GuHCl and 2-ME concentrations. Results are expressed as Mean value ± SD of OD ratio relative to the same antibody dilution without agents (ratio = 1). Duplicate results from one representative experiment are shown
In a capture ELISA (Fig. 5), treatment of gliadin samples with 2-ME at 0.02% or higher concentrations, alone or combined with GuHCl, made antigen undetectable for both 3B4H1/1B4E9 and 1B4E9/2A1C4.
Effect of treatment with 2-ME and GuHCl on gliadin determination by capture ELISA using monoclonal antibodies. Standard gliadin solutions at 500 μg/ml were incubated for 40 min at 50 °C in buffer containing different concentrations of 2-ME (0–2%) and GuHCl (0–2 mol/L). After 1,000-fold dilution in buffer without denaturing agents, concentration was determined by capture ELISA using 3B4H1 for antigen capture and biotinylated 1B4E9 mAb. a Interference of GuHCl (0–2 M) in the presence of 0, 0.02, 0.5 and 2% 2-ME. b Interference of 2-ME (0–2%) in the presence of 0, 0.02, 0.5 and 2 mol/L GuHCl. c 3D plot for combinations of GuHCl and 2-ME concentrations. Similar analysis for 1B4E9 mAb and biotinylated 2A1C4 mAb (d, e and f). Results are expressed as Mean value ± SD of OD ratio relative to the same antibody dilution without agents (ratio = 1). Duplicate results from one representative experiment are shown
Use of denaturing and reducing agents in the extraction procedure
The use of 2-ME and GuHCl for prolamin extraction was investigated using wheat-based cookies and wheat flour as model systems for processed and non-processed food samples, respectively.
Samples were extracted with 60% ethanol (conventional extraction procedure) or with 60% ethanol containing 2% 2-ME, 2 mol/L GuHCl or both together. Prior to ELISA analysis, samples were diluted at least 1,000-fold in order to avoid a direct effect of these agents on antigen/antibody interactions. Total protein content was also determined to assess protein recovery under each extraction condition.
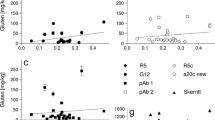
Compared with results for extracts obtained with ethanol alone, extraction of wheat flour with ethanol in the presence of GuHCl produced an increase in protein recovery which correlated with competitive ELISA results, but not with capture ELISA data (Fig. 6). Similarly, extracts from cookies showed a slight increase in protein content when GuHCl was used. The use of 2-ME did not produce a relevant increase in protein recovery, while antigen became almost undetectable in capture and competitive ELISA. When the extraction procedure for wheat flour or wheat-based cookies was performed in the presence of 2-ME and GuHCl, an increase in protein recovery was observed. However, detection by either capture or competitive ELISA was severely impaired by the presence of 2-ME and GuHCl.
Gliadin determination in extracts from model samples obtained with solvents containing 2-ME and GuHCl. Wheat-based cookies and wheat flour, used as model for processed and unprocessed food, were extracted with 60% ethanol, or 60% ethanol containing 2 mol/L GuHCl, 2% 2-ME or both. Histograms correspond to the protein content determined employing a BCA assay kit (black bars). Gliadin concentration determined by competitive ELISA using polyclonal antibodies (gray bars). Gliadin concentration determined by capture ELISA using 1B4E9 mAb for antigen capture and biotinilated 2A1C4 mAb for detection (white bars). Mean value ± SD of duplicate experiments is shown
Thus, despite the increase in protein recovery when 2-ME and GuHCl were used in the extraction procedure, these agents significantly altered ELISA measurements, leading to an underestimation of the actual gliadin content. When capture ELISA was used, antigen became almost undetectable.
Discussion
Patients suffering from coeliac disease, a life-long gastrointestinal disorder must follow a strict gluten-free diet. Good dietary compliance usually results in a rapid and good clinical response and reduces the risk of further long-term complications observed in untreated patients [6].
By ex vivo studies using organ culture and in vitro assays assessing the activation of T cell clones, the most commonly used techniques to identify toxic sequences, a small number of causative peptides have been identified in the gliadin and glutenin fraction [18–23]. Owing to the individual variation and ethical concerns, studies enrolling a high number of subjects aimed at determining the maximal amount of gluten tolerated by coeliac patients are very difficult to perform [6, 14]. More recent studies have shown that 30 mg gluten/day is safe [15] while 50 mg gliadin/day induced histopathological changes in the intestinal mucosa [16].
To certify gluten-free products, the complete extraction of gliadins from food sample is required. However, this process is very far from being complete when the conventional extraction solvent (ethanol 60%) is applied to heat-processed products. Increased temperature during the extraction procedure, as well as addition of reducing and disaggregating agents are required for a complete gliadin recovery from these samples [24]. A commercially available test, R5, is able to quantify gliadins in food samples extracted with the buffer provided with the kit, which contains 2-ME and GuHCl [33, 35]. However, studies by Ellis et al. [28] showed that even after 100-fold dilution of samples extracted with reducing agents a strong interference with quantification was evident.
Since the expected amount of prolamins in gluten-free products is low, the sample is usually diluted 1:40 or less, prior to analysis. Consequently, the residual amount of denaturing agents is high enough to cause severe interference with the quantification. As result, the quantitative assay may fail to detect the presence of toxic prolamins in these products leading to an analytical error with serious consequences.
In previous studies, we observed interference with immunoassays using either anti-gliadin polyclonal or monoclonal antibody when dithiotreitol or 2-ME [36] and 2-ME and GuHCl [37] were added to samples. According to previous results, the effect of denaturating agents appears to depend on the antibody and ELISA format employed. Therefore, we extended the analysis by assessing the combined effect of 2-ME and GuHCl in competitive and capture ELISAs using different antibody systems [38]. Altogether, these studies allow assessment of the effects of 2-ME and GuHCl on antigen/antibody interaction at different stages of the immunoassays and, consequently, on the performance of the quantitative test.
As reported for several proteins, 2-ME and GuHCl produce conformational changes leading to a decrease in the biological activity. Studies performed on different enzymes demonstrated the effect of denaturating agents (such as GuHCl) in producing inactivation due to partial unfolding of the molecule. It was suggested that the active site is more easily perturbed and more flexible than the protein as a whole, thus explaining the loss of enzymatic activity with concentrations lower than those necessary for the complete unfolding of the molecule [39, 40]. Similarly, antibodies showed loss of antigen recognition after treatment with GuHCl at concentration considerably lower than those required to change the global antibody conformation [41]. It was observed that protein denaturation occurs at higher concentrations of GuHCl when this agent is used alone, and that the non-native disulphide bonds were more susceptible to reduction than native bonds. Since an intact disulphide network appears to preserve the overall structure and increase conformational stability, most proteins are more resistant to denaturation when they are treated with agents such as GuHCl in the absence of reducing agents [42]. Results presented in this study (Figs. 1, 2, 3, 4, 5, 6) support these observations. The antigen (in this case, a complex mixture of homologous proteins) is strongly affected by treatment with 2-ME and becomes almost undetectable by ELISA when treated with 2-ME and GuHCl together. Similarly, interference with antigen/antibody interaction is enhanced by the presence of 2-ME with or without GuHCl.
Competitive and capture ELISAs produced very different estimates of gliadin concentration compared with the actual gliadin value. The scheme in Fig. 7, illustrates how the presence of these agents leads to different degrees of interference. In the case of competitive ELISA, overestimation and underestimation can be observed by the effect of agents at two different levels in the immunoassay. Underestimation is observed when denaturing agents interfere mainly with immunocomplex formation during the preincubation step, because of antigen denaturation. Conversely, when the residual amount of agents interferes with antibody binding to antigen-coated wells, an overestimation of the actual gliadin concentration is observed. Depending on the composition of the extraction solvent and the dilution factor employed for sample analysis, both effects will have a different impact on final results. Antigen denaturation, and consequently loss of recognition by specific antibodies, as well as interference with antibody binding by antibody denaturation contributed to the underestimation of the gliadin concentration in all the capture ELISAs tested in this study.
Remarkably, when samples were treated and then 1,000-fold diluted prior to analysis in order to avoid the effect of the agents on antigen/antibody interactions, a variable level of underestimation was observed. The alteration of antigen conformation during the extraction procedure is enough to cause a severe reduction in the quantified amount of gliadin by both Competitive and Capture ELISA. Moreover, antigen became almost undetectable when Capture ELISA was used, probably due to a strict requirement for native antigen conformation in fluid phase to be recognized by monoclonal antibodies. As a consequence, although an increase in gliadin recovery was observed when denaturing agents (2% 2-ME and 2 mol/L GuHCl) were included in the extraction solvent for model samples such as wheat-based cookies and wheat flour, the concentration determined by ELISA was lower than that obtained with the conventional extraction procedure (i.e. 60% ethanol).
Furthermore, it has recently been reported that glutenins might also be toxic for coeliac patients [23]. Consequently, uncertainty about the efficiency of the extraction procedure for gliadins and glutenins from food samples, the interaction of antibodies with the very complex protein system of gliadins and glutenins, and the presence of toxic peptides in both fractions, raise new analytical challenges.
In conclusion, when food samples are extracted with 60% ethanol containing 2% 2-ME and 2 mol/L GuHCl for certification of gluten-free food by immunoassays, different effects can lead to a false estimation of the gliadin content. Firstly, recognition by specific antibodies of partially or totally denatured gliadins can be severely impaired. Secondly, residual amounts of denaturing agents alter antibody binding. Thirdly, since quantitative results are obtained by comparison with a calibration curve using a native antigen in the absence of denaturing agents, there is no equivalent antigen/antibody interaction between gliadin in the sample and the standard gliadin. Finally, the magnitude of the interference depends on the antibodies used and the ELISA format. Given the well-known effects of 2-ME and GuHCl on the native conformation of proteins, we suggest considering not only the effects of these agents on antigen during the extraction procedure, but also the effects of residual amounts of these agents on the antigen/antibody interaction when a quantitative immunoassay is performed.
Abbreviations
- 2-ME:
-
2-Mercaptoethanol
- GuHCl:
-
Guanidinium chloride
- mAb:
-
Monoclonal antibody
References
Koning F (2005) Celiac disease: caught between a rock and a hard place. Gastroenterol 129:1294–1301
Londei M, Ciacci C, Ricciardelli I, Vacca L, Quaratino S, Maiuri L (2005) Gliadin as a stimulator of innate responses in celiac disease. Mol Immunol 42(8):913–918
Brandtzaeg P (2006) The changing immunological paradigm in coeliac disease. Immunol Lett 105(2):127–139
Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, Macneil J, Mack D, Patel D, Moher D (2005) The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterol 128(4 Suppl 1):S57–S67
Sollid LM, Khosla C (2005) Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol 2(3):140–147
Ciclitira PJ, Ellis HJ, Lundin KE (2005) Gluten-free diet—what is toxic?. Best Pract Res Clin Gastroenterol 19(3):359–371
Rashid M, Cranney A, Zarkadas M, Graham ID, Switzer C, Case S, Molloy M, Warren RE, Burrows V, Butzner JD (2005) Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 116(6):754–759
Abdulkarim A, Burgat L, See J, Murray JA (2002) Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol 97(8):2016–2021
Johnston SD, Rodgers C, Watson RG (2004) Quality of life in screen-detected and typical coeliac disease and the effect of excluding dietary gluten. Eur J Gastroenterol Hepatol 16(12):1281–1286
Zarkadas M, Cranney A, Case S, Molloy M, Switzer C, Graham ID, Butzner JD, Rashid M, Warren RE, Burrows V (2006) The impact of a gluten-free diet on adults with coeliac disease: results of a national survey. J Hum Nutr Diet 19(1):41–49
Kupper C (2005) Dietary guidelines and implementation for celiac disease. Gastroenterology 128(4 Suppl 1):S121–S127
Hopman EG, le Cessie S, von Blomberg BM, Mearin ML (2006) Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J Pediatr Gastroenterol Nutr 43(1):102–108
Case S (2005) The gluten-free diet: how to provide effective education and resources. Gastroenterology 128(4 Suppl 1):S128–S134
Hischenhuber C, Crevel R, Jarry B, Makis M, Moneret-Vautrin DA, Romano A, Troncote R, Ward R (2006) Safe amounts of gluten for patients with wheat allergy or coeliac disease. Alim Pharm Ther 23:559–575
Collin P, Thorell L, Kaukinen K, Maki M (2004) The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment Pharmacol Ther 19(12):1277–1283
Catassi C, Fabiani E, Iacono G, D’ Agate C, Francavilla R, Biagi F,Volta U, Accomando S, Picarelli A, De Vitis I, Pianelli G, Gesuita R,Carle F, Mandolesi A, Bearzi I, Fasano A (2007) A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 85(1):160–166.
Shewry PR, Tatham AS (1990) The prolamin storage proteins of cereal seeds: structure and evolution. Biochem J 267:1–12
Howdle PD, Ciclitira PJ, Simpson FG, Losowsky MS (1984) Are all gliadins toxic in coeliac disease? An in vitro study of alpha, beta, gamma, and w gliadins. Scand J Gastroenterol 19(1):41–47
Sturgess RP, Hooper LB, Spencer J, Hung CH, Nelufer JM, Ciclitira PJ (1992) Effects of interferon-gamma and tumor necrosis factor-alpha on epithelial HLA class-II expression on jejunal mucosal biopsy specimens cultured in vitro. Scand J Gastroenterol 27(11):907–911
Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P (1995) Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut 37(6):766–776
Molberg O, Mcadam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 4(6):713–717
Tollefsen S, Arentz-Hansen H, Fleckenstein B, Molberg O, Raki M, Kwok WW, Jung G, Lundin KE, Sollid LM (2006) HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest 116(8):2226–2236
Dewar DH, Amato M, Ellis HJ, Pollock EL, Gonzalez-Cinca N, Wieser H, Ciclitira PJ (2006) The toxicity of high molecular weight glutenin subunits of wheat to patients with coeliac disease. Eur J Gastroenterol Hepatol 18(5):483–491
Wieser H (1998) Investigations on the extractability of gluten proteins wheat bread in comparison with flour Z. Lebensm Unter Forshc A 207:128–132
Wieser H, Antes S, Seilmeier W (1998) Quantitative determination of gluten protein types in wheat flour by reversed-phase high performance liquid chromatography. Cereal Chem 75(5):644–650
Freedman AR, Galfre G, Gal E, Ellis HJ, Ciclitira PJ (1987) Monoclonal antibody ELISA to quantitate wheat gliadin contamination of gluten-free foods. J Immunol Meth 98(1):123–127
Chirdo FG, Añón MC, Fossati CA (1995) Optimization of a competitive ELISA for quantification of prolamins in food. Food Agric Immunol 7(4):333–343
Ellis J, Rosen-Bronson S, O´Reilly N, Ciclitira PJ (1998) Measurement of gluten using a monoclonal antibody to a coeliac toxic peptide of A-gliadin. Gut 43:190–195
Ferranti P, Mamone G, Melck D, Tafuro M, Picarriello G, Addeo F (2002) Combined mass spectrometric and immunological strategies for detection of gliadins in wheat varieties from Europe. In: Proceeding of the 16th Meeting of the European Working Group in Prolamin analysis and Toxicity, pp 29–36
Henterich N, Osman A, Mendez E, Mothes T (2003) Assay of gliadin by real-time immuno-polymerase chain reaction. Nahrung/Food 5:343–346
Valdés I, García E, Llorente M, Méndez E (2003) Innovative approach to low level gluten determination in foods using a novel sandwich ELISA protocol. Eur J Gastroenterol Hepatol 15(5):465–474
Spaenij-Dekkin EHA, Kooy-Winkelaar EMC, Nieuwenhuizen WF, Drijfhout JW, Koing F (2004) A novel and sensitive method for the detection of T cell stimulatory epitopes of α/β and γ-gliadin. Gut 53:1267–1273
Mendez E, Vela C, Immer U, Janssen FW (2005) Report of a collaborative trial to investigate the performance of the R5 enzyme linked immunoassay to determine gliadin in gluten-free food. Eur J Gastroenterol Hepatol 17(10):1053–1063
Kahlenberg F, Sanchez D, Lachmann I, Tuckova L, Tlaskalova H, Mendez E, Mothes T (2006) Monoclonal antibody R5 for detection of putatively celiac-toxic gliadin peptides. Eur Food Res Technol 222:78–82
Garcia E, Llorente M, Hernando A, Kieffer R, Wieser H, Mendez E (2005) Development of a general procedure for complete extraction of gliadins for heat processed and unheated foods. Eur J Gastroenterol Hepatol 7:529–539
Margheritis AI, Doña V, Fossati CA, Chirdo FG (2003) Effect of reducing agents on the immunochemical detection of gliadin. In: Stern M (ed) Proceeding of 17th meeting of the European working group on prolamin analysis and toxicity, 2–5 October, Stockholm, Sweden. 2003. Zwickau Germany: Verlag Wissenschaftliche Scripten 2004, pp 51–58
Doña V, Fossati CA, Chirdo FG (2003). Interference of denaturing and reducing agents on gliadin/antibody interaction. In: Stern M (ed) Proceeding of the 18th meeting of the European working group on prolamin analysis and toxicity, 2–5 October 2003, Stockholm, Sweden. 2003. Zwickau Germany: Verlag Wissenschaftliche Scripten 2004, pp 51–58
Chirdo FG, Añón MC, Fossati CA (1998) Development of high sensitive enzyme immunoassays for gliadin quantification using the streptavidin–biotin amplification system. Food Agric Immunol 10(2):143–155
Yang HJ, Tsou CL (1995) Inactivation during denaturation of ribonuclease A by guanidinium chloride is accompanied by unfolding at the active site. Biochem J 305(Pt 2):379–384
Wang GF, Cao ZF, Zhou HM, Zhao YF (2000) Comparison of inactivation and unfolding of methanol dehydrogenase during denaturation in guanidine hydrochloride and urea. Int J Biochem Cell Biol 32:873–878
Wang XD, Luo ZQ, Zhou JM, Tsou CL (1997) Perturbation of the antigen-binding site and staphylococcal protein A-binding site of IgG before significant changes in global conformation during denaturation: an equilibrium study. Biochem J 325:707–710
Singh RR, Chang JY (2004) Investigating conformational stability of bovine pancreatic phospholipase A2: a novel concept in evaluating the contribution of the “native framework” of disulphides to the global conformational stability of proteins. Biochem J 377:685–692
Author information
Authors and Affiliations
Corresponding author
Additional information
V.V. D is postgraduate fellow of CONICET. C.A.F. and F.G.Ch. are members of the Researcher Career of CONICET. This study was supported by the Grant PICT 9800 from ANPCyT and a Grant from the Comisión Investigaciones Científicas de la Provincia de Buenos Aires.
Rights and permissions
About this article
Cite this article
Doña, V.V., Fossati, C.A. & Chirdo, F.G. Interference of denaturing and reducing agents on the antigen/antibody interaction. Impact on the performance of quantitative immunoassays in gliadin analysis. Eur Food Res Technol 226, 591–602 (2008). https://doi.org/10.1007/s00217-007-0597-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0597-9