Abstract
Quality and shelf life of non-irradiated and irradiated (2.5 and 5 kGy) sea bream in ice conditions and stored at +4 °C were investigated by measurement of microbiological, chemical and sensory analysis. Microbial counts for non-irradiated sea bream samples were higher than respective irradiated fish. Total volatile base nitrogen (TVB-N) values increased value of 38.64 mg/100 g for non-irradiated, sea bream during iced storage whereas for irradiated fish lower values of 13.48 and 12.06 mg/100 g were recorded at 2.5 and 5 kGy, respectively (day 19). Trimethylamine (TMA-N) values and thiobarbituric acid (TBA) values for irradiated samples were lower than non-irradiated samples. Acceptability scores for odour, taste and texture of cooked decreased with storage time. The sensory scores of sea bream stored in control and 2.5–5 kGy at +4 °C were 13 and 15 days, respectively. The results obtained from this study showed that the shelf life of sea bream stored in ice, as determined by overall acceptability all data, is 13 days for non-irradiated sea bream and 15 days for 2.5 kGy irradiated and 17 days for 5 kGy irradiated sea bream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gamma irradiation has been employed for decontamination and/or sterilization of dehydrated vegetables, fruits [1, 2] seasonings [3], and animal foods [4–8] and then to prolong the storage period of irradiated food. There is ample published evidence showing the enormous potential of this method for extending shelf life of chicken meat by eliminating certain spoilage and pathogenic organisms [9, 10]. Irradiation of food up to an overall dose of 10 kGy is accepted in several countries for commercial food processing [11]. Many researchers recognized and reported that gamma irradiation in low doses, below 10 kGy; kill most organisms without deterioration of food quality [12]. Application of gamma-radiation up to a dose level of 10 kGy can be used to eliminate or greatly reduce the numbers of food spoilage micro-organisms as well as food-borne pathogens in food products without compromising the nutritional or sensory quality [10, 13–16]. A combination of hurdles can ensure stability, microbial safety, and sensory quality of food [9]. The most important hurdles used in food preservation are temperature (high or low), water activity (aw), acidity, redox potential, preservatives, and irradiation [17]. Effect of irradiation on shelf life of pork and chicken [12, 18], poultry [19], beef jerky [20], and semi-dried seafood [4] has been reported.
The effect of gamma-radiation (2.5 and 5 kGy) and post-irradiation ice storage on sea bream (Sparus aurata) muscle microbiological, chemical and sensory characteristics was studied using analysis.
Fish samples and storage conditions
For preparation of the samples and storage conditions, aqua-cultured fresh sea bream (Sparus aurata) were cultivated in net cages in a Turkish fish farm (PINAR Company) and harvested during the period of May 2005. Thirty kilograms of samples were used for the experiment. The fish were slaughtered by immersing in ice-cold water (hypothermia) and delivered to the laboratory (whole) within 12 h of harvesting, packed in separate insulated polystyrene boxes with ice. Whole ungutted fishes were divided into three lots; control samples (0 kGy) and the irradiated batches 2.5 kGy and 5 kGy were kept iced in an industrial refrigerator with controlled temperature (4±1 °C) and the ice replenished when necessary. The ice samples were placed in plastic film bags packaged by vacuum packaging machine (Henkovac model E-173, vacuum-packaging machine, Switzerland). Fish samples were delivered to the irradiation plant in insulated polystyrene boxes with ice within 1.5 h of harvesting.
Irradiation
Samples were irradiated at the GAMMAPAK Company, Çerkezköy, Tekirdağ, Turkey using a 60Cobalt radiation source (MDS, Nordion, Canada). The applied doses in this study were 2.5 and 5.0 kGy. To minimize variations in radiation-dose absorption, the boxes were turned 180 ° halfway through the irradiation process of each experiment. Exposure time was 120 and 240 min. The absorbed dose was monitored by polymethyl methacrylate dosimeters (Harwell Amber Perspex dosimeter, Batch R Type 3042 Range 1–30 kGy, UK). The absorbance signal was measured using a Camspec M 201 UV spectrophotometer at 640 nm. The treatment was performed at 2–4 °C for sample temperature at the beginning and 18–20 °C for internal temperature of the facility. Fish samples were maintained at 2±1 °C during irradiation by using ice covering the samples. After irradiation, the fish were transported to the laboratory in ice via insulated polystyrene boxes, within 1.5 h, and maintained at 4±1 °C until microbiological, chemical and sensory analyses were conducted.
Microbiological analysis
Sample preparation
Sea bream flesh (25 g) obtained from each fillet, were transferred aseptically to a Stomacher bag (Seward Medical, London, UK) containing 225 ml of 0.1% peptone water (Merck, Cat No.: 107228) and homogenized for 60 s using a Lab Blender 400, Stomacher at high speed, (Stomacher, IUL Instrument, Spain). Microbiological media and enumeration for microbial enumeration 0.1 ml samples of serial dilutions (1:10, diluent, 0.1% peptone water) of fish homogenates were spread on the surface of dry media. Total viable counts were determined using plate count agar (PCA, Merck, Cat No.: 105463) after incubation for 24–48 h at 37 °C. Plate count agar was used for psychrotrophic bacteria and incubated at 7 °C for 10 days [21]. Pseudomonads were enumerated on cetrimite agar (Merck, Cat No.: 105284) incubated at 37 °C for 2 days; H2S-producing bacteria (including Shewanella putrefaciens) were determined on Iron agar (Peptone from casein 5 g; yeast extract 2.5 g; glucose 1 g; agar-agar 14 g, 0.3 g iron(III) citrate; 0.48 g sodium thiosulphate, 3 g NaCl)) after incubation 25 °C, 2–3 days. VRGB agar (Merck, Cat No.: 110275) was used for Enterobacteriaceae and incubated 37 °C, 1 day [22, 23]. Results are expressed as a logarithm of colony forming units (log CFU) per gram of sample.
Chemical analysis
Proximate composition: The fish samples were analysed in triplicate for proximate composition: lipid content of sea bream by Weilmeier and Regenstein [24] method, moisture content by Mattissek et al. [25] method, the ash content by AOAC [26] method, total crude protein by Kjeldhal method [27], and carbohydrate content of fish by the Merrill and Watt [28] method. pH was determined at room temperature on homogenates of filleted muscle in distilled water (1/10 w/w) [29]. pH was monitored using a WTW-pH-Meter (ino Lab pH Level 1 model, Weilheim, Germany). Total volatile base nitrogen (TVB-N) was determined according to the method of Antonacopoulos and Vyncke [30]. Trimethylamine (TMA) analysis was carried out according to the method proposed by AOAC [31]. Thiobarbituric acid (TBA) was determined according to the method proposed by Weilmeier and Regenstein [24].
Sensory assessment
Sensory evaluation of raw fish: Five experienced panellists analysed fish at days 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19, according to EU fish sensory schema for whitefish [32], and for the EU scheme. The mean points of each panellist were calculated and the fish were classified according to the following correspondence between points and quality bands: Extra Quality E≥2.7; A Quality 2.0≤A<2.7; B Quality 1.0≤B<2.0; Unacceptable C<1.0 [33].
Sensory analysis of cooked fish: The attributes of cooked fish (filleted) were evaluated by a panel of five experienced judges on each day of sampling. Fish samples (200 g of fish fillets) were cooked individually in a microwave oven at full power (600 W), for 5 min including defrosting time and immediately presented to the panellists. Panellists were laboratory trained. Sensory evaluation was conducted in individual booths under controlled conditions of light, temperature and humidity. Panellists were asked to score odour, taste, and texture of fish using a 10–9=excellent, 8.9–8=very good, 7.9–6=good, 5.9–4=sufficient, 4=limit of acceptable, <4=unacceptable [34].
Statistical analysis
Significant differences between the samples were calculated by Excel XP 2003 by one-way analysis of variance (ANOVA) using a significance level of p<0.05 by Tukey's test [35].
Results and discussion
Microbiological analysis
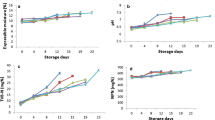
The shelf life of fresh fish is limited by the growth and biochemical activities of Gram-negative, psychrotrophic strains of Pseudomonas, Achromabacter, Flavobacterium and Moraxellla species in the presence of atmospheric O2 [36]. Initial bacterial population and packaging materials are important factors affecting shelf life of fish in packages. The microbiological condition of fish muscle is directly related to fishing ground and environmental factors [37]. The present study focused on the monitoring of the following species of micro-organisms: psychrotrophic bacteria count, mesophilic aerobic bacteria count, H2S-producing bacteria count, Enterobacteriaceae and Pseudomonads (Fig. 1A–E). Initial psychrotrophic bacteria counts of non-irradiated and irradiated sea bream were 4.7 log cfu/g, respectively (day 0). Psychrotrophic counts reached 6.2 log cfu/g for non-irradiated samples after 13 days of storage. On day 13 of storage, psychrotrophic bacteria counts of irradiated sea bream were <4 log cfu/g, respectively. Irradiated fish samples increased after 19 days of storage in ice demonstrated populations of ca. 7.4 and 7.2 log cfu/g, respectively. Non-irradiated and irradiated (2.5 and 5 kGy) samples stored under ice conditions psychrotrophic bacteria counts exceeded the value of 6 log cfu/g), considered as the upper acceptability limit for marine species [38], on 13, 15 and 17 storage day at +4 °C.
The initial mesophilic aerobic bacteria count of sea bream was ca 4 log cfu/g (day 0). Total viable count exceeded the value of 7 log cfu/g, which is considered as the upper acceptability limit for freshwater and marine species as defined by ICMSF [39], on day 17 (non-irradiated sea bream samples). Non-irradiated and 2.5–5 kGy irradiated sea bream samples did not reach this value throughout the 19-day storage period. After 19 days of storage, the 5 kGy irradiated samples and 2.5 kGy irradiated sea bream had a significantly lower (p<0.05) mesophilic aerobic bacteria count than the control samples. Jeevanandam et al. [40] reported that initial total viable count of non-irradiated and irradiated (1 and 2 kGy) sea bream 4.64, 3.36, and 2.75 log cfu/g reached the counts of 8 log cfu/g at day 20 in non-irradiated samples, at day 30 for irradiated group samples. Savvaidis et al. [41] reported counts of 7 log cfu/g for vacuum-packed trout after 9, 14 and 24 days for non-irradiated and irradiated samples at 0, 0.5 and 2 kGy, respectively. Total mesophilic counts for salted vacuum-packed, refrigerated control and irradiated sea bream reached an average value of 7 log cfu/g after 14 days (0 kGy), 23 days (1 kGy) and 40 days (3 kGy) days as reported by Chouliara et al. [42]. Irradiation doses ranging from 1 to 3 kGy have been suggested for shelf-life extension of fresh fish [43–45]. van Cleemput et al. [46], Chen et al. [47], Ouattara et al. [48], and Mendes et al. [49] reported that mesophilic bacteria count of irradiated shrimp, crab and fish are lower than those in non-irradiated samples during the storage at 4 °C. Chouliara et al. [50] found initial aerobes bacterial counts as 5.4, 2, and 4 log cfu/g in vacuum-packed non-irradiated and irradiated (1 and 3 kGy) sea bream. They also stated that the aerobes bacterial counts increased to value of 7 log cfu/g in control samples in 9 days, 1 kGy irradiated samples in 18 days and 3 kGy irradiated samples in 26 days.
Initial H2S-producing bacteria count was ca. 4.4 log cfu/g, while a count of 7 log cfu/g was exceeded on day 9 (non-irradiated samples). The irradiated sea bream samples reach this value throughout the 17- and 19-day storage period. Of the bacteria examined in the present study, H2S-producing bacteria (typical Shwanella putrefaciens) and Pseudomonas spp constituted a large proportion of the microflora of sea bream fillets in agreement with the findings of Koutsoumanis and Nychas [51]; Savvaidis et al. [41]; Papadopoulos et al. [52] for fresh fish stored aerobically, under vacuum and ice under refrigeration, respectively.
Pseudomonas spp. count of non-irradiated sea bream reached 6 log cfu/g after 19 days (limit of acceptability), respectively. After 19 days of storage, irradiation (2.5 and 5 kGy) reduced the Pseudomonas spp. from 5.2 and 4.7 log cfu/g by almost 1 and 2 log cycles (p<0.05) respectively. H2S-producing bacteria (including S. putrefaciens) were also a dominant bacterial species in fish spoilage. This is in agreement with the elimination of H2S-producing bacteria in freshwater and marine fish (tilapia and Spanish mackerel) at a dose of 1.5 kGy [13]. Chouliara et al. [50] also reported that 3 kGy dose was effective in eliminating the growth of H2S-producing bacteria and Pseudomonas spp. in vacuum-packed sea bream for 9 days under refrigerated storage.
The initial Enterobacteriaceae count of <4 log cfu/g increased to 5.6, 4.6, and 5.5 log cfu/g after 19 days of storage control and irradiated samples. The contribution of Enterobacteriaceae to the microflora of fish and its spoilage potential must be taken into consideration especially in the case of polluted water or delay in chilling after catch [42].
Chemical analysis
Increases in pH indicate the accumulation of alkaline compounds, such as ammonia compounds and TMA, mainly derived from microbial action [53]. The pH of live fish muscle is close to the value 7.0. However, post-mortem pH can vary from 6.0 to 7.1 depending on season, species and other factors [54]. In our work regarding pH, statistically significant differences (p<0.05) were determined between groups control, irradiated sample (2.5 and 5 kGy) at the end of the storage period. pH values of groups non-irradiated and irradiated were 6.69 at the beginning and 7.14, 6.91 and 6.88 at the end of the storage period (19 days), respectively (Fig. 2). The pH increases are in agreement with the findings of Kyrana et al. [55], Kyrana und Lougovois [56], Masyinom et al. [57], Tejada and Huidobro [58], Papadopoulus et al. [52], Grigorakis et al. [59], and Taliadourou et al. [60] for sea bream species stored in ice.
TVB-N content of non-irradiated, 2.5 and 5 kGy irradiated sea bream stored in ice at +4 °C is shown in Fig. 3. At the beginning of storage, the TVB-N value was 15.65 mg/100 g flesh for sea bream stored in ice. TVB-N values increased according to time of storage. At the end of the storage period of 19 days, TVB-N values reached 38.64±1.57, 13.48±0.60 and 12.06±0.67 mg/100 g for non-irradiated and 2.5–5 kGy irradiated sea bream, respectively. The statistical analysis of the TVB-N data showed that significant differences (p<0.05) were found between sea bream stored at +4 °C in control −2.5 kGy irradiated, control −5 kGy irradiated samples after 3 days of storage.
TVB-N levels for non-irradiated sea bream exceeded 25 mg/100 g [61], which is considered as the maximum level for acceptability in three Sparidae species during refrigerated storage, after 9 and after 8 days, respectively, in agreement with TVB-N levels of sea bream stored in ice [62, 63]. The TVB-N for gilthead sea bream increased with the time of storage. However, the increase was small, from 15 to 25 mg/100 g tissue after 15 days of ice storage [59]. Chouliara et al. [42] found initial TVB-N value of vacuum-packed salted, non-irradiated and 1–3 kGy irradiated sea bream, 25.31, 24.08, and 22.28 mg/100 g. This value increased to 27.22 mg/100 g after 14 days (rejection time) of storage in control samples and 32.34 and 31.87 mg/100 g after 28 days (rejection time) in irradiated samples. Al-Kahtani et al. [64] reported TVB-N level of 1.5–10 kGy irradiated tilapia and mackerel are lower than non-irradiated tilapia and mackerel samples during the storage at 2 °C. Similarly, von Amin et al. [65] determined in irradiated freshwater fish, Jo et al. [66] in irradiated fish, and van Cleemput et al. [46] in irradiated shrimp lower TVB-N level than control non-irradiated samples. Jeevanadam et al. [40] found initial TVB-N value of sea bream, 8 mg/100 g. This value increased to 80 mg/100 g after 29 days of storage in control samples and 44 and 38 mg/100 g in irradiated samples. Chouliara et al. [50] reported that initial TVB-N level of vacuum-packed irradiated (1–3 kGy) stored under refrigeration sea bream 27.5, 27.3, and 25.1 mg/100 g, reached the acceptable limits at day 10 in control, at days 21 and 28 for 1 and 3 kGy irradiated sea bream. Mendes et al. [49] reported that initial TVB-N level of chilled horse mackerel, which is 15.6 mg/100 g, reached the limit levels of 30–35 mg/100 g at day 12, in 1 and 3 kGy irradiated samples at day 20, 13.6, and 12.7 mg/100 g, respectively.
Trimethylamine-N-oxide (TMAO) is found in most marine fish and can reach high concentrations [67]. It is reduced to trimethylamine (TMA-N) by spoilage bacteria giving rise to the characteristic pungent smell of iced fish, and TMA is therefore an indicator of spoilage [68, 69]. In marine fish, TMA-N is the main component responsible for an unpleasant “fishy” odour [70]. The quantitative level of TMA-N in fish is considered to be a major index of the quality of marine fish [71, 72].
TMA-N content varies with species, season and type of storage [73]. Initial average value of TMA-N was found to be 3.22 mg/100 g muscle for sea bream, whereas final values of TMA-N were 9.66 (control), 5.70 (2.5 kGy), and 4.97 mg /100 g muscle (5 kGy) (Fig. 4). TMA-N values showed significant (p<0.05) increase for all groups during storage. Values of TMA-N showed statistically significant (p<0.05) differences between all groups during the post 7-day period of storage. TMA-N values were acceptable during the first 9 days of storage in control group samples and the 17 days of storage in irradiated (2.5 kGy) sea bream samples. Similar results were found by Cakli et al. [62] in ice sea bream after 9 days (5.95 mg/100 g TMA-N) during the storage. Initial value of TMA was in agreement with those found for sea bream and sea bass [55, 56, 74]. The slow increase in TMA during the shelf life of gilthead sea bream invalidates this parameter as a freshness indicator for this species. Similar results have been reported by other authors for Sparidae species [55, 75]. The level of TMA was typically around 5 mg TMA-N/100 g in aerobically stored fresh fish rejected by sensory panels [76]. Chouliara et al. [42] reported that TMA production was significantly (p<0.05) reduced by 1 and 3 kGy irradiation. The concentration of TMA was found to be 3.92 and 2.96 mg/100 g in sea bream kept in vacuum-packed for 35 days at 4 °C. Mendes et al. [49] found highest concentration of TMA non-irradiated horse mackerel, followed by horse mackerels stored in refrigerated and the lowest in irradiated samples (1 and 3 kGy) for 24 days at 3–5 °C. Chouliara et al. [50] also refer that TMA formation is lower in irradiated and cold-stored sea bream than in non-irradiated fish.
The extent of lipid oxidation in the fish during ice storage is depicted in Fig. 5. At the beginning of the storage period, TBA values of non-irradiated sea bream and irradiated sea bream were determined as 0.003 mg MA/kg. In the case of non-irradiated fish, the TBA value increased to a maximum during storage up to the ninth day and decreased. The TBA value of irradiated samples increased during the storage. The decrease in TBA values after day 9 of storage may represent the breakdown of the malonaldehyde to tertiary degradation. At the end of the storage period of 19 days, TBA values of 2.5 and 5 kGy irradiated sea bream were found to be 1.120±0.05 and 0.928±0.02 mg MA/kg, respectively. Similar results have been obtained in irradiated anchovy and threadfin bream [40, 42, 77].
Sensory evaluation
Sensory parameters of raw sea bream were scored as A Quality during the first 9 days of storage and B Quality were obtained between days 9–13 of storage for control group samples (Table 3). The limit of acceptability of quality was reached after 7 days for the control samples and after approximately 9 days for the 2.5 kGy and 13 days for the 5 kGy irradiated samples. Unacceptable limit for quality was reached after approximately 13 days for the control, 17 days (2.5–5 kGy) for the irradiated sea bream (Fig. 6). Acceptability scores for texture, odour, and taste of cooked sea bream stored in ice storage conditions decreased with time of refrigerated storage (Fig. 7). All three sensory attributes showed a similar pattern of decreasing acceptability. The limit of acceptability of texture and taste was reached after 15 days for the control sea bream samples and after ca. 17 days for the irradiated samples. The limits of acceptability of odour were reached after 13 days for the control, after 17 days for 2.5 and 5 kGy irradiated samples.
Food irradiation at a dose of up to 10 kGy has been used in both animal and vegetable foods as an effective, safe and economical method of food preservation posing no nutritional, toxicological or microbiological problems [16]. The application of low-dose irradiation has been previously reported to extend the shelf life of foods. Low-dose irradiation in the range of 1–3 kGy known as “radurization” has been previously used to extend the shelf life of fishery products [43]. van Cleemput et al. [46] reported that the stored at +6 °C and 5 kGy irradiated shrimp was still unacceptable after 8 days, although the control sample was unacceptable after 4 days of storage. The shelf life of non-irradiated crab stored in ice was found to be 3 days, and 14 days for 3 kGy irradiated crab in ice [47]. Ghadi and Venugopal [78] reported a shelf life of 12 days for non-irradiated fish under refrigeration, and an extension of shelf life of up to 25 days using low-dose irradiation (1.5 kGy).
In another study by Icekson et al. [79], ionizing radiation in combination with refrigeration (0–2 °C) was used for shelf-life extension of carp (Cyprinus carpio). Based on sensory evaluation, a shelf life of 31 days (1.5 kGy, 0–2 °C) was obtained for freshwater carp, compared to a shelf life of 15 days for the non-irradiated fish. Lakshmanan et al. [77] studied the bulk preservation of whole anchovy (Stolephorus commersonii) using low-dose gamma irradiation (2 kGy) under various storage conditions. The treated fish were stored in ice in insulated boxes that were held at 13 °C. Non-packaged irradiated fish had a shelf life of 17 days as compared to a shelf life of 13 days for the non-irradiated (non-packaged) samples. With regard to packaged and irradiated fish, a shelf life of 20 days was obtained; however, packaging caused drip accumulation and resulted in poor appearance of the fish. Jeevanandam et al. [40] also reported a shelf-life extension for sea bream in ice from 8 to 12 days (1 kGy) or to 22 days (2 kGy). Ouattara et al. [48] determined shelf life of pre-cooked and 3 kGy irradiated shrimp as 11 days, and of non-irradiated shrimp as 5 days. In another study on preservation of whole salted vacuum-packaged trout using low-dose irradiation (0.5 and 2 kGy) in combination with refrigeration (4 °C), a shelf life of 28 days was obtained for irradiated (2 kGy), salted, vacuum-packaged trout samples, as compared to a shelf life of 7 days for the salted, non-irradiated sample, as determined by sensory evaluation [41]. The combined use of salting and gamma irradiation (0–2 kGy) on the shelf-life extension of threadfin bream (Nemipterus japonicus) was reported by Jeevanandam et al. [40]. A shelf life of 14 and 28 days was obtained for salted and irradiated fish (1 and 2 kGy), respectively, in comparison to a shelf life of 9 days for the salted, non-irradiated fish. The storage life of fish is affected by the initial microbial load of the fish, storage temperature and packing methods [80]. The combined use of vacuum-packed, salting and gamma irradiation (1 and 3 kGy) on the shelf-life extension of sea bream was reported by Chouliara et al. [42]. A shelf life of 27 and 28 days was obtained for irradiated fish (1 and 3 kGy), respectively, in comparison to a shelf life of 14–15 days for the non-irradiated fish. The combined use of vacuum-packed and gamma irradiation (1–3 kGy) on the shelf-life extension of sea bream (Sparus aurata) was reported by Chouliara et al. [50]. A shelf life of 17 and 28 days was obtained for vacuum-packed and irradiated fish (1 and 3 kGy), respectively, in comparison to a shelf life of 9–10 days for the non-irradiated fish. Irradiated (1 kGy) and non-irradiated horse mackerel were sensorial acceptable on the 12–14th days of storage in refrigerator, respectively [49].
The effects of different dose irradiation on microbiological, chemical, and sensory properties of sea bream (Sparus aurata) stored in ice storage were studied. In the present study, a shelf life of 15 and 17 days were recorded for sea bream irradiated 2.5 and 5 kGy in ice, compared with a shelf life of 13 days for the non-irradiated sea bream based on sensory, chemical and microbiological evaluation.
References
Yu SF, Zhang YH, Cheng BS, Zheng SQ (1993) Radiat Phys Chem 42:339–341
Zhang ZZ, Liu XM, Li GF, Yang YT, Tian LM (1993) Radiat Phys Chem 42:331–332
Chen QX, Xu PS, Chen H, Chen LH, Dong SB (1993) Radiat Phys Chem 42:323–326
Chwla SP, Kim DH, Jo C, Lee JW, Song HP, Byun MW (2003) J Food Prot 66(11):2093–2096
Fu J, Shen W, Bao J, Chen Q (2000) Radiat Phys Chem 57:345–348
Kamat A, Warke R, Kamat M, Thomas P (2000) Int J Food Microbiol 62:27–35
Mahrour A, Caillet S, Nketsa-Tabiri J, Lacroix M (2003) J Food Prot 66(11):2156–2159
Yang SF, Perng FS, Liou SE, Wu JJ (1993) Radiat Phys Chem 42:319–322
Leistner L (2000) Int J Food Microbiol 55(1–3):181–186
Jo C, Leea NY, Kanga HJ, Shinb DH, Byun MW (2004) Food Microbiol 21:543–548
Lacroix M, Quattara B (2000) Food Res Int 33:719–724
Thayer DW, Boyd G, Fox JR, Lakritz L, Hamson JW (1995) J Food Sci 60:63–67
Abu-Tarboush HM, Al-Khatami HA, Atia M, Abou-Arab AA, Bajaber AS, El-Mojaddidi MA (1996) J Food Prot 59:1041–1048
Durante RW (2002) Radiat Phys Chem 63:289–294
Morehouse KM (2002) Radiat Phys Chem 63:281–284
Badr HM (2006) Food Chem 97:285–293
Javanmard M, Rokni N, Bokaie S, Shahhosseini G (2006) Food Cont 17:469-473
de Azevedo GH, da Silva EN, Cardello HMAB, Cipolli KMVAB (2003) Meat Sci 65:919–926
Thayer DW, Boyd G (1994) J Food Prot 57:758–764
Albright SN, Kendall PA, Avens JS, Sofos JN (2003) Lebensm-Wiss Technol 36:381–389
Baumgard J (1986) Mikrobiologische Untersuchung von Lebensmitteln. Ed: Jürgen Baumgart, unter Mitarbeit von Jürgen Firnhaber, Gottfried Spicher. Behr's Verlag, Hamburg. ISBN: 3-922528-91-0
Pichhardt K (1998) Lebensmittelmikrobiologie. Grundlagen für die Praxis. Springer-Verlag, Berlin Heidelberg. ISBN: 3540633804
Lagrange F, Jark U, Etzel V, Feldhusen F (2003) Arch Lebensmittelhyg 54(3):65–70
Weilmeier DM, Regenstein JM (2004) J Food Sci 69:FCT16–FCT23
Mattissek R, Schnepel MF, Steiner G (1992) Lebensmittelanalytik, Grundzüge. Methoden. Anwendungen. Zweite, korrigierte Auflage. Springer, Berlin Heidelberg New York. ISBN: 3-540-54684-7
AOAC (1998a) Official Method 938.08. Ash of seafood, chapter 35, p 6. Fish and other marine products, James M. Hungerford, Chapter Editor. Official Methods of Analysis of AOAC International, Edited by Patrica Cunniff. ISBN: 0-935584-54-4, ISSN: 1080-0344
AOAC (1998b) Official Method 955.04. Nitrogen (total) in seafood, chapter 35, p 6. Fish and other marine products, James M. Hungerford, Chapter Editor. Official Methods of Analysis of AOAC International, Edited by Patrica Cunniff. ISBN: 0-935584-54-4, ISSN: 1080-0344
Merrill AL, Watt BK (1973) Energy value of foods, …basis and derivation. Agriculture research service. United States Department of Agriculture, Agriculture handbook no 74, p 2
Vyncke W (1981) In: Proceedings of the 12th Western European Fish Technologists’ Association (WEFTA) Meeting, Copenhagen, Denmark
Antonocoupoulos N, Vyncke W (1989) Determination of volatile basic nitrogen in fish. Z Lebensmit Unters 189:309–316
AOAC (1998c) Official Method 971.14. Trimethylamine nitrogen in seafood colorimetric method, chapter 35, p 7. Fish and other marine products, James M. Hungerford, Chapter Editor. Official Methods of Analysis of AOAC International, Edited by Patrica Cunniff. ISBN: 0-935584-54-4, ISSN: 1080-0344
EC Regulation (1996) Council Regulation (EC) N 2406/96 of 26 November 1996 laying down common marketing standards for certain fishery products (OJ L 334, 23.12.1996), p 1–15
Huss HH (1988) Fresh fish quality and quality changes. FAO fisheries series no 29, FAO, Rome
Karl H, Meyer C, Münkner W (2001) Inf Fish Fischereifor 8(3):139–143
Sümbüloğlu K, Sümbüloğlu V (2002) Biyoistatistik. Hatipoğlu Basım ve Yayım San. Tic. Ltd. Şti. 10. Baskı, Ankara. ISBN: 975-7527-12-2
Huss HH (1994) Assurance of seafood quality. FAO fisheries technical paper 334, FAO, Roma
Ward D R, Baj N J (1988) Food Technol 42:85–89
Lapa-Guimarães J, Aparecida Azavedo da Silva M, Eduardo de Felicio P, Contreras Guzman E (2002) Lebensm-Wiss Technol 35:21–29
ICMSF International Commission on Microbiological Specifications for Foods (1986) Sampling plans for fish and shellfish in microorganisms in foods. Sampling for microbiological analysis: principles and scientific applications, 2nd edn, vol 2. University of Toronto Press, Toronto, pp 181–196
Jeevanandam K, Kakatkar A, Doke SN, Bongiwar V, Venugopal V (2001) Food Res Int 34:739–746
Savvaidis IN, Skandamis P, Riganakos K, Panagiotakis N, Kontominas MG (2002) J Food Prot 65:515-522
Chouliara I, Sawaidis LN, Riganakos K, Kontaminas MG (2004) Food Microbiol 21(3):351–359
Venugopal V, Doke SN, Thomas P (1999) Crit Rev Food Sci Nutr 39:391–440
Molins RA, Motarjemi Y, Käferstein FK (2001) Food Cont 12:347–356
Jo C, Lee WD, Kim DH, Kim JH, Ahn HJ, Byun MW (2004) Food Cont 15:435–440
van Cleemput O, Debevere J, Debevere P, Baert L (1980) Lebensm-Wiss Technol 13:322–323
Chen YP, Andrews LS, Grodner RM (1996) J Food Sci 61(6):1239–1242
Ouattara B, Sabato, SF, Lacroix M. (2001) Int J Food Microbiol 68:1–9
Mendes R, Silva HA, Nunes ML, Empis JMA (2005) Eur Food Res Technol 221:329–335
Chouliara I, Sawaidis LN, Riganakos K, Kontaminas MG (2005) J Sci Food Agric 85(5):779–784
Koutsoumanis K, Lampropoulou K, Nychas GJE (1999) J Food Prot 62:398–402
Papadopoulos V, Chouliara I, Badeka A, Savvaidis IN, Kontominas MG (2003) Food Microbiol 20:414–420
Schormüller J (1968) Handbuch der Lebensmittelchemie (Band III/2). Springer Verlag, Berlin Heidelberg New York
Simeonidou S, Govaris A, Vareltzis K (1998) Food Res Int 30:479–484
Kyrana VR, Lougovois VP, Valsamis DS (1997) Int J Food Sci Technol 32:339–347
Kyrana VR, Lougovois VP (2002) Int J Food Sci Technol 37:319–328
Masyinom P, Benjakul S, Visessanguan W (2002) J Sci Food Agric 82:873–880
Tejada M, Huidobro A (2002) Eur Food Res Technol 215:1–7
Grigorakis K, Taylor KD, Alexis M (2003) Food Chem 81(2):263–268
Taliadourou D, Papadopoulos V, Domvridou E, Savvaidis I, Kontominas GM (2003) J Sci Food Agric 83:1373–1379
EC Regulation (1996) Council Regulation (EC) N 2406/96 of laying down common marketing standards for certain fishery products (OJ L 334, 23.12.1996), pp 1–15
Cakli S, Kilinç B, Cadun A, Dinçer T, Tolasa S (2006) Eur Food Res Technol 222(5–6):719–726
Cakli S, Kilinç B, Cadun A, Dinçer T, Tolasa S (in press) Food Cont. DOI 10.1016/j.foodcont.2005.11.005 Available online 26 January 2006
Al-Kahtani HA, Abu-Tarboush MH, Bajaber AS, Atia M, Abou-Arab AA, El-Mojaddidi MA (1996) J Food Sci 61849:729–733
von Amin MH, Jamil Qureshi M, Haq I, Ashraf Chaudhry M (1978) Arch Lebensmittelhyg 29(2):54–56
Jo C, Lee WD, Kim DH, Kim JH, Ahn HJ, Byun MW (2004) Food Cont 15:435–440
Oetjen K, Karl H (1999) Deut Lebensmit Rundschau 95:403–407
Timm M, Jørgensen BM (2002) Food Chem 76:509–518
López-Caballero ME, Gonçalves A, Nunes ML (2002) Eur Food Res Technol 214:192–197
Debevere J, Devlieghere F, von Sprundel P, De Meulenear B (2001) Int J Food Microbiol 68:115–123
Parkin K, Wells MJ, Brown Wo996D (1981) J Food Sci 47:181–184
Zhang Y, Lu H, Levin RE (2003) Food Microbiol 20:87–90
Ababouch LH, Souibri L, Rhaliby K, Ouadhi O, Battal M, Busta FF (1996) Food Microbiol 13:123–132
Huidobro A, Mendes R, Nunes, ML (2001) Eur Food Res Technol 213:267–272
Huidobro A, Mendes R, Nunes ML (2001b) Eur Food Res Technol 213:267–272
Sikorski ZE, Kolakowska A, Burt J R (1990) Post harvest biochemical and microbial changes seafood. In: Sikorski ZE (ed) Resources nutritional composition and preservation. CRC Press-Inc, Boca Raton, FL, pp 55–75
Lakshmanan R, Venugopal V, Venketashvaran K, Bongiwar DR (1999) Food Res Int 32:707–713
Ghadi SV, Venugopal V (1991) Int J Food Sci Technol 26:397–401
Icekson I, Pasteur R, Drabkin V, Lapidot M, Eizenberg E, Klinger I, Gelman A (1996) J Sci Food Agric 72:353–358
Özoğul F, Taylor KDA, Quantick P, Özoğul Y (2000) Food Chem 71:267–273
Acknowledgements
This study was supported by the Research Fund of the University of Istanbul (Project Nos.: T-516/21102004 and UDP-816/10072006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özden, Ö., İnuğur, M. & Erkan, N. Preservation of iced refrigerated sea bream (Sparus aurata) by irradiation: microbiological, chemical and sensory attributes. Eur Food Res Technol 225, 797–805 (2007). https://doi.org/10.1007/s00217-006-0484-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0484-9